Ex Situ Conservation and Ornamental Evaluation of the Endangered Amberboa moschata (Asteraceae) in Armenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site and Cultivation Techniques
2.2. Morpho-Phenological and Ornamental Traits Assessment
2.3. Chromosome Analysis
2.4. Pollen Fertility Analysis
2.5. Eco-Physiological Features Evaluation
3. Results and Discussion
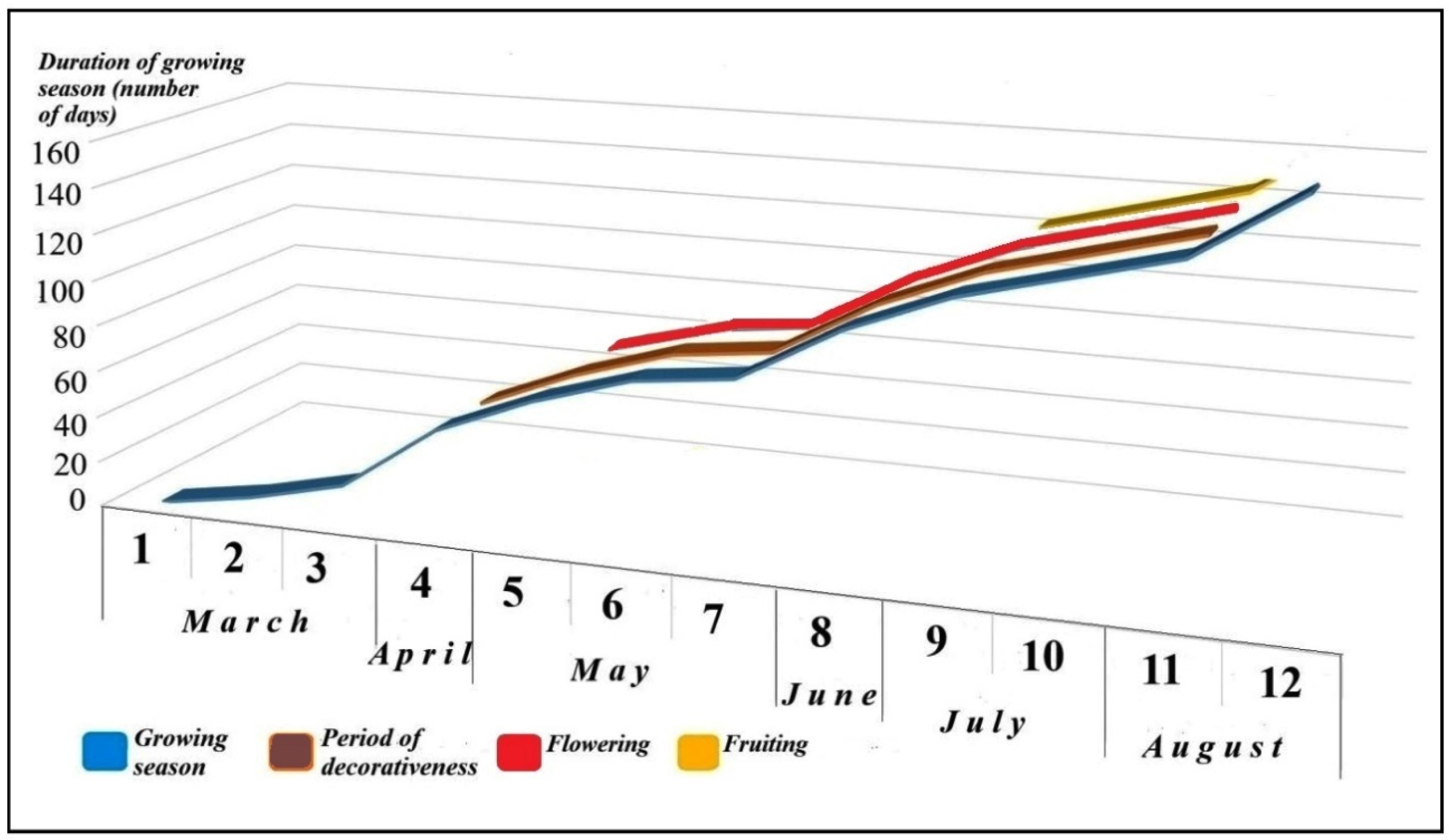
3.1. Morpho-Phenological Characteristics
3.2. Cytogenetics and Pollen Fertility
3.3. Ecological–Physiological Characteristics
3.4. Ornamental Traits and Practical Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gabrielian, E.T. Amberboa (Pers.) Less. In Caucasian Flora Conspectus; Part 1; KMK Scientific Press Ltd.: St. Petersburg, Russia, 2008; Volume 3, pp. 282–285. [Google Scholar]
- Murtazaliev, R.A. A new species of the genus Amberboa (Asteraceae) from Dagestan. Turczaninowia 2024, 27, 81–91. [Google Scholar] [CrossRef]
- Tamanyan, K.; Fayvush, G.; Nanagulyan, S.; Danielyan, T. (Eds.) The Red Book of Plants of the Republic of Armenia. Higher Plants and Fungi, 2nd ed.; Ministry of Nature Protection of Republic of Armenia: Yerevan, Armenia, 2010; 592p.
