1. Introduction
Harmful algal blooms (HABs) are increasing worldwide from abnormally warm ocean and climatic conditions that have far-reaching negative effects on wildlife and humans [
1]. Of highest current concern along the west coast of North America are blooms of the diatom
Pseudo-nitzschia sp. that produce the neurotoxic metabolite domoic acid (DA) [
2,
3]. DA enters the food chain via small fish that feed on algal blooms and shellfish that filter the water, and cause disease (DA toxicosis) and sometimes death in a wide range of exposed animals, including humans [
2]. It is currently the leading cause of neurological abnormalities in pinnipeds on the west coast of the United States [
4,
5,
6].
DA acts directly on the brain, and is a potent agonist of glutamate receptors, particularly the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate subtypes, which are over-represented in the medial temporal lobe [
7,
8]. DA has a 100-fold higher affinity than glutamate and binds to these receptors, displacing glutamate and kainate, increasing the concentration of glutamate to bind to other ligand-gated channels that regulate Ca
2+, Na
+, and K
+, in addition to N-methyl-D-aspartate (NMDA), AMPA, and kainate receptors [
2,
7,
8]. The binding of excess glutamate initiates voltage changes in the membrane and activates nerve transmission in adjacent neurons, resulting in an acute hyperexcitable state [
2,
7,
8]. The activation of voltage-gated calcium channels results in flooding post-synaptic tissue with calcium, which in excess causes progressive neuron death [
2,
7,
8]. Direct and indirect exposure of DA in utero or nursing or from consumption of DA as a weaned yearling or adult animal, as shown in laboratory mice and rehabilitated sea lions, can lead to varied excitotoxicity changes in the developing and mature brain with a wide range of health, behavioral, and neurobiological effects, including heart disease, chronic epilepsy, brain damage, and memory deficits depending on the dose and chronicity of DA exposure [
7,
8].
Although acute effects of DA are well-studied with dose–response curve pharmacology in lab animals and increasing reports described in wildlife, the long-term chronic effects in wildlife have been difficult to ascertain [
9]. The most significant chronic effect of DA toxicity is the extensive neuronal degeneration that occurs in the hippocampus at the site of highest concentrated areas of glutamate and kainate receptors, resulting in the progressive loss of memory, spatial awareness, and learning [
2,
8]. This is of concern both for animals living in the wild after toxic exposure and for stranded marine life placed in long-term managed care. The fate of animals placed in permitted facilities is of current interest, as many of these stranded animals are deemed non-releasable by governing agencies following preliminary veterinary care and assessment [
10]. Decisions are deliberated about the viability of these animals, if stabilized, for long-term housing in display and research facilities. A survey of facilities housing non-releasable sea lions examined the medical assessment and outcomes of 171 animals between 2000 and 2016, 15% exhibited neurological signs, two thirds of which developed neurologic signs post-placement [
11]. During the survey period, ~60% of sea lions with neurological symptoms died or were euthanized. Findings mirrored laboratory rodent models in which early exposure can lead to delayed onset epilepsy [
4].
Therefore, is it justified to rehabilitate and take juvenile or subadult stranded otariids with neurological symptoms (or possible asymptomatic DA exposure) into long term managed-care facilities? If so, what should be the standard of care? Here we present a novel exemplary case study in another otariid species, the Guadalupe fur seal (GFS), that may cast some light on these issues.
While California sea lions have been the main focus of examinations into DA’s effects on Otariidae over the last thirty years, Guadalupe fur seals occupy the same range and prey on similar fish and squid species. Guadalupe fur seals are, despite the name, in the Otariidae suborder and more closely related to sea lions than to true seals. The common ancestor of California sea lions and Guadalupe fur seals was extant between 6 and 7 million years ago, according to genetic analyses, and the two species have similar foraging ecology and risk for DA exposure [
12,
13]. Guadalupe fur seals have increased their population along the West coast; however, they are still listed as a threatened species under the U.S. Endangered Species Act (ESA), and as a Depleted and Strategic species under the U.S. Marine Mammal Protection Act (MMPA) [
14]. GFS in the Pacific Northwest are increasingly subject to stranding due to emaciation, infectious disease, trauma/fishery interactions, and toxic algal blooms [
12]. The range of the Guadalupe fur seal is centered around Guadalupe Island, Mexico, yet it extends south to waters off Baja California and north to the Channel Islands off Southern California up to Oregon and Washington [
14]. The California Stranding Network facilities and NOAA scientists have observed that Guadalupe fur seals are seldom seen on the coast; however, yearlings or juveniles will also occasionally strand malnourished, moribund to infectious disease, obtunded secondary to DA toxicity, or wounded by fishery interactions (entanglement or gunshot) along the coast of California [
15]. An increase in Guadalupe fur seal strandings led to a defined Unusual Mortality Event (UME) from January 2015 to September 2021, where a total of 715 seals stranded in California, Oregon, and Washington [
15]. NOAA scientists attributed the main cause of stranding of juvenile Guadalupe fur seal pups and juveniles (<2 years of age) to be malnutrition secondary to unprecedented ocean warming in the Northeast Pacific that reduced prey availability [
15]. The anomalous marine heatwaves caused extreme warm sea surface temperatures in 2014–2016 and 2019–2020. In 2015–2016, an El Niño aided in producing some of the warmest sea surface temperatures reported in November 2016 [
15]. That makes this case study of broad interest, as during rehabilitation, fur seals, like sea lions, can show atypical behavior, decreased mentation, seizures, and/or slow developmental stages (i.e., fish prehension) following exposure to DA [
15].
