Abstract
For the effective management of captive animals, monitoring their behavior and interactions within the exhibit is essential. This approach provides insights into their welfare and helps identify behavioral differences compared to conspecifics in the wild. This ex situ study aimed to provide more insights about the behavior of a pair of siamangs residing at the Wildlife Park “Le Cornelle” in Valbrembo, Italy. The focus was on their behavior, and observations were conducted during two distinct periods (May–June 2021 and December 2021) to identify any seasonal differences. In both periods, the most frequent behaviors observed were resting, feeding, and allogrooming. Compared to conspecifics in the wild, allogrooming and singing occurred more frequently. Spatial analysis indicated a higher utilization of outdoor spaces than the tunnel and indoor areas, with statistically significant variations for both periods. The spatial analysis of singing behavior showed an equal use of outdoor and indoor areas during the first observation period. However, in the second observation period, a preference for indoor spaces was observed, likely due to lower recorded temperatures. More observations are needed to identify the factors influencing behavior, but it is clear that continuous monitoring plays a vital role in promptly addressing deviations or anomalies in animal behavior.
1. Introduction
The survival of both plant and animal species is under significant threat due to rapid global population growth and depletion of natural resources. Consequently, the preservation of biological and genetic diversity has become essential. In this context, wildlife parks play a crucial role by providing the so-called “ex situ conservation” [1]. Since the adoption of the Convention on Biological Diversity in 1992, these institutions have expanded their mission to include social responsibilities, with a focus on conservation, education, and compliance with regulations that ensure animal welfare and public awareness of biodiversity [2]. To achieve these goals, it is important to consider the concept of animal welfare, which includes not only physical health but also overall well-being. This encompasses environmental quality and the opportunity to express a variety of natural behaviors [3].
Captive animals should be free from prolonged negative emotional states, which requires environments that support natural behavioral repertoires and physiological balance. Environmental enrichments, designed to meet physical, physiological, and cognitive needs, enhance welfare by increasing behavioral diversity, reducing abnormal behaviors, and fostering positive interactions with the captive environment [4].
This field is indispensable for designing exhibits that meet animals’ needs. Information obtained from studies of animal behavior is then used to improve animal care practices and living conditions, as well as raise awareness in society [5].
The design of captive environments often aims to simulate or replicate aspects of the animals’ natural habitats [6], while feeding plans are tailored to meet the nutritional needs of each species. Enrichment programs are implemented to provide additional stimulation and encourage species-specific behaviors [7]. Research into the biology and ethology of species, both in captivity and the wild, provides critical insights for conservation management. The expression of species-typical behaviors akin to those observed in the wild serves as an indicator of an animal’s well-being, the suitability of its environment, and overall health [8].
Symphalangus syndactylus (Raffles, 1821), commonly known as “siamang”, is a small primate, considered endangered (EN) according to the IUCN 2020 Red List. It is primarily found on the Indonesian island of Sumatra and also occurs in the southern portion of the Malaysian Peninsula, typically at altitudes up to 2300 m [9], although a study has suggested that their range can extend to areas as high as 3800 m [10]. They usually prefer tree heights ranging from 20 to 30 m with canopy connectivity of 50–70% [11]. The most significant threat to this species is the loss of habitat, which is being fragmented and degraded mainly due to activities like logging for agriculture and road construction [12]. In Malaysia, as in Sumatra, the expansion of oil palm plantations is a key driver of forest loss, and deforestation rates have increased in recent years, with predictions that up to 60% of suitable habitat may be lost by the middle of this century [13].
The siamang is protected throughout its range by local laws, and international trade is prohibited due to its inclusion in Appendix I of CITES. However, the future of the species remains uncertain, even within protected areas, and will depend heavily on strengthened conservation efforts. A key conservation strategy is to preserve the species within wildlife parks, where public awareness and educational activities can be conducted, thereby improving knowledge of its status and conservation needs. Currently, a large population of captive individuals is housed across 96 zoological collections, making it essential to implement effective management strategies [13]. Furthermore, to ensure optimal animal welfare in captivity, it is essential to address not only the general requirements of the species, but also the specific needs of each individual [14].
While there are many studies in the literature focusing on intergroup social relationships, vocalization, and brachiation [15,16,17,18,19,20] in siamangs, there are relative few studies that examine the totality of behavioral pattens in relation to available space [21]. These kinds of studies are important, as they not only contribute to our understanding of the ethology of the species but also provide valuable insights for designing exhibits that promote animal welfare [22].
Thus, the aims of this study are threefold: first, to determine the time budget of activities by analyzing the behavior of the two studied siamangs and exploring their interactions and relationship with the surrounding environment; second, to assess space utilization by examining how the entire exhibit is used to evaluate whether it allows the animals to fully express the behavioral repertoire characteristic of their species; and finally, to conduct an in-depth investigation of their singing behavior, identifying the contexts and conditions under which it occurs in a controlled environment.
2. Materials and Methods
2.1. Pair of Siamangs
The subjects of this study were a pair of siamangs residing at the Wildlife Park “Le Cornelle” in Valbrembo (BG), Italy. The data of the individuals are summarized in Table 1.

Table 1.
Main identifying features of the two specimens.