- Vardanyan, Z.h.H.; Danelyan, T.S.; Fayvush, G.M.; Ghukasyan, A.G.; Nanagyulyan, S.G. Fifth National Report to the Convention on Biological Diversity; Ministry of Nature Protection: Yerevan, Armenia, 2015; 106p.
- Neves, K.G. Botanic Gardens in Biodiversity Conservation and Sustainability: History, Contemporary Engagements, Decolonization Challenges, and Renewed Potential. J. Zool. Bot. Gard. 2024, 5, 260–275. [Google Scholar] [CrossRef]
- Heywood, V.H. Conservation and sustainable use of wild species as sources of new ornamentals. Acta Hortic. 2003, 598, 43–53. [Google Scholar] [CrossRef]
- Akopian, J.A. Conservation of native plant diversity at the Yerevan Botanic Garden, Armenia. Kew Bull. 2010, 65, 663–669. [Google Scholar] [CrossRef]
- Akopian, J.; Hovakimyan, Z.; Paravyan, Z. Bio-morphological features of somerare and endangered sandy and gypsy desert plant species of Armenia. Biol. Zhurn. Armen. 2017, 3, 39–46. [Google Scholar]
- Akopian, J.A.; Ghukasyan, A.G.; Elbakyan, A.H.; Martirosyan, L.Y. Indigenous Flora as a Source of Arid Ornamental Horticulture in Armenia; Gitutiun NAS RA: Yerevan, Armenia, 2023; 213p. [Google Scholar]
- Akopian, J.; Elbakyan, A.; Martirosyan, L.; Hovakimyan, Z.; Ghukasyan, A. Biological aspects of threatened Psephellus erivanensis in the context of ex-situ conservation. Iran. J. Bot. 2023, 29, 165–174. [Google Scholar] [CrossRef]
- Gosling, P.G. Viability testing, Chapter 24. In Seed Conservation: Turning Science into Practice; Smith, R.D., Dickie, J.B., Linington, S.H., Pritchard, H.W., Probert, R.G., Eds.; Royal Botanic Gardens: London, UK, 2003; pp. 445–481. [Google Scholar]
- Baskin, C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014; pp. 150–162. [Google Scholar]
- Beydeman, I.N. Methods of Studying the Phenology of Plants and Plant Communities. In Methodical Instructions; Science: Novosibirsk, Russia, 1974; 155p. [Google Scholar]
- Bylov, V.N. Fundamentals of Comparative Variety Evaluation of Ornamental Plants. In Introduction and Selection of Flower Plants; Nauka: Moscow, Russia, 1978; pp. 7–32. [Google Scholar]
- Pausheva, Z.P. Workshop on Plant Cytology; Kolos: Moscow, Russia, 1980; 304p. [Google Scholar]
- Dospekhov, B.A. Field Experiment Methodology; Kolos: Moscow, Russia, 1973; 336p. [Google Scholar]
- Wolf, V.G. Statistical Data Processing; Kolos: Moscow, Russia, 1966; 254p. [Google Scholar]
- Sheremetyev, S.N. Grasses on a Soil Moisture Gradient; Partnership of Scientific Publications KMK: Moscow, Russia, 2005; 271p. [Google Scholar]