Stranded sea lions and fur seals that show neurologic signs can be stabilized with antiepileptic drugs (AED) until appropriate diagnostics like blood analyses, radiographs, serologic testing, and advanced imaging are performed to rule-out infectious disease, parasitism, trauma, neoplasia, or suspect toxicity [
16]. Patient response to treatment for successful rehabilitation and return to the wild is often case-by-case depending on the underlying problem and time to recover from illness. With domoic acid toxicity, it is difficult to ascertain if toxicity is acute or acute-on-chronic, based on initial presentation, therefore additional criteria are needed, such as the oceanographic presence of an algal bloom, increased numbers of animals stranding or cohorts of stranding events, clinical signs of seizures, ataxia, decreased responsiveness, statis epilepticus, or acute death [
8,
15]. Adult pinnipeds presenting with generalized seizures or comatose and in excellent body condition are more likely to have been exposed to higher acute DA exposure than those that have been exposed chronically [
8]. Ramsdell and Gulland (2015) found a strong sex and age distribution of 551 DA sea lion cases between 1998 and 2006, with 70% being adult female [
8]. Male sea lions will leave the breeding and pupping rookeries around the Channel Islands, while the females remain to raise their young and are subject to increased exposure to DA blooms and legacy pollutants in the region [
8]. These adult females are exposed to chronic levels of DA in their food supply and may encounter new blooms with increased toxicity that exacerbate their chronic condition, such as hippocampal atrophy, that may affect their foraging ability and these animals often present in thin body condition [
8]. In addition, chronic exposure that is secondary to in utero ingestion of amniotic fluid, nursing from an intoxicated dam or cumulative toxin exposure is often displayed by disorientation, ataxia, tremors, difficult prehension with secondary malnutrition, dyspnea, lethargy, and/or progressive seizures at time of stranding and during rehabilitation [
16,
17]. Additional information regarding regional ocean temperature changes, algal bloom demographics, and stranding reports along the coast of California can aid in determining likelihood of toxin level exposure as the cause of stranding. Ultimately, marine mammal stranding centers receive guidance from NOAA to make the most appropriate disposition decision for stranded marine mammals. Due to the threatened status of Guadalupe fur seals, more effort is given to the rehabilitation and return of these animals back into the wild population. Rehabilitating patients that present or develop seizures secondary to mild DA exposure that are managed with AEDs may be deemed non-releasable due to the need for chronic therapy. These animals may be displayed with permit authorization in managed-care facilities and serve to inform the public about population biology, conservation status and ocean conditions. Pinnipeds treated with AEDs may have break-through or uncontrolled seizures; for these patients, the prognosis for long-term survival and placement in managed-care would be considered poor, and euthanasia may be indicated due to the quality of life.
In this case, after 17 years with an unremarkable health history apart from managed epilepsy secondary to DA toxicity, this Guadalupe fur seal began a progressive decline in health, ultimately deeming a poor prognosis, which promoted humane euthanasia. Comparative MR images were obtained at the time of the initial diagnosis and following post-mortem examination of the brain, essentially twenty years apart. This case is of great interest for examining the long-term adaptive changes and effects of managed DA toxicosis in a managed otariid species.
2. Detailed Case Description
In the fall of 2001, an emaciated subadult male Guadalupe fur seal (
Arctocephalus townsendi, NOAA ID: NOA0006136), weighing 41.8 kg and estimated to be 3–5 years of age, stranded in southern California and was taken to SeaWorld of California (SWC) Marine Mammal Rescue Center for supportive care and rehabilitation. Within five days of stranding, he began to have full body tremors that progressed to a generalized seizure, with vertical nystagmus, and post-ictal lethargy. A blood analysis revealed mild inflammation and the fur seal was treated empirically with broad-spectrum antibiotics, Ampicillin (22 mg/kg) per os (PO) twice daily (BID) then Chloramphenicol (22 mg/kg) PO BID for 1 month. Over the following 2 months, the fur seal continued to progress through rehabilitation with improved appetite after vomiting up pieces of a shoe, but had intermittent mild focal seizures, consisting of focal muscle fasciculations, dull mentation and lethargy. With persistent neurologic signs, a recheck physical exam and radiographs were conducted to rule-out any anatomical defect or injury. With no significant physical or radiographic findings, a further workup with advanced imaging and cerebrospinal fluid (CSF) tap analysis was conducted. The MRI showed a focally extensive area of encephalitis involving the right parietal/temporal lobes and right cerebellum, swelling of the left cerebrum in the hippocampal and thalamic regions with compression of the lateral ventricle. The CSF analysis was unremarkable. Serum morbillivirus and protozoal (sarcocystis and toxoplasma) antibody titers were also analyzed and found to be negative at 1:40, respectively. His presumptive diagnosis was focal encephalitis and hippocampal degeneration secondary to domoic acid exposure and toxicity. Other differentials considered for the source of focal encephalitis included focal trauma (i.e., blunt force/penetrating foreign body, such as a pellet/bullet, sting ray spine, etc.), parasitism/larval migrans, other bacterial/viral/fungal infection, lymphohistocytic encephalitis, congenital malformation, cerebral/cerebellar vascular event/stroke and vitamin deficiency (i.e., thiamine) secondary to malnutrition. Diagnostic testing (blood and CSF analysis, viral and protozoal screening, radiographs, and advanced imaging) ruled out most other possible disease processes. Unfortunately, a urine sample was not collected at the time of intake to test for the presence of DA in urine. An electroencephalogram (EEG) was not performed, as it is difficult to conduct and a recent study in canines found only 25% of dogs with Idiopathic epilepsy (IE) had EEG evidence supporting diagnosis [
18]. Chronic DA toxicity was considered most-likely due to similar presentation characteristics of MRI findings to that of California sea lions [
17].