2.2. Exhibit
The exhibit is placed in the northern part of the park and it includes three parts:
- The outdoor part of the exhibit has three sides and the roof is closed by a wire mesh, while the other side has three glass windows. This section has a trapezoidal shape with dimensions of 10 m × 12 m × 10 m × 14.5 m and a height ranging from 4 m to 6 m;
- The wire meshed tunnel, connecting indoor and outdoor spaces, has a maximum height of 5 m and a total length of 20 m;
- The indoor part of the exhibit has a rectangular shape with approximate dimensions of 3 m × 2 m and a height of 2.5 m.
Data collection was based on previous experimental designs [23]. The outdoor area was divided into six levels, each of which was further subdivided into 1 m3 cells. Subsequently, a unique system of coordinates was established for each cell within the outdoor exhibit area by assigning numbers from 1 to 10 for the width and letters from A to Q for the length (e.g., 01D7).
Within the outdoor space, various elements are present, including Chusan palms (Trachycarpus fortunei), a scaffold featuring horizontal, vertical, and oblique poles, 8 ropes, and other smaller objects used as enrichments. The levels of the outdoor exhibit area were nearly the same during the two observation periods, the only significant differences being the arrangement of some horizontal poles at the fourth and fifth levels.
The same subdivision method reported in Sacchet [23] was also applied to the indoor space. Given the small size, it was not the necessary to subdivide the space through a grid with coordinates. Instead, 4 levels and some reference zones and elements were identified and labeled with the lowercase letter “i” (indoor) followed by the level number: for example, the shelf (shelf) located on the second level had the coordinate:“i08-s”.
During the first two observation periods, the indoor elements, such as the 3 shelves and ropes, remained unchanged. The plans used for data collection are in the Supplementary Materials (Figures S3–S8).
2.3. Ethogram
The ethogram utilized in the current study was adapted from the one developed for the aforementioned work by Sacchet [23]. The ethogram consisted of 29 micro-categories organized into 13 macro-categories, as detailed in Table 2. Figure 1 shows some behavioral modules.

Table 2.
Ethogram used for scan sampling.
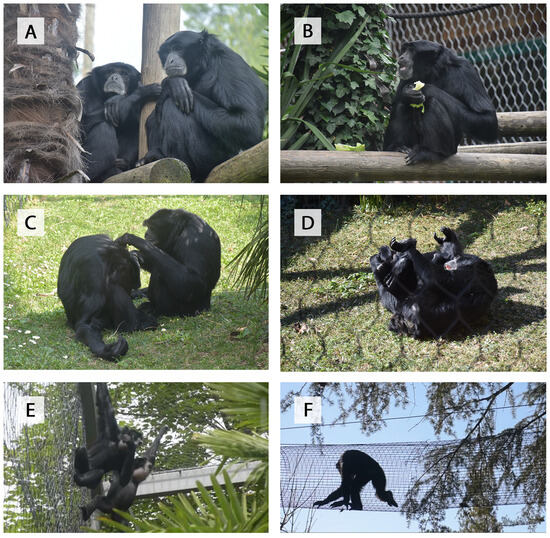
Figure 1.
Examples of behavioral modules (A–F): (A) rest; (B) feed; (C) allogrooming; (D) play; (E) sing; and (F) move.
The following were the micro-categories that constituted the ethogram, detailing the various behaviors observed.
Grooming behaviors
- Scratching referred to the use of fingers or nails to scratch various parts of the body, often through quick and repetitive movements.
- For allogrooming (T-N) and (N-T), the notation (T-N) indicated that Tomas was the grooming individual, providing allogrooming to Nina, whereas (N-T) signified the reverse, with Nina grooming Tomas.
Play behaviors
- Acrobatic swing: In solitary play, the siamang moved independently on ropes, sometimes hanging upside down or using ropes like a swing, often rapidly and in combination with other play behaviors. In social play, this occurred within a chase session (Chase)
- Chase: Either individuals engaged in playful pursuit, chasing one another or, less frequently, external objects.
- Grapple/Bite: Individuals grasped and rolled together while playfully biting, usually on the ground.
- Grunt/Growl: Playful wrestling was combined with grunting and growling vocalizations, typically occurring on the ground.
- Manipulation play: The individual manipulated an object, often a detached palm frond, while moving on ropes, not for locomotion but as part of play.
- Roll: The individual curled up and rolled by pushing off with its forelimbs.
- Somersault: The individual performed a full-body rotation, typically while in the air, completing a flip.
Conflict behaviors
- Slap: A quick, forceful strike was made with an open hand to another individual.
- Open mouth: The individual opened its mouth widely, approaching the face of another individual while displaying its teeth.
Singing behaviors
- Singing: For example, SI-AM indicated that the micro-categories reflected the behaviors observed during vocalizations.
Manipulative behaviors
- Manipulation: The siamang interacted with an object.
- Catching insects: The individual captured insects using its limbs, often consuming them afterward.
Resting behaviors
- Look: The individual remained still while observing the surroundings, occasionally turning its head in different directions.
- Hanging rest: The siamang hung motionless for an extended period, either from the mesh or from pole.
- Lie down: The siamang was in a fully reclined position.