- Salnikov, A.I.; Maslov, I.L. Physiology and Biochemistry of Plants: Workshop; Publishing House of FGBOU VPO Perm State Agricultural Academy: Perm, Armenia, 2014; 300p. [Google Scholar]
- Mezhunts, B.K.; Navasardyan, M.A. Method for Determining the Content of Chlorophylls a, b and Carotenoids in Plant Leaf Extracts; Intellectual Property Agency of the Republic of Armenia: Yerevan, Armenia, 2010. [Google Scholar]
- Shlyk, A.A. Definition of Chlorophylls and Carotenoids in Extracts of Green Leaves. In Biochemical Methods in the Physiology of Plants; Nauka: Moscow, Russia, 1971; pp. 154–170. [Google Scholar]
- Nikolaeva, M.G. Peculiarities of germination of seeds of plants from the subclasses Dilleniidae, Rosidae, Lamiidae and Asteridae. Bot. Zhurn. 1989, 74, 651–668. [Google Scholar]
- Borisova, I.V. Types of germination of seeds of steppe and semi-desert plants. Bot. Zhurn. 1996, 81, 9–22. [Google Scholar]
- Tonyan, T.R. The chromosome number of some species of the genus Centaurea. Biol. J. Armen. 1968, 21, 86–96. [Google Scholar]
- Tonyan, T.R. The relationship between the number of chromosomes and some morphological characters in representatives of the subtribe Centaureinea Less. Biol. J. Armen. 1980, 33, 522–554. [Google Scholar]
- Avetisian, E.M.; Tonyan, T.R. Palynomorphology and number of chromosomes of some of the species of the subtribe Centaureinae Less. Akad. Nauk. Arm. SSSR 1975, 16, 45–49. [Google Scholar]
- Garcia-Jacas, N.; Susanna, A.; Vilatersana, R.; Guara, M. New chromosome counts in the subtribe Centaureinea (Asteraceae, Cardueae) from west Asia, II. Bot. J. Linn. Soc. 1998, 128, 403–412. [Google Scholar] [CrossRef]
- Gupta, R.C.; Gill, B.S. Compositae. In Chromosome number reports LXXI. Taxon 1981, 30, 508–519. [Google Scholar]
- Gupta, R.C.; Gill, B.S. Cytopalynology of North and Central Indian Compositae. J. Cytol Genet. 1989, 24, 96–105. [Google Scholar]
- Moore, D.M. Index to Plant Chromosome Numbers 1967–1971; JSTOR: Utrecht, The Netherlands, 1973; 539p. [Google Scholar]
- Kharina, T.G.; Pulkina, S.V. Features of the biology of flowering Serratulacoronata, L. In Proceedings of the International Scientific Conference Dedicated to the 140th Anniversary of the Siberian Botanical Garden of Tomsk State University: “Botanical Gardens as Centers for the Study and Conservation of Phytodiversity”, Tomsk, Russia, 28–30 September 2020; pp. 203–205. [Google Scholar] [CrossRef]