Approximately three months after the MRI, the fur seal was continuing to gain weight and progressing in rehabilitation for a potential return to the wild in the spring season, until he had a generalized seizure consisting of full body myoclonic contractions, opisthotonos, disorientation and rapid eye blinking lasting approximately 3 min in length. This generalized seizure was the first significant seizure for this animal, therefore he was started on oral antiepileptic drug (AED) therapy, phenobarbital, at approximately 0.57 mg/kg per os (PO) twice daily (BID). There were no further clinical signs of focal or generalized seizure activity and the fur seal resumed normal behavior, mentation, and appetite after the seizure event. He was maintained on AED therapy with rechecks of AED drug levels annually, 2002–2004, and deemed non-releasable by NOAA in 2004. He was moved to a mixed pinniped species exhibit on public display at SWC having reached an adult size and weighing 150 kg. This male fur seal was the first Guadalupe fur seal to be granted an enhancement display permit, as a threatened species under the Endangered Species Act 1973. He was castrated in 2004 to satisfy ESA display permit requirements. Over the years, the fur seal experienced mild medical conditions, such as: mild superficial alopecia (biopsy results indicated non-progressive hair follicle growth on nape of neck related to abnormal molt), nasal pediculosis, and progressive degenerative changes (cataracts and osteoarthritis) in later years. He had a focal seizure in 2005, characterized by full body stiffness and flipper tremoring with normal respirations. The seal was observed to have normal activity and appetite immediately following and was moved to a back area, to monitor and facilitate a future physical exam and blood collection. An exam conducted five days later found normal blood analytes and the trough phenobarbital level to be 16.7 µg/mL. No benzodiazepines were administered, and the maintenance phenobarbital dose was increased by 25%. He remained at a 0.83 mg/kg BID phenobarbital dose until the spring of 2015, when he lost 30 kg to decrease heavy body condition and his dose was adjusted back down to ~0.60 mg/kg PO BID phenobarbital. In 2018, at an estimated age of 22–24 years old, he presented with acute vomiting and lethargy. A physical exam was conducted under anesthesia to obtain radiographs and blood analysis for additional diagnostic testing with fungal antibody immunodiffusion (Candida sp. Antibody (AB), Aspergillus sp. AB, Coccidioides sp. antigen (AG), Histoplasma sp. AB, Cryptococcus sp. AG, Blastomyces AB), thyroid hormone level, and West Nile Virus titers (Negative@1: <4)). All additional tests were negative and measured to rule-out any other possible cause of decreased drug response. A trough phenobarbital level was analyzed to be 25.1 µg/mL. The physical exam findings included mild degenerative changes in the joints (i.e., osteoarthritis) and immature cataract formation. He received supportive treatment with antiemetics (maropitant, 1 mg/kg), antacids (famotidine, 0.1 mg/kg), antibiotics (ceftiofur 5 mg/kg), and anti-inflammatory (meloxicam, 0.1 mg/kg), and fluid therapy (lactated ringers solution, 25–30 mL/kg) to regain normal behavior and appetite in a week.
There are no standard therapeutic doses or ranges of AEDs in pinnipeds. The standard phenobarbital dose in canines is 2–4 mg/kg BID and the documented therapeutic range approximately 20–45 µg/mL but can vary based on the variation of dog breeds [
19]. It is recommended that phenobarbital levels be monitored at two and six weeks after initiation of therapy and then every three to six months [
20]. With a large adult fur seal in a mixed pinniped exhibit, consistent monitoring to acquire a diagnostic blood sample of adequate volume was challenging. With daily husbandry assessment of an animal on consistent effective low-dose therapy, the management decision was to intervene with any subtle change in behavior or appetite. The dose of phenobarbital chosen was based on the lowest effective dose that prevented seizure activity from occurring, this was ~0.6 mg/kg BID, which was much lower than a standard canine dose of 2–4 mg/kg BID. With the long-term effects of phenobarbital treatment known to potentiate hepatotoxicity in canine patients, the lowest effective dose was desired to prevent drug side-effects [
19,
20]. Also, with the lowest effective dose, there was more discretion and safety in increasing the dose if needed. In the case study fur seal, trough drug levels were collected at times of seizure break-through and if significant changes to the dose (>25% increase or decrease) were made. Overall, the fur seal’s clinical condition of mild seizure break-through responded to incremental supplemental phenobarbital dosing (i.e., up to 1.25–1.5 mg/kg PO in PM BID) with seasonal weight fluctuation.