- Sit: The individual remained seated without movement, appearing to rest but with open eyes.
- Sleep: The siamang was completely at rest with their eyes closed.
Feeding behaviors
- Feed keeper: This referred to food being provided directly by keepers during feeding sessions. It included specific items offered to the animals as part of their diet in a controlled environment.
- Feed weed: This referred to all food sources that were not part of the provided diet by keepers, but rather those that the animals obtained independently, such as grass from the outdoor area, palm fruits, leaves, and other naturally available plants.
Contact behaviors
- Touch indicated a light and slow contact made with a limb on another individual.
- Hug indicated that the animals embraced, with one or more limbs fully wrapping around another individual.
Locomotor behaviors
- Hang move indicated that the siamang moved slowly while hanging from mesh or pole.
- Walk indicated a slow, bipedal or quadrupedal movement, occurring either on the ground or while balancing on poles.
- Swing indicated a rhythmic, repetitive motion while suspended from ropes or palm fronds.
- Acrobatic move indicated a rapid and agile movement, involving leaps between poles, ropes, palm fronds, or the mesh.
Other behaviors
- Defecate indicated all physiological elimination behaviors.
- Sexual behavior included mounting and copulation.
2.4. Data Collection
Data were collected over two different periods: the first from 17 May 2021 to 7 June 2021, while the second from 11 December 2021 to 20 December 2021 (32 days in total). For the first period, data collection took place from 9:00 AM to 5:30 PM, totaling 8.5 h. As for the second period, scans were performed from 9:00 AM to 4:00 PM, totaling 7 h. Overall, data collection spanned a total of 496 h. Data collection was performed using scan sampling, i.e., the position of the animals was recorded every five minutes along with a description of the behavior exhibited during that time.
2.5. Data Analysis
Activity budgets and space use across various levels were calculated as the percentage of time spent in each behavior and at each spatial level, respectively, by each individual. The calculations were performed using standard methods, such as pivot tables or summary statistics, depending on the specific analysis.
For the in-depth study of the behavioral sing module, we focused on four key aspects:
- Singing frequency;
- Spatial analysis of the relative spatial distribution of singing behavior around the exhibit space;
- Time of day in one-hour time slots;
- Duration, subdivided into six durational categories.
To complement the descriptive analyses, statistical tests were performed to assess potential differences in behavior between the two individuals. In particular, the Chi-square test was used to determine whether there were significant differences in the frequency of behavioral modules exhibited by each individual. Additionally, the test was applied to evaluate whether the spatial use of the exhibit was non-random, assessing whether the observed preferences for certain areas were statistically significant. These analyses provided valuable insights into individual behavioral patterns and space use within the environment.
3. Results
3.1. Spatial Analysis and Time Budget
The analysis revealed that the differences observed between the two individuals were not statistically significant (χ2 = 0.40; p = 0.52; df = 1), leading to the discussion of results for the pair as a whole. Individual data are summarized in Tables S1 and S2 in the Supplementary Materials. Table 3 and Figure 2 provide a summary of the spatial analysis and temporal balance for the pair of individuals, considering both periods together.

Table 3.
Percentages of pair’s behavioral modules at the various levels of the exhibit.
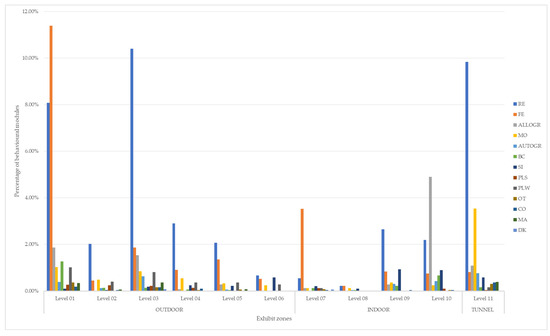
Figure 2.
Percentage of pair’s behavioral modules at the various levels of the exhibit.
3.2. Singing
The individuals performed vocalizations most frequently in the indoor area, followed by the outdoor area, and least in the tunnel. The specific percentages for each level are summarized in Table 4 and Figure 3.

Table 4.
Percentages of vocalizations performed by the two siamangs on the different levels.
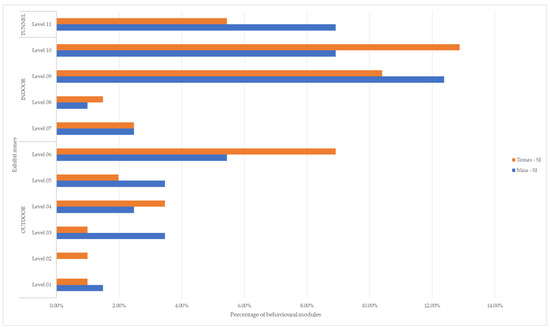
Figure 3.
Percentage of vocalizations performed by the two siamangs at the different levels.
Most of the singing took place between 9:00 AM and 3:00 PM, with two peak periods of vocal activity recorded between 10:00–10:55 AM and 11:00–11:55 AM. In contrast, much lower levels of vocalization were detected in the early afternoon. No vocalizations were detected outside this time range (Table 5).

Table 5.
Percentages of vocalizations made by the two siamangs in relation to time slots.