- Elbakyan, A.H. Pollen fertility in some interspecific hybrids of tomato. In Proceedings of the Sixth International Solanaceae Conference, Madison, WI, USA, 23–27 July 2006. [Google Scholar]
- Elbakyan, A.H. Pollen fertility in some species and interspecific hybrids of tomato. Fl. Veget. Plant Res. Armen. 2007, 16, 94–95. [Google Scholar]
- Yandovka, L.F.; Shamrov, I.I. Pollen fertility in Cerasus vulgaris and C. tomentosa (Rosaceae). Bot. Zhurn. 2006, 91, 206–218. [Google Scholar]
- Kruglova, N.N. Assessment of the pollen grains quality in flowering plants (overview). Bull. State Nikita Botan. Gard. 2020, 135, 50–56. [Google Scholar] [CrossRef]
- Rigamoto, R.R.; Tyagi, A.P. Pollen Fertility Status in Coastal Plant Species of Rotuma Island. S. Pac. J. Nat. Sci. 2002, 20, 30–33. [Google Scholar] [CrossRef]
- Qureshi, S.J.; Khan, M.A.; Arshad, M.; Rashid, A.; Ahmad, M. Pollen Fertility (Viability) Status in Asteraceae Species of Pakistan. Trakia J. Sci. 2009, 7, 12–16. [Google Scholar]
- Belyaeva, T.N.; Leshchuk, R.I. The Pollen Fertility and the Features of Seed Germination of Some Perennial Decorative and Medicinal Plants from Family Asteraceae Dumort. in Culture in the Siberian Botanical Garden. Belgorod State Univ. Sci. Bull. Nat. Sci. 2011, 187, 188–192. [Google Scholar]
- Gibson, A.C. Photosynthetic Organs of Desert Plants. BioScience 1998, 48, 911–920. [Google Scholar] [CrossRef]
- Mircea, D.M.; Calone, R.; Shakya, R.; Saavedra, M.F.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Use of Multivariate Analysis in Screening for Drought Tolerance in Ornamental Asteraceae Species. Agronomy 2023, 13, 687. [Google Scholar] [CrossRef]
- Eftekhari, M.S. Impacts of climate change on agriculture and horticulture. In Climate Change; Springer International Publishing: Cham, Switzerland, 2022; pp. 117–131. [Google Scholar]
- Cicevan, R.; Al Hassan, M.; Sestras, A.F.; Prohens, J.; Vicente, O.; Sestras, R.E.; Boscaiu, M. Screening for drought tolerance in cultivars of the ornamental Genus Tagetes (Asteraceae). PeerJ 2016, 4, e2133. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.A.; Martínez-Sánchez, J.J.; Fernández, J.A.; Bañón, S. Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments. J. Hortic. Sci. Biotechnol. 2006, 81, 3–17. [Google Scholar] [CrossRef]
- Juan Vicedo, J.; Laguna, E.; Ríos, S.; Casas, J. Ornamental potential of the coastal plant Lapiedra martinezii Lag. (Amaryllidaceae): The role of its revalorization in xero-gardening and ex-situ conservation. Nereis Interdiscip. Ibero-Am. J. Methods Model. Simul. 2021, 13, 211–226. [Google Scholar] [CrossRef]