Historically, a phenobarbital dose at the lower limit of the dose range was effective at seizure control. In fall of 2018, the fur seal’s behavior was observed to be periodically lethargic with a lack of interest in prehending fish. A mild weight increase was reported, and subsequently the phenobarbital dosage was decreased to the original dosage (phenobarbital 0.57 mg/kg PO BID), which improved the fur seal’s demeanor and appetite. A return of weight gain during spring of 2019 resulted in a mild behavioral change (mild ataxia), but no tremors or seizures were observed, therefore the phenobarbital dosage was increased (1.25 mg/kg PO BID). Phenobarbital levels were not obtained in the fall of 2018 or spring of 2019 due to the historical treatment adjustments that were made seasonally with weight change to manage phenobarbital side-effects of sedation vs. allowing a sudden, involuntary, synchronous discharge of brain neurons or seizure event. In February 2020, he presented with vomiting, anorexia and mild tremor activity that was initially treated with injectable AED therapy, phenobarbital (0.57 mg/kg intramuscular (IM)) and antinausea agent, maropitant (1.0 mg/kg subcutaneous (SC)), to stabilize his condition. His blood analysis showed no significant abnormalities in complete blood count (CBC) and serum chemistries. The phenobarbital levels were measured to be acceptable at 31.6 µg/mL. A physical exam showed mild cataract progression in both eyes with blepharospasm in the right eye, as well as mild obesity. The phenobarbital dosage (0.8 mg/kg) was increased and administered IM BID to stabilize his condition. Further empiric treatment with anti-inflammatory meloxicam (0.2 mg/kg SID) was observed to improve mobility, and the dose was subsequently decreased to 0.1 mg/kg for chronic administration. In late summer of 2020, he presented again with lethargy and vomiting. Blood analysis showed a mild increase in white blood cell count (WBC) (6000 cells/µL with left shift of 9% immature band neutrophils), increased fibrinogen (463 mg/dL), a mild decrease in serum iron (117 µg/dL), and a static serum phenobarbital level (33.1 µg/mL). He had small multifocal ulcerative lesions, suggestive of contact dermatitis, on the chest and fore flippers. A cursory ultrasound exam revealed a few mild hyperechoic comet tails extending from the right thoracic pleura and normal echocardiogram. A subsequent examination one week later was performed due to persistent lethargy and poor appetite. Radiographs and recheck blood analysis were conducted under anesthesia and revealed marked osteoarthritis of bilateral scapulohumeral joints and coxofemoral joints, chronic cataracts, and small focal dermal lesions (biopsy confirmed a focal bacterial dermatitis). Patient recovery from anesthesia was prolonged and, in the following days, the patient required supportive care and injectable treatment to aid poor appetite and manage AED compliance. Recheck blood analysis showed persistent inflammation with increased WBC (6500 cell/µL with 13% band neutrophils), mild azotemia (BUN 58 mg/dL, Cr 1.65 mg/dL), hemoconcentration (HCT 57%), and hyperproteinemia, total protein (TP) (8.3 g/dL). Empiric therapy with injectable antibiotics (ampicillin-sulbactam, 15 mg/kg PO) and steroid anti-inflammatory therapy (dexamethasone, 0.25 mg/kg IM) were given. Progressive decreased mobility was observed when hauled-out on the exhibit deck, though he appeared comfortable in water by displaying normal grooming and surface resting behavior. However, due to chronic arthritis and decreased mobility on land, his overall quality of life was evaluated to be compromised despite supportive anti-inflammatory treatment. Two days later, he presented again with no appetite, lethargy, decreased grooming behavior and the decision was made to humanely euthanize due to his recurrent progressive declining medical condition. A necropsy and brain MRI were conducted post-mortem to investigate the underlying disease.
2.1. Histopathology
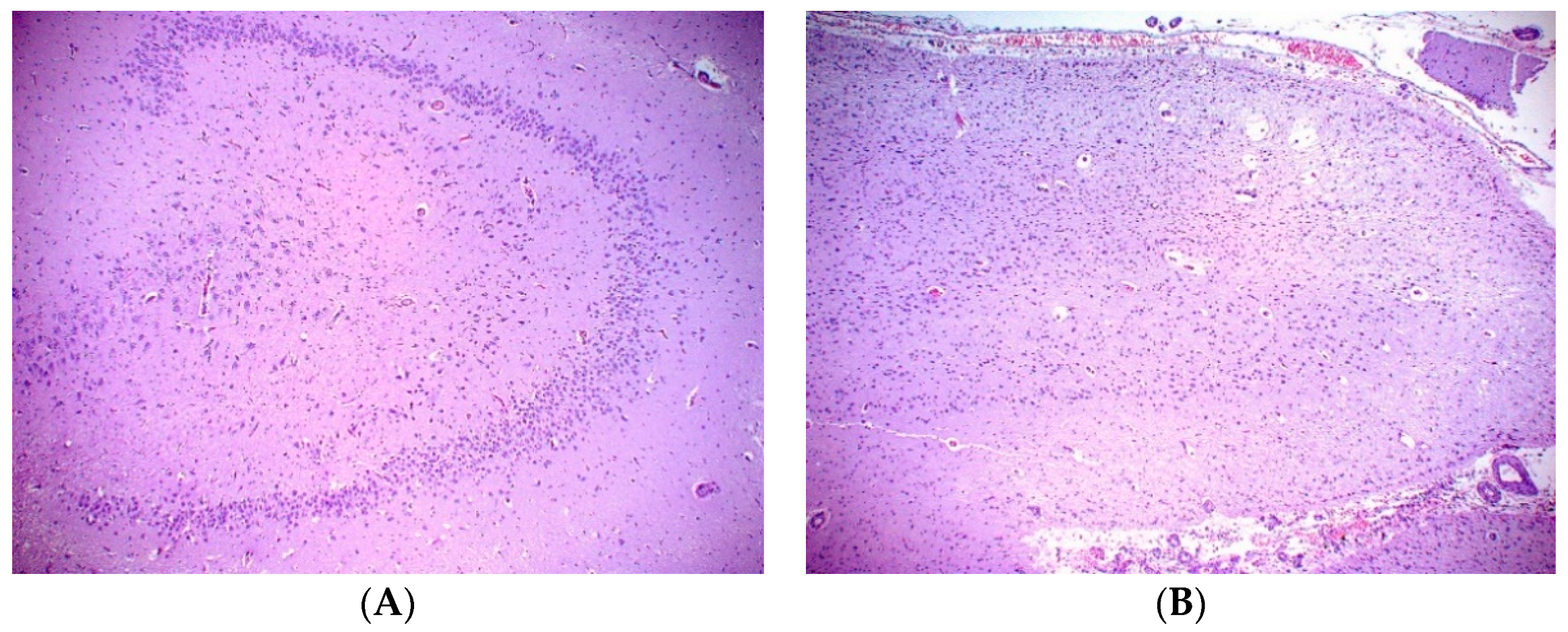
A necropsy examination was conducted within hours after humane euthanasia and found the animal to be in good nutritional condition weighing 148 kg. Gross findings included mild mottling of the lung parenchyma, mild liver lobe congestion on the recumbent side and mottled gastrointestinal serosa. The most significant histopathologic finding was chronic marked to severe atrophy of the right hippocampus and right temporal lobe. There was also chronic mild atrophy of the left hippocampus. The right dentate gyrus was not present, but instead consisted of reduced loose neuropil and large astrocytes with small numbers of poorly organized neurons compared to the left dentate gyrus (