The analysis of song durations revealed that no vocalizations with durations between 1 and 5 min or 26–30 min were recorded. The percentages are presented in the following table (Table 6).

Table 6.
Percentages of vocalizations made by the two siamangs in relation to the time categories.
4. Discussion
4.1. Time Budget and Spatial Analysis
As reported in Table 3, the greatest percentage of behavior was spent resting (41.56%), in agreement with the literature, where it was found that, in the wild, siamangs spend 25–45% of their time resting [24], and with data recorded for the same individuals during previous works: 40.8% [23] and 48.3% [25]. In particular, the most used levels for this activity were 03 and 11 (tunnel). This can be explained by the presence of supports (poles and wire mesh) providing individuals with high and safe places to rest [26].
The second greatest percentage of behavior was feeding (22.61%). This was lower than the percentage reported in the literature in the wild, which was 35–50% [24], but in agreement with ex situ studies: 21% [25] and 17.84% [23]. This phenomenon can be explained by the fact that animals reared in situ, accustomed to receiving a consistent amount of food at regular intervals, likely spend less time searching for food. Additionally, in the wild, the diet comprises 42.2% whole leaves [24], which necessitate greater consumption to meet daily nutritional requirements, thereby increasing the time spent feeding. The levels on which this behavioral module occurred most frequently were 01 and 07. In particular, the former was characterized by the presence of wild grass used by the individuals as a supplement to their diet. Level 07, on the other hand, corresponded to the indoor level where the meal was placed, twice a day.
The third most common behavior was allogrooming, representing (10.14%) of the behavioral scans. It is important for hygienic, social, and communication purposes, to relieve stress and strengthen relationships [17]. Level 10 appeared to be most suitable for allogrooming since it had the highest positioned shelf, thus providing a safe place for social and grooming activities.
An important difference found with siamangs in the wild was singing. In the wild, the behavior occupies only 1% of the day, whereas in captivity, siamangs spend an average of 4.70% of their time singing solo [27] or 5.80% [28] duetting. The percentage of time the focal pair spent vocalizing (4.08%) was in agreement with previously cited works. It is important to emphasize that, in nature, vocalizations occur on 30% of observation days with a very high variability, probably due to food availability, breeding season [24], and the presence or absence of conspecifics in neighboring territories [27]. In the present study, 75.18% of the observation days were characterized by one or more vocalization. This difference may be due to the fact that, in a controlled environment, the animals are subjected to many unfamiliar external stimuli such as noises and singing from neighboring pairs. The most commonly used levels during vocalizations were those positioned at higher elevations, consistent with natural behavior, as they provided better sound propagation and greater control over the territory.
The time budget analysis conducted by Pearson et al. (2010) [29] indicated that the most frequent behaviors were resting and feeding, findings which are consistent with the present study. The frequency of the singing behavior was also comparable. However, it is important to note that their sample included a mixed-species group, with both a pair of siamangs and a pair of orangutans in the same exhibit. While the presence of an additional species could potentially influence the distribution and frequency of various activities, the results from the present study, which involved siamangs in a single-species group, did not suggest any significant role for group composition in the observed behavioral patterns.
Taking the spatial analysis into consideration, it can be stated that the individuals preferred the outdoors (60.26%), in particular the most used levels were 01 (26.28%) and 03 (17.39%). This is in disagreement with the ecology of the species, which is rarely observed on the ground [24]; in this case, the presence of grass on level 01 and the stable supports on level 03 meant that these individuals made greater use of the lower levels. The subjects in this study used the ground for allogrooming or play sessions, grasping and biting each other with or without growls (grapple/bite or grunt/growl). This is consistent with previous reports in the literature for captive individuals [30]. Indoor areas (21.70%) and tunnels (18.04%) showed similar percentages of use. Despite their relatively small surface area, these structures still exhibited high usage rates. These areas provided essential shelter (indoor areas) and elevated vantage points for observing other parts of the exhibit (tunnels), making them indispensable components of the overall exhibit. Notably, level 11 (tunnel) ranked as the second most frequently used area, further highlighting its significance within the exhibit.
4.2. Analyses of Singing
Spatial analyses focusing exclusively on the singing behavioral module revealed that the percentages of vocalizations differed across the areas, with a higher usage of the indoor area (51.98%), followed by the outdoor area (33.66%) and the tunnel (14.36%). Interestingly, in most instances, singing sessions commenced in the outdoor area, then extended into the tunnel, and ultimately concluded in the indoor area, or vice versa, covering all regions of the exhibit. The indoor area’s level that was most frequently utilized during duets was 09 (22.77%). This level is not the highest, but it offers a connection to the tunnel, and the animals frequently used this exit to project their vocalizations outside. The second most utilized level was level 10 (21.78%), which is the highest level. At this height, the individuals often made use of the shelf, held onto the ropes, or remained suspended from the upper part of the aluminum structure in the outdoor area. Notably, Tomas, in particular, frequently struck his hind legs to create noise during duets. Notably, the tunnel exhibited a usage percentage of 14.36%, and the highest outdoor level (06) showed the same usage percentage (14.36%) (Table 4). In all instances, these findings align with what has been reported in the existing literature, indicating that individuals tend to choose higher and acoustically favorable locations for sound propagation [26,31].