- Nikolić, M.; Stevović, S. Family Asteraceae as a sustainable planning tool in phytoremediation and its relevance in urban areas. Urban For. Urban Green. 2015, 14, 782–789. [Google Scholar] [CrossRef]
- Wróblewska, A.; Stawiarz, E.; Masierowska, M. Evaluation of Selected Ornamental Asteraceae as a Pollen Source for Urban Bees. J. Apic. Sci. 2016, 60, 179–191. [Google Scholar] [CrossRef]





| Specimen Number | Locality |
|---|---|
| ERE 130754 | Abovyan distr., Zovashen, vicinity of the Azat reservoir, hammada. 14 June 1985, E. Gabrielian |
| ERE 139664 | Abovyan distr., between villages Djrvej and Shorbulakh, on dry clay slopes, 1100–1500 m a.s.l. 27 June 1985, E. Gabrielian |
| ERE 130754 | Abovyan distr., Zovashen, vicinity of the Azat reservoir, hammada. 14 June 1985, E. Gabrielian |
| ERE 139664 | Abovyan distr., between villages Djrvej and Shorbulakh, on dry clay slopes, 1100–1500 m a.s.l. 27 June 1985, E. Gabrielian |
| ERE 145154, 145156 | Nubarashen, on clay slopes. 2 July 1997, E. Gabrielian |
| ERE 151800 | Near Nubarashen, on tertiary red clays. 24 May 2000, E. Gabrielian |
| ERE 153193 | Kotayk province, Abovyan distr., between villages Shorbulakh and Vokhchaberd, 3 km SSW of Vokhchaberd, Erebuni reserve, mountain steppe, 1350 m a.s.l., 1 July 2003. M. Barkworth, F. Smith, E. Gabrielian, A. Nersesyan, M. Oganesyan |
| ERE 202314 | Yerevan, southern border of city at Sovetashen, 1040 m, 40°07′22 N/44°32′36″ E 11.07.2003, M. Oganesian, H. Ter-Voskanyan, E. Vitek |
| ERE 161, 644 | Sovetashen, 1190 m a.s.l. 40°06′100″ N/44°33′25″ E. 26.05.2006. K. Tamanyan, G. Fayvush |
| ERE 190335 | Kotayk marz, vicinity of Vokhchaberd village, on the territory of the Erebuni Nature Reserve, on clays. 5 June 2008. J. Akopian |
| ERE 182109 | Vedy region, near v. Urtcadzor, on dry clay slopes, 1100 m. 24 May 2011, E. Gabrielian |
| ERE 202313 | Ararat province, slope between river and road Vedi to Lusashogh, 3.5 km SE of Urtsadzor, 1165 m, 39°53′50″ N/40°50′58″ E 17 May 2017, E.Vitek, M.Oganesian, M.Sargsyan, A.Khachatryan |
| ERE 202334 | Ararat Marz 5.6 km from Lanjasar, near Azat reservoir, 40°05′13″ N/44°38′06″ E, 1110 m to 40°05′17″ N 44°38′05″ E, 1130 m, 4 June 2018, E.Vitek, P. Escobar-Garcia, G. Fayvush |
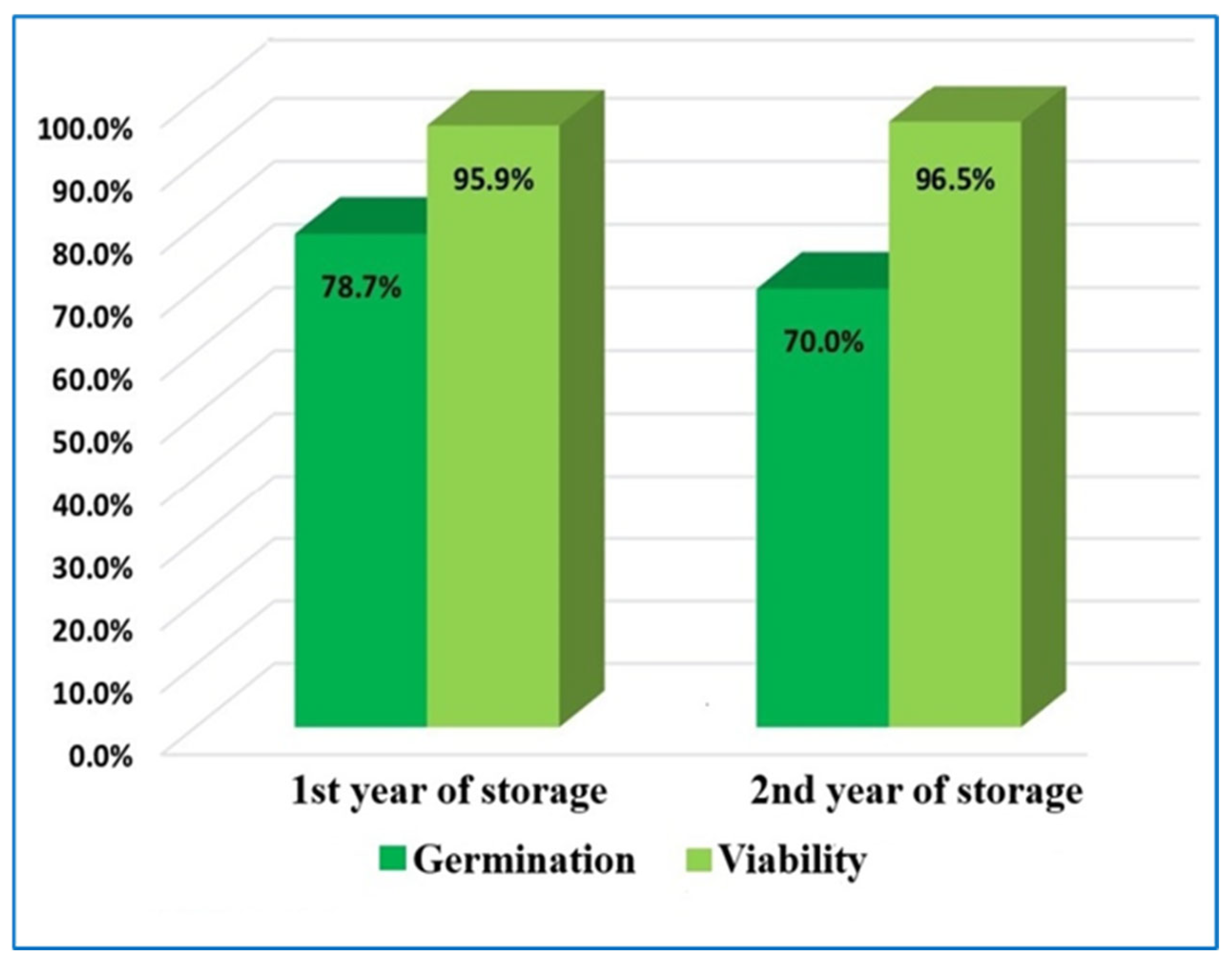
| Experiment Repetition Number | Seed Germination (%) | Seed Viability (%) | ||