Figure 1). All findings are consistent sequelae from chronic domoic acid toxicity [
21]. There was additionally focal chronic severe necrosis and atrophy of distal cerebellar folia, with acute hemorrhage with no cause identified. There was no microscopic evidence of any previous or ongoing infectious or inflammatory disease in the brain or other tissues. While domoic acid exposure has additional documented adverse effects to the myocardium that often progresses to degenerative cardiomyopathy, there was no microscopic evidence of persistent damage to the heart related to previous DA exposure and no microscopic evidence of cardiac insufficiency in this fur seal case [
21,
22].
2.2. MRI Investigations
With an estimated age of 20–22 years of age, this senior fur seal patient is unique in having received MR imaging both during early rehabilitation as a young adult and post-mortem almost two decades later. This allows one of the first meaningful assessments of the long-term neural effects of managed domoic acid toxicosis in a large mammal. The first imaging was conducted in vivo under anesthesia in 2001 at Helen Woodward Animal Hospital, Rancho Sante Fe, CA on a Philips Gyroscan T5 series II 0.5 tesla (T) scanner. Standard T1-weighted spin echo images were acquired. The MRI showed a focally extensive area of encephalitis involving the right parietal/temporal lobes and right cerebellum, swelling of the left cerebrum in the hippocampus and thalamic areas with compression of the lateral ventricle. The cerebellar lesion may be secondary to seizure activity, as cerebellar Purkinje cell layer lesions are often superficial and may result from secondary tissue hypoxia, and lead to ischemic necrosis [
23]. Reversible MRI changes have also been reported in humans with seizure or epilepsy disorder, unfortunately sequential antemortem MRIs of this animal were not obtained but could be evaluated in other seizure disorder patients [
24].
Post-mortem brain MR imaging was initially conducted at a small animal referral hospital (Veterinary Specialty Hospital, Sorrento Valley, CA, USA) with a Siemens Magnetom Avanto Tim MR scanner operating at 1.5 T. T2 weighted (T2W) images were obtained, with dorsal sagittal and transverse to oblique sequences acquired with 3.5–4 mm thickness. Findings included atrophy of the right temporal lobe, right side of the cerebellum and right hippocampus and parahippocampal gyrus with secondary hydrocephalus ex vacuo. There was mild left hippocampal atrophy with normal parahippocampal gyrus and slight enlargement of the temporal horn of the lateral ventricle. The right hippocampus and parahippocampus gyri were T2W hyperintense centrally. No other parenchymal changes were observed within the limitation of T2W images.
A follow-up brain MRI was conducted in 2021 on a Siemens Tim/TRIO MRI scanner operating at 3 T. A 32-channel receive-only coil was used to maximize image quality. For imaging, the brain was removed from the fixative solution, drained, and dried with paper towels. The brain was placed in an airtight plastic container on top of a low-density foam pad to center it, and additional foam pads were placed around the brain to prevent movement. The container was then filled with a perfluoropolyether (PFPE) (HT-200, TMC Industries, Inc., Waconia MN, USA), sold as an industrial heat transfer fluid, which has a similar magnetic susceptibility to brain tissue. Similar PFPE fluids have been used previously to eliminate edge artifacts in postmortem human brain imaging [
25]. The PFPE contains no hydrogen and is fully fluorinated, yielding no MR signal in conventional imaging. The specific weight of PFPE also tends to expel air bubbles from within brain cavities, further reducing a common source of postmortem imaging artifacts.
For delineation of GM and WM we optimized a T
2-weighted SPACE scan with TE = 145 ms. Voxel dimensions were (0.7 mm
3). Two averages were collected to boost SNR. For diffusion tractography we used a DW-SSFP sequence developed at Oxford University for post-mortem human imaging that has subsequently successfully been applied to pinniped and cetacean brains [
26,
27]. This sequence maximizes SNR per unit time, negating some of the costs of low T2 in fixed tissue. A total of fifty-two diffusion weighted (DW) images were collected in blocks of 11, 12, 14 and 15 directions, with minimally diffusion weighted images acquired before and after each block. The DWI were collected with q value of 266 cm
−1, while minimally diffusion weighted scans used q = 20 cm
−1. Image resolution was (1 mm
3). At the completion of the DW-SSFP acquisitions, T
1 and T
2 maps were collected to be used in modeling the diffusion signal to take into account tissue changes after death and fixation [
28].