The analysis of vocalization time slots revealed that the highest activity occurred between 10:00 and 11:55, accounting for 60.39% of vocalizations (Table 5). It is worth noting that, in the wild, siamangs tend to vocalize early in the morning, after foraging, and in the late afternoon [15,31]. However, studies conducted on the same individuals in captivity showed two peaks, one between 12:00 and 12:55 and the other at 14:00–14:55 [28]. The placement of AudioMoth in previous studies ruled out the occurrence of vocalizations during times prior to our first time slot, which began at 9:00. The variation in behavior compared to the wild may be attributed to the fact that the late morning hours coincide with an increased number of visitors and, consequently, more noise that might trigger singing. The difference observed compared to the previous study might have been due to seasonality, as well as variations in group composition or differences in visitor frequency across the different study periods. To fully elucidate the underlying factors, further detailed investigation would be necessary [23,25].
Regarding the duration of the singing sessions, the highest percentage was recorded in the 16–20 min category, accounting for 50.00% of singing sessions (Table 6). In the wild, the average duration of singing session for this species typically ranges between 11.8 min [31] and 15–16 min [24]. In captivity, singing sessions usually range between 11–15 min (30.61%) and 16–20 min (28.57%) [28]. In this study, the average singing session duration for the siamang pair was 18 min, which aligns with both wild and captive observations previously conducted on siamangs.
4.3. Comparisons Between First and Second Observation Periods
The time budget comparison revealed an increase in resting frequency in December (47.41%) compared to the May–June period (39.76%). This shift was likely a result of the lower temperatures recorded during autumn, with minimum temperatures dropping to −1 °C. It is important to note that, throughout both observation periods, the animals were not confined indoors due to weather conditions and were instead granted unrestricted access to all three areas of the exhibit, allowing them to choose whether to remain indoors or outdoors. In response, the animals tended to conserve energy by resting more. This hypothesis support a reduction in movement activity, which decreased from 8.18% to 6.81%, and of play activity, which decreased from 4.49% in the spring period to 0.34% in the autumn period. The table containing the percentages of usage of the levels and behavioral modules for each observation period can be found within the Supplementary Materials (Table S5).
Notably, the frequency of the solitary play behavior increased from 0.98% during the spring period to 1.72% in the autumn period. This slight increase may be attributed to the fact that, during the autumn observation period, the park was often closed to the public due to icy conditions. As a result, the keeper introduced enrichment activities at regular intervals to prevent the animals from becoming bored. Additionally, unlike the spring period, a specific behavioral module, rolling, was observed in Nina upon the arrival of the keepers for feeding. However, the most frequent behaviors remained resting, feeding, and allogrooming.
In the comparison of spatial analysis, there was a significant difference in the utilization of level 01, which was the most frequently used during the spring period (31.90%), while in the autumn observation period, its frequency of use dropped to 7.93% (Table S5). This difference may be attributed to climate-related factors, as the animals, due to lower temperatures, might have sought higher locations to avoid soil humidity. This hypothesis was supported by the overall trend of increased utilization of higher levels, both in the outdoor and indoor areas. For instance, the frequency of level 10 usage increased from 7.76% in the spring period to 18.28% in the autumn period.
The greater use of the upper levels in the outdoor area could have also been influenced by the addition of horizontal poles in the wooden scaffolding. While the pair still preferred the outdoors, the difference was less pronounced during the autumn period, and they displayed a preference for upper levels over lower ones.
4.4. Singing Comparisons Between the First and Second Observation Periods
Analyzing the time budgets of the two observation periods and focusing on the singing behavior module, it appeared that the percentage of vocalizations remained stable (4.17% in spring, 3.79% in autumn). This is in agreement with what has been reported in the literature for captive siamangs [28].
The most significant difference in singing behavior laid in the spatial analysis, which indicated that, during the autumn period, the individuals exclusively vocalized in the indoor (88.64%) and tunnel (11.36%) areas, particularly on level 10, with a utilization percentage of 56.82%. In contrast, during the spring period, the percentage of usage for the indoor (41.77%) and outdoor (43.04%) areas was very similar. The table showing the usage of the levels during singing for each observation period is included in the Supplementary Materials (Table S6). This result further supports the notion that environmental conditions, particularly outdoor temperatures, play a significant role in vocalization patterns, with vocalization position height and call propagation also being crucial factors.
Comparing the temporal distribution of the singing bouts, it can be noted that, in both seasons, no singing events occurred after 15:00, with the peak of vocalizations occurring between 11:00 and 11:55. In the autumn period, no singing events were recorded during the first morning hours of observation (9:00–9:55 and 10:00–10:55) (Table S7 and Figure S1). This absence of early-morning singing could also be attributed to the low temperatures recorded, especially during the initial hours of data collection. The duration of autumn singing sessions, similar to the spring observation period, exhibited two peaks, 11–15 and 16–20 min. However, during the spring period, singing sessions were also recorded with durations both shorter and longer than the ranges during which the primary peaks were observed. In contrast, during the autumn period, singing sessions were exclusively concentrated within the two peaks (11–15, 16–20 min). This indicates a higher degree of selectivity in the duration of vocalizations during the autumn period (Table S8 and Figure S2).