|---|---|---|---|---|
| Seeds from the 1st Year of Storage | Seeds from the 2nd Year of Storage | Seeds from the 1st Year of Storage | Seeds from the 2nd Year of Storage | |
| I | 82 | 70.5 | 96 | 94.4 |
| II | 73.3 | 67.6 | 98 | 100 |
| III | 81.3 | 71.8 | 93.8 | 94.8 |
| Average | 78.7 ± 3.22 | 70 ± 1.3 | 95.9 ± 1.2 | 96.5 ± 2.1 |
| Amberboa moschata | Pollen Grain Size, Μm | Pollen Fertility Percentage | ||
|---|---|---|---|---|
| Range | Average Size, μm | Range | Average Fertility, % | |
| Yerevan Botanical Garden | ||||
| Cultivated specimens | 61.4–63.2 | 62.1 ± 0.6 | 92–100 | 96.7 ± 0.9 |
| Herbarium samples (ERE) collected from natural habitats | ||||
| N 130754 | 62.4–67.1 | 64.1 ± 1.1 | 93–100 | 96.0 ± 2.2 |
| N 139664 | 60.2–62.8 | 61.7 ± 0.5 | 96–100 | 97.8 ± 0.7 |
| N 145154 | 59.8–61.6 | 60.6 ± 0.4 | 95–100 | 98.2 ± 1.1 |
| N 151800 | 59.4–62.4 | 60.6 ± 0.6 | 95–99 | 97.4 ± 0.9 |
| N 153193 | 62.4–63.6 | 62.6 ± 0.4 | 95–100 | 97.6 ± 1.3 |
| N 202314 | 57.4–62.6 | 59.8 ± 1.3 | 92–100 | 96.8 ± 1.4 |
| N 161644 | 58.2–62.4 | 59.7 ± 0.8 | 98–100 | 99.4 ± 0.5 |
| N 182109 | 58.8–63.0 | 60.6 ± 0.8 | 94–100 | 96.6 ± 1.3 |
| N 202313 | 60.2–62.8 | 61.7 ± 0.5 | 95–99 | 95.8 ± 1.6 |
| N 202334 | 60.3–64.5 | 62.2 ± 1.1 | 95–100 | 98.1 ± 1.2 |
| Plant Species and Habitat | Total Water Content, % | Water Deficit, % | Intensity of Transpiration, mg CO2 dm2/h | Photosynthetic Productivity, mg/g Wet Weight, h |
|---|---|---|---|---|
| Amberboa moschata cultivated in the Yerevan Botanical Garden | 50.02 ± 0.91 c | 35.83 ± 0.82 d | 135.62 ± 0.92 b | 2.13 ± 0.902 cd |
| Amberboa moschata in the natural habitat of the Erebuni State Reserve | 48.01 ± 1.08 d | 37.81 ± 0.81 b | 130.43 ± 0.91 b | 2.03 ± 0.821 d |
| Optical density of chlorophyll “a”, λ 663 | 0.932 ± 0.015 d |
| Optical density of chlorophyll “b”, λ 645 | 0.847 ± 0.001 c |
| Optical density of carotenoids, λ 440.5 | 1.261 ± 0.014 d |
| Chlorophyll “a” content, per wet leaf (mg/g) | 22.308 ± 0.114 c |
| Chlorophyll “b” content, per wet leaf (mg/g) | 26.612 ± 0.112 c |
| Chlorophyll “a” + ”b” | 48.920 ± 0.102 cd |
| Chlorophyll “a”/“b” | 0.8 ± 0.022 c |
| Carotenoids content, per wet leaf (mg/g) | 6.85 ± 0.271 d |
| Traits of Decorativeness | Traits Value and Score (Points) | Trait Significance Coefficient | Number of Points |
|---|---|---|---|
| 1 | 2 | 3 | 4 |
| Inflorescence color and stability | Color is bright, stable or slightly unstable (5) | 3 | 15 |