High resolution T
2 images were used for hippocampal segmentation and volumetry in the program 3Dslicer [
29]. Hippocampal segmentation followed anatomical criteria previously established in Cook et al., 2018 and Cook et al., 2021 [
30,
31]. Briefly, the hippocampal GM was segmented in an oblique orientation (between dorsal and transverse) perpendicular to the longitudinal axis of the hippocampus and was cross-referenced slice-by-slice against a sagittal view, in which the longitudinal extent of the hippocampus was visible. Included in the segmentation were CA subfields, the dentate gyrus and alveus, and a portion of the subiculum. Septal and temporal boundaries relied on visible extent of the hippocampus in the sagittal view. The lateral boundaries were determined predominantly by the lateral ventricle of the temporal horn. Medial boundaries were set at the medial most extension of the subicular cortex. Hippocampal tissue was traced by the author PC and for the purposes of computing interobserver reliability, by the author IM. PC’s values are reported except where specified.
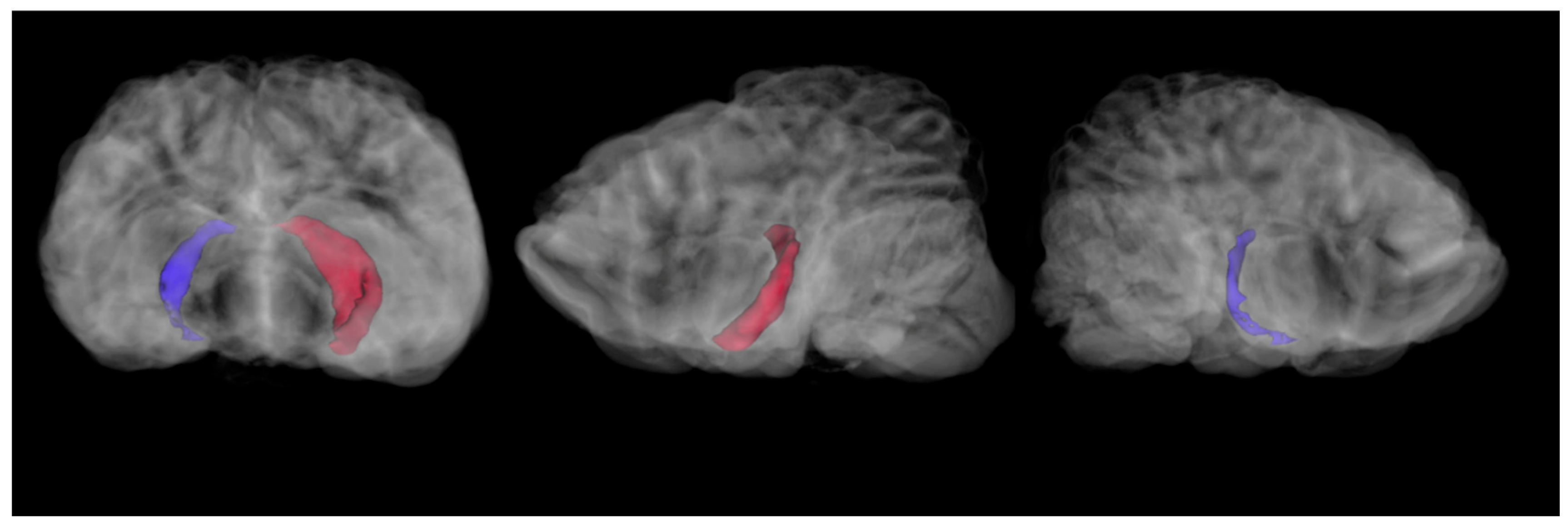
Hippocampal volumes were computed using the segment statistics module in 3D Slicer. For the 2021 imaging sequence, the right hippocampus was 273.6 mm
3 and the left hippocampus was 879.6 mm
3 (
Figure 2).
A subsection of the 2021 hippocampal segmentations were compared to a matched slice from the 2001 imaging. The 2001 images were only available as MR films, so they were digitized. The digitized scans did not have voxel-by-voxel data preserved, so they could not be used for assessing specific volumetrics. To compare the 2001 scans with the 2021 scans, a representative slice of a proton density image sequence acquired in the dorsal plane was matched by multiple anatomical criteria to three contiguous slices in the 2021 T
2 image sequence. The right and left hippocampi were traced in the 2001 scans, and then computed as a percentage of the total brain area for that slice. The average right and left hippocampal volume from the three comparable 2021 scans (acquired with much smaller voxels and thus thinner slices in all planes) were computed as a percentage of brain volume for those three slices. This allowed a meaningful comparison, although the underlying imaging geometry could not be precisely matched. In 2001, the section of the right hippocampus made up 0.39% of the total brain area in that slice, while the left hippocampus took up 0.38% of the area. In 2021, the right hippocampus took up 0.18% of the total brain volume for those slices and the left took up 0.47% (
Figure 3).
Acknowledging the uncertainty due to inability to compute by-voxel volumetrics on the original image sequence, as well as the imperfectly matched scan orientation and necessity of comparing PD to T2 images, the relative tissue loss in the right hippocampus is very evident in the post-mortem scans. Interrater reliability was moderate to high for the four computed hippocampal volumes (2001 right: 74%, left: 74%; 2021 right: 72%, left: 87%). Notably, both raters measured the right hippocampus in the 2021 scan as having a far lower volume than the other three volumes (PC: 0.18, IM: 0.13). In addition, while there were signs of cerebellar encephalitis in the 2001 scan series, there was no evidence of gross cerebellar damage. In contrast, the left cerebellum showed evidence of atrophy in the 2021 scan series.