5. Conclusions
In conclusion, the most frequently observed activities in both observation periods were resting, feeding, and allogrooming. However, a significant difference was noted in play behavior: during the second observation period, there was a decrease in group play and an increase in solitary play. The reduction in group play could be attributed to environmental factors, such as lower temperatures, or to a decrease in the stimuli provided by the presence of visitors, considering that, during autumn, the park was closed to visitors for several days or experienced lower attendance compared to spring.
An interesting aspect to investigate further is the potential correlation between the frequency of observed behaviors and visitor attendance. This could be interpreted in the context of the so-called “visitor effect”, as conceptualized in a previous study [22,32], which suggests that the presence of visitors can have positive, negative, or neutral effects on animal behavior [33].
Regarding the increase in solitary play, it is worth considering the role of the keeper–animal relationship. For example, during December, the roll behavior was observed in the individual Nina near feeding times, when keepers were present in the foraging area. This behavior was not recorded during May–June. Future studies could help clarify this potential relationship.
In the spatial analysis, a preference for outdoor areas was observed in both observation periods, with predominant use of the lower levels. This result, seemingly at odds with the species’ natural biology, might be explained by the absence of some elements in the higher levels (levels 05–06), such as the reduced presence of poles in those levels. The explanation we propose is also supported by findings from a previous study [23], where level 05 contained all the typical exhibit elements (partially absent in the present study) and its frequency of use was entirely comparable to that of level 03. Therefore, restoring the exhibit to optimal conditions is essential to determine whether the preference observed in the present study was genuinely influenced solely by design or represents an adaptation to other factors that may have emerged subsequently, such as changes in social dynamics, climatic conditions, or human-related disturbances.
Finally, regarding singing, no outdoor vocalizations were observed during the second observation period. This phenomenon warrants further investigation to determine whether it is related to seasonal factors or other environmental elements.
The design of exhibits for captive animals must align with the specific needs of the species and the individuals, ensuring their well-being across all areas, including those individuals exposed to the public and those housed in “off-exhibit” spaces. Outdoor spaces facilitate natural behaviors such as foraging, exploration, and social interaction. However, indoor areas and tunnels also play a critical role. Indoor zones provide shelter, controlled environments, and quiet spaces for grooming and rest. Tunnels, in particular, aerial tunnels, serve more than as mere passageways: they offer elevated vantage points, allowing animals to explore their surroundings from different perspectives, fostering both physical and cognitive enrichment. Indeed, both in May–June and especially in December, the spatial analysis of level use revealed that level 11 (tunnel) was the location with the highest frequency of use for the ‘rest’ behavioral module compared to the other levels, indicating that the animals deliberately selected it for this purpose.
Despite their relatively small size, these elevated areas in the exhibit were often preferred for specific behaviors, emphasizing how the contribution of a well-rounded habitat is essential. To optimize exhibit design, modifications should be implemented based on spatial analysis studies of the target species, ensuring that all the zones of an exhibit meet their biological, psychological, and behavioral needs, regardless of visitor visibility.
This study highlights a significant shift in the concept of animal welfare. While “wellness” primarily focuses on basic physical needs such as nutrition, veterinary care, and physical activity, “well-being” adopts a more complex approach, encompassing the animals’ emotional and psychological state [34]. By monitoring animal behavior and the environments they inhabit, valuable insights were gained into their physical and psychological needs, helping environmental or social preferences. This approach reflects a modern trend in animal management, emphasizing overall well-being, not just physical health, and underscores the need for developing specific protocols aimed at enhancing the animals’ quality of life. Additionally, continuous monitoring plays a vital role in promptly addressing any deviations or anomalies in the behavior of the individuals under study. This information can lead to more effective management strategies that prioritize the well-being of the animals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jzbg6020023/s1, Table S1. Percentages of Nina’s behavioural modules in the various levels of the exhibit (May–June 2021); Table S2. Percentages of Tomas’ behavioural modules in the various levels of the exhibit (May–June 2021); Table S3. Percentages of Nina’s behavioural modules in the various levels of the exhibit (December 2021); Table S4. Percentages of Tomas’ behavioural modules in the various levels of the exhibit (December 2021); Table S5. Percentages of pair’s behavioural modules in the various levels of the exhibit in both periods; Table S6. Percentages of vocalisations performed by the two siamangs in the different levels; Table S7. Percentages of vocalisations made by the two siamangs in relation to time slots. Table S8. Percentages of vocalisations made by the two siamangs in relation to time categories; Figure S1. Percentages of vocalisations made by the two siamangs in relation to time slots; Figure S2. Percentages of vocalisations made by the two siamangs in relation to time categories; Figure S3. First level plan of the outdoor area. Cell color represents the number of times the animals was observed within that cell; Figure S4. Second level plan of the outdoor area. Cell color represents the number of times the animals was observed within that cell; Figure S5. Third level plan of the outdoor area. Cell color represents the number of times the animals was observed within that cell; Figure S6. Fourth level plan of the outdoor area. Cell color represents the number of times the animals was observed within that cell; Figure S7. Fifth level plan of the outdoor area; Cell color represents the number of times the animals was observed within that cell. Figure S8. Sixth level plan of the outdoor area. Cell color represents the number of times the animals was observed within that cell.