| Inflorescence shape | Large fringed basket (5) | 2 | 10 |
| Inflorescence size (diameter and height) | Diameter 5–7 cm, height from 3.5–4.5 cm (5) | 2 | 10 |
| Petal quality | Dense, retaining shape under adverse weather conditions (5) | 1 | 5 |
| Number of inflorescences on one generative shoot | One inflorescence (5) | 2 | 10 |
| Number of simultaneously open inflorescences on a plant | In the mass flowering phase about 70% (5) and more or about 50% (4) | 3 | 15 |
| Inflorescence density | Dense, compact (5) | 2 | 10 |
| Shoot strength | Not subject to deformation under the influence of external factors (5) | 2 | 10 |
| Shoot coloring | Bright (5) or middle bright (4) | 1 | 4 |
| Leaf color stability | Stable (5) or slightly unstable (4) | 2 | 8 |
| Durability of leaf decorativeness | Most decorative during the phases of budding and flowering (5) | 1 | 5 |
| Fruit decorativeness | Fruits slightly enhance the decorative effect (5) | 3 | 9 |
| General condition of plants during the flowering period | Presence or absence of breaks during flowering (5) | 2 | 10 |
| Plant originality | Habitus attractiveness (5) | 1 | 5 |
| Period of decorativeness | From the phase of formed vegetative habit of the plant until the end of flowering (5) | 1 | 5 |
| Sum of points | 131 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akopian, J.; Ghukasyan, A.; Elbakyan, A.; Martirosyan, L.; Hovakimyan, Z. Ex Situ Conservation and Ornamental Evaluation of the Endangered Amberboa moschata (Asteraceae) in Armenia. J. Zool. Bot. Gard. 2025, 6, 26. https://doi.org/10.3390/jzbg6020026
Akopian J, Ghukasyan A, Elbakyan A, Martirosyan L, Hovakimyan Z. Ex Situ Conservation and Ornamental Evaluation of the Endangered Amberboa moschata (Asteraceae) in Armenia. Journal of Zoological and Botanical Gardens. 2025; 6(2):26. https://doi.org/10.3390/jzbg6020026
Chicago/Turabian StyleAkopian, Janna, Anahit Ghukasyan, Araksya Elbakyan, Lora Martirosyan, and Zhanna Hovakimyan. 2025. "Ex Situ Conservation and Ornamental Evaluation of the Endangered Amberboa moschata (Asteraceae) in Armenia" Journal of Zoological and Botanical Gardens 6, no. 2: 26. https://doi.org/10.3390/jzbg6020026
APA StyleAkopian, J., Ghukasyan, A., Elbakyan, A., Martirosyan, L., & Hovakimyan, Z. (2025). Ex Situ Conservation and Ornamental Evaluation of the Endangered Amberboa moschata (Asteraceae) in Armenia. Journal of Zoological and Botanical Gardens, 6(2), 26. https://doi.org/10.3390/jzbg6020026