High resolution diffusion tensor imaging was collected on the post-mortem brain in the 2021 imaging protocol. DTI was not available for the 2001 scan. Cook et al. (2018) found that California sea lions with signs of domoic acid toxicosis and hippocampal atrophy had lower fractional anisotropy (FA) values in the body and pillar of the fornix than animals without clinical signs of domoic acid toxicosis [
30]. Here we used the same protocol to trace the fornix and quantify FA values, and detailed protocol is available in Cook et al., 2018 [
30]. Briefly, separate left and right fornix body ROIs were drawn in FSLeyes. These were placed just ventral to the ventral boundary of the corpus callosum, and rostral to the medial projection of the hippocampal tails. ROIs were placed in voxels with apparent forward directionality of white matter as per overlaid V1 maps. Then, FSL’s FMRIB Diffusion Toolbox (FDT) was used to conduct probabilistic tract traces from both masks. These were conducted with 5000 samples per voxel and standard settings. For viewing and quantitative analysis, the tracts produced were thresholded at a lower bound of 10% of the waytotal, then binarized and multiplied by FA maps (
Figure 4). The mean value of each image was then computed with
fslstats, providing a mean FA value within well-defined forniceal tissue bilaterally. Reduced FA in tracts can be associated with neurological damage—in particular, epilepsy tends to lead to reduced FA values in limbic tracts. Here, the mean FA was 0.36 in both the left and right fornix.
2.3. Differential Diagnoses and Treatment
The cause for marine mammals presenting with seizure disorders follow similar characteristics of most mammals; however, some conditions exist that are unique due to their fish diet and the marine environment. Differential diagnoses include developmental (i.e., hydrocephalus), nutritional disorders (i.e., hypoglycemia, thiamine deficiency, mercury toxicity), inflammation (i.e., meningitis, encephalitis, amyloidosis), bacterial infection (i.e., brucella), parasitic migration (i.e., Anisakis, Nasitrema), protozoal infection (i.e., Toxoplasma, Sarcocystis), fungal infection (i.e., aspergillosis, cryptococcosis, coccidiomycosis, mucormycosis), viral infection (i.e., distemper, morbillivirus, West Nile virus), trauma (i.e., barotrauma, gunshot, sting ray barb, contusion/concussion), degenerative (i.e., infarct, aneurysm, protein storage disease, lipid/cholesterol accumulation, plaques), toxins/metabolites (i.e., domoic acid, brevotoxin, uremia, ketosis) and certain pharmaceuticals (i.e., ivermectin, haloperidol) [
5,
16,
32,
33,
34]. Awareness of unusual mortality events (UME), regional stranding epizootics and changes in the environment can help rule-in or rule-out predisposing conditions. Most animals presenting with seizure disorder will undergo first-line physical and neurologic examination with diagnostic testing of blood and urine analysis, and radiographs to identify the underlying cause of the disorder. Antemortem neurologic disease without obvious causative agents or understandable pathology often requires further advanced diagnostics such as imaging (i.e., MRI, CT, angiography), serology testing (i.e., Toxoplasma antibody), heavy metal analysis, vitamin analysis, drug levels, microbial culture or antigen PCR/DNA probe techniques to further identify the underlying problem. Unfortunately, domoic acid has a very rapid metabolism with renal elimination from the body, but its pathologic effects cause long-standing damage to the brains of sea lions, specifically the hippocampus, with linked behavioral alterations [
35,
36,
37]. Other high trophic marine predators such as harbor seals, northern fur seals, common dolphins, southern sea otters, California brown pelicans, shearwaters, and cormorants have been reported to exhibit neurologic effects (disorientation, ataxia, aggression, focal and generalized seizures) and acute death related to domoic acid toxicity [
2]. When available, diagnostic imaging can reveal suggestive evidence of hippocampal atrophy that tends to accompany chronic domoic acid toxicosis [
30,
35,
37].
Following the diagnosis of a seizure disorder, treatment should begin immediately to stop the seizure and treat the underlying condition. Initial treatment, if the patient is normoglycemic, typically begins with administration of a benzodiazepine, such as diazepam, midazolam (0.5–1 mg/kg, IV, 2–4 mg/kg, rectally) or lorazepam (0.1–0.2 mg/kg IM PRN), to control the seizure. If the seizure continues or does not stop, then an adjunct AED, such as phenobarbital (1–3 mg/kg, IM/PO, start low-end of dose), potassium bromide (20–40 mg/kg/day PO once or divided into 2 doses), or levetiracetam (10–20 mg/kg PO/IV TID), is administered to effect, as directed by veterinary consultation. Once the seizure disorder is controlled, then the treatment can be titrated or modified in accordance with the patient’s mentation and overall neurological function. Additional anti-inflammatory, antibiotic, antifungal, antiparasitic, or nutritional supplement treatment may be warranted depending on the underlying cause of the seizure disorder. Maintenance therapy is dictated by serum drug level analysis and clinical effects of the AED on the patient. Advanced diagnostics to rule-out infectious agents and imaging for central nervous system disease will aid the estimation of treatment duration.
3. Discussion
This is the first case of a stranded Guadalupe fur seal presenting with neurologic disease from a suspected DA exposure with bookended brain imaging almost two decades apart in managed care in a zoological facility. The case highlights that while brain lesions were progressive and seizures were controlled medically, the animal had normal behavior and apparently normal cognitive function well past normal life expectancy for wild pinnipeds. The maximum life expectancy of Guadalupe fur seals is reported between 17 and 20 years of age, while the median lifespan for wild male and female California sea lions is approximately 7.7 and 11.5 years of age, respectively [
38,
39]. The median life expectancy of a sea lion born in a zoological park or aquarium is approximately 23–25 years [
40].