Author Contributions
Conceptualization, R.C., V.R. and E.S. (Emilio Sperone); methodology, R.C., G.G., F.L.L. and E.S. (Emilio Sperone); validation, R.C. and E.S. (Emilio Sperone); formal analysis, C.C., E.S. (Elisa Sacchet), A.M. and L.M.; investigation, C.C., R.C., E.S. (Elisa Sacchet), A.M. and L.M.; resources, V.R. and E.S. (Emilio Sperone); data curation, C.C., R.C., G.G., F.L.L. and E.S. (Emilio Sperone); writing—original draft preparation, C.C., R.C. and E.S. (Emilio Sperone); writing—review and editing, E.S. (Elisa Sacchet), A.M., L.M., F.L.L., G.G. and V.R.; supervision, R.C., V.R. and E.S. (Emilio Sperone); project administration, R.C., V.R. and E.S. (Emilio Sperone) All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval.
Data Availability Statement
Data are available on request due to restrictions, e.g., privacy or ethical.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nebasifu, A.A. Ex-Situ Conservation: Primate Protection in the Limbe Wildlife Centre. J. Zool. Stud. 2015, 2, 12–21. [Google Scholar]
- Miranda, R.; Escribano, N.; Casas, M.; Pino-del-Carpio, A.; Villarroya, A. The Role of Zoos and Aquariums in a Changing World. Annu. Rev. Anim. Biosci. 2023, 11, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S.M. Assessment of Welfare in Zoo Animals: Towards Optimum Quality of Life. Animals 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Sueur, C.; Pelé, M. Importance of Living Environment for the Welfare of Captive Animals: Behaviours and Enrichment. In Proceedings of the Animal Welfare: From Science to Law, Palazzo dell’UNESCO, Parigi, France, 10–11 December 2015. [Google Scholar]
- Gil-Dolz, J.; Ayuso, P.R.; Riba, D.; Crailsheim, D. Neighbors, Pros and Cons: Impact of Intergroup Interactions on the Welfare of Captive Chimpanzee Groups (Pan troglodytes). Ecologies 2024, 5, 279–295. [Google Scholar] [CrossRef]
- Coe, J.C. Naturalizing Habitats for Captive Primates. Zoo Biol. 1989, 8, 117–125. [Google Scholar] [CrossRef]
- Williams, E.; Cabana, F.; Nekaris, K.A.I. Improving Diet and Activity of Insectivorous Primates in Captivity: Naturalizing the Diet of Northern Ceylon Gray Slender Loris, Loris Lydekkerianus Nordicus. Zoo Biol. 2015, 34, 473–482. [Google Scholar] [CrossRef]
- McPhee, M.E.; Carlstead, K. The Importance of Maintaining Natural Behaviors in Captive Mammals. In Wild Mammals in Captivity: Principles and Techniques for Zoo Management, 2nd ed.; Kleiman, D.G., Thompson, K.V., Charlotte, K.B., Eds.; University of Chicago Press: Chicago, IL, USA, 2010; pp. 303–313. ISBN 978-0-226-44010-1. [Google Scholar]
- Yanuar, A. The Population Distribution and Abundance of Siamangs (Symphalangus syndactylus) and Agile Gibbons (Hylobates agilis) in West Central Sumatra, Indonesia. In The Gibbons; Springer: New York, NY, USA, 2009; pp. 453–465. ISBN 978-0-387-88603-9. [Google Scholar]
- Malone, N.; Fuentes, A.; White, F.J. Variation in the Social Systems of Extant Hominoids: Comparative Insight into the Social Behavior of Early Hominins. Int. J. Primatol. 2012, 33, 1251–1277. [Google Scholar] [CrossRef]
- Hankinson, E. Spatial Distribution and Density of the Lar Gibbon Hylobates Lar and Siamang Symphalangus Syndactylus in Relation to Canopy Structure and Disturbance in a Lowland Dipterocarp Forest, Sumatra. Master’s Thesis, Bournemouth University, Poole, UK, 2016. [Google Scholar]
- O’Brien, T.G.; Kinnaird, M.F.; Nurcahyo, A.; Prasetyaningrum, M.; Iqbal, M. Fire, Demography and the Persistence of Siamang (Symphalangus syndactylus: Hylobatidae) in a Sumatran Rainforest. Anim. Conserv. 2003, 6, 115–121. [Google Scholar] [CrossRef]
- Nijman, V.; Geissmann, T.; Traeholt, C.; Roos, C.; Nowak, M.G. Symphalangus Syndactylus. In The IUCN Red List of Threatened Species 2020; IUCN: Gland, Switzerland, 2015; E.T39779A17967873. [Google Scholar]
- Langbein, J.; Nawroth, C. Editorial: Captive Animal Behavior: Individual Differences in Learning and Cognition, and Implications on Animal Welfare. Front. Vet. Sci. 2022, 9, 1102122. [Google Scholar] [CrossRef]
- Chivers, D.J. Communication Within and Between Family Groups of Siamang (Symphalangus syndactylus). Behaviour 1976, 57, 116–135. [Google Scholar] [CrossRef]