Harmful algal blooms (HABs) have been responsible for mass mortalities and morbidity of marine mammals, fish, and seabirds from central California to the southern tip of Baja California for decades [
41]. Domoic acid toxicity and epileptic disease in California sea lions has been well characterized over the last two decades since this disease was first identified in the late 1990s [
8,
16,
23,
30,
35,
36,
37,
42]. Since Guadalupe fur seals occupy the same range as sea lions, it is not surprising that fur seals are also at risk of domoic acid exposure [
12]. Lefebvre et al. (2010) found comparable evidence of unilateral or bilateral hippocampal atrophy, loss of granular cells in dentate gyrus, loss of hippocampal pyramidal cells, neuronal necrosis, and intense gliosis with domoic acid toxicity in Northern fur seals stranded in Central California between 2005–2007 [
43].
Numerous California sea lions and several fur seals (Northern and Guadalupe) with suspected domoic acid exposure and toxicity have been placed in long-term care facilities under chronic treatment with AED therapy [
11]. Early reports suggest a fairly high prevalence (~15%) of neurological symptoms in these animals, but there are no long-term studies of outcome [
11]. Of additional concern, a recent case study identified an emergent epileptic disease after puberty in a captive California Sea Lion most likely exposed to domoic acid in utero [
17]. Research in humans and rodents suggests outcomes of developmental seizure disorders can vary greatly depending on the timing and course of treatment [
3]. The outcome of the current case, although anecdotal, presents a more hopeful picture in which Otariidae with toxic algal exposure can be managed in captivity, allowing them to serve as animal ambassadors for their species. Daily observation from experienced animal care specialists, like domestic pet owners, cannot be ignored in the care of managed wild animals, where subtle behavioral changes in posture, appetite, food prehension, response to training and mobility are noticed by animal caretakers that can be communicated to veterinary staff. From the authors’ perspective, the model of seizure management applied in this case is well supported from evidence in domestic small animals with idiopathic seizure disorders.
While the fur seal discussed here had minimal difficulty living in a long-term managed-care setting, it is important to highlight potential variability in outcomes of toxic algal exposure. The outcome of domoic acid intoxication may have variable results based on the laterality of hippocampal damage. Prior evidence shows clear spatial memory deficits scaling with the extent of right hippocampal damage in wild sea lions with domoic acid toxicosis. In addition, damage to the ventral right hippocampus has been associated with behavioral disinhibition and reactivity in this population of animals [
37,
44]. Although sea lions with domoic acid toxicosis present with both unilateral right and left hippocampal lesions, no behavioral test to date has shown deficits correlated specifically with decreased left hippocampal volume. The fur seal in the current case showed strong unilateral right hippocampal atrophy. He did not show obvious signs of behavioral disinhibition or any difficulty with basic husbandry behavior. Associative learning and operant conditioning, which may support the bulk of husbandry behaviors, do not rely on the medial temporal system and related cognitive mechanisms [
45,
46].
In the current study, the fur seal’s diffusion marker of tract integrity in the hippocampal fornices (FA value) was relatively high for both left and right hemispheres. The lack of symmetrical variation is not surprising given the results in Cook et al. (2018), where the laterality of hippocampal damage did not predict the laterality of forniceal FA values [
30]. However, in that study, animals with clear signs of domoic acid toxicosis had lower FA values overall, in the mid-0.2 s. The values obtained in the current study are more in line with the FA values from the control animals from the 2018 study. Because the tract tracing and FA valuation protocol was the same, this could be due either to differences in disease progression or brain development between those sea lions and the fur seal in the current study, or to differences in brain fixation and tissue preservation. It is not clear whether this bears on relative cognitive sparing in this animal. Finally, the loss of the dentate gyrus in the right hippocampus most likely had some cognitive effect, albeit not one that clearly manifested for the study animal. The dentate gyrus has been linked with pattern separation—that is, distinguishing similar stimuli and episodes from each other in both humans and rodents [
47,
48]. This type of distinction may be of minor importance in a stable and predictable managed care setting.
Although there were no obvious behavioral alterations due to hippocampal damage in the current case, more specialized behavioral tests may be necessary to identify subtle behavioral deficits in trained animals in managed care. Some tools, such as, behavioral maze tests could be used to assess spatial recognition and memory in DA-exposed pinnipeds, possibly to detect more subtle deficits in behavior that may be secondary to DA toxicity. Previous work with exposed sea lions, in rehabilitation settings and the laboratory, has indicated spatial memory deficits tracking with extent of hippocampal damage, and sensitization of sensory defensive responses. Variants of these tests could be used with animals in long-term captivity to track behavioral and cognitive alterations over time [
37]. Further studies examining the dose of DA necessary to produce clinical symptoms in laboratory experiments may be helpful, although, as discussed above, dose and progression of brain pathology may produce different signalments.
In a recent high-profile case, a managed epileptic California sea lion with breakthrough seizure activity and signs of rapid neural degeneration was successfully treated with pig inhibitory neuron progenitor cells transplanted into its hippocampus [
49]. While that animal remains on AED therapy, the cell implant treatment and other novel treatments may be worth considering for animals in long term managed care, particularly for those showing evidence of rapid disease progression, behavioral impairment, break-through seizure activity and poor treatment response. The current case indicates that for animals with a stable presentation, more standard supportive veterinary care, and regular drug level monitoring can also support a positive outcome.