- Lappan, S. Male Care of Infants in a Siamang (Symphalangus syndactylus) Population Including Socially Monogamous and Polyandrous Groups. Behav. Ecol. Sociobiol. 2008, 62, 1307–1317. [Google Scholar] [CrossRef]
- Lappan, S.; Morino, L. Mating in the Presence of a Competitor: Audience Effects May Promote Male Social Tolerance in Polyandrous Siamang (Symphalangus syndactylus) Groups. Behaviour 2014, 151, 1067–1089. [Google Scholar] [CrossRef]
- Morino, L. Social Correlates of Androgen Levels in a Facultatively Monogamous Ape (Symphalangus syndactylus): A Test of the Challenge Hypothesis. Behav. Ecol. Sociobiol. 2015, 69, 243–251. [Google Scholar] [CrossRef]
- Morino, L.; Pasquaretta, C.; Sueur, C.; MacIntosh, A.J.J. Communication Network Reflects Social Instability in a Wild Siamang (Symphalangus syndactylus) Population. Int. J. Primatol. 2021, 42, 618–639. [Google Scholar] [CrossRef]
- Dewi, D.P.; Iskandar, E.; Perwitasari-Farajallah, D. The Feeding Behavior and Food Preferences of Siamang (Symphalangus syndactylus Raffles, 1821) at Taman Safari Indonesia Bogor. IOP Conf. Ser. Earth Environ. Sci. 2023, 1271, 012048. [Google Scholar] [CrossRef]
- Vaglio, S.; Kaburu, S.S.K.; Pearce, R.; Bryant, L.; McAuley, A.; Lott, A.; Sheppard, D.J.; Smith, S.; Tompkins, B.E.; Elwell, E.J.; et al. Effects of Scent Enrichment on Behavioral and Physiological Indicators of Stress in Zoo Primates. Am. J. Primatol. 2021, 83, e23247. [Google Scholar] [CrossRef]
- Hosey, G.; Ward, S.; Melfi, V. The Effect of Visitors on the Behaviour of Zoo-Housed Primates: A Test of Four Hypotheses. Appl. Anim. Behav. Sci. 2023, 263, 105938. [Google Scholar] [CrossRef]
- Sacchet, E.; Milesi, A.; Preatoni, D.; Castiglioni, R. Symphalangus syndactylus (Raffles, 1821) in Ambiente Controllato. Comportamento Vocale e Sociale, uso Dello Spazio e Prossemica. Bachelor’s Thesis, Università Studiorum Insubriae, Varese, Italy, 2020. [Google Scholar]
- Mittermeier, R.A.; Rylands, A.B.; Wilson, D.E. Handbook of the Mammals of the World; Lynx: Barcelona, Spain, 2013; Volume 3. [Google Scholar]
- Poletti, S.; Castiglioni, R.; Gamba, M. Comportamento Sociale e Vocale di una Coppia di Siamanghi (Symphalangus syndactylus) in Ambiente Controllato. Master’s Thesis, Università degli Studi di Torino, Turin, Italy, 2020. [Google Scholar]
- Harrison, N.J.; Hill, R.A.; Alexander, C.; Marsh, C.D.; Nowak, M.G.; Abdullah, A.; Slater, H.D.; Korstjens, A.H. Sleeping Trees and Sleep-Related Behaviours of the Siamang (Symphalangus syndactylus) in a Tropical Lowland Rainforest, Sumatra, Indonesia. Primates 2021, 62, 63–75. [Google Scholar] [CrossRef]
- Orgeldinger, M. Protective and Territorial Behavior in Captive Siamangs (Hylobates syndactylus). Zoo Biol. 1997, 16, 309–325. [Google Scholar] [CrossRef]
- Castiglioni, R.; Sacchet, E.; Milesi, A.; Preatoni, D. Singing Behavior of Siamang Group Living in Captivity. In Proceedings of the XII Convegno Nazionale della Ricerca nei Parchi, Bussolengo, Spain, 7–9 October 2022. [Google Scholar]
- Pearson, E.L.; Davis, J.M.; Litchfield, C.A. A Case Study of Orangutan and Siamang Behavior Within a Mixed-Species Zoo Exhibit. J. Appl. Anim. Welf. Sci. 2010, 13, 330–346. [Google Scholar] [CrossRef]
- Liebal, K.; Pika, S.; Tomasello, M. Social Communication in Siamangs (Symphalangus syndactylus): Use of Gestures and Facial Expressions. Primates 2004, 45, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Zulamri Morning Call of Siamang (Symphalangus syndactyllus) in Subayang River, Riau—Indonesia. Int. J. Ecophysiol. 2019, 1, 125–130. [CrossRef]
- Chamove, A.S.; Hosey, G.R.; Schaetzel, P. Visitors Excite Primates in Zoos. Zoo Biol. 1988, 7, 359–369. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Hemsworth, P.H. The Visitor Effect on Zoo Animals: Implications and Opportunities for Zoo Animal Welfare. Animals 2019, 9, 366. [Google Scholar] [CrossRef]
- Maple, T.L.; Perdue, B.M. Wellness as Welfare. In Zoo Animal Welfare; Maple, T., Perdue, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 49–67. ISBN 978-3-642-35955-2. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).