Bird Welfare in Zoos and Aquariums: General Insights across Industries
Abstract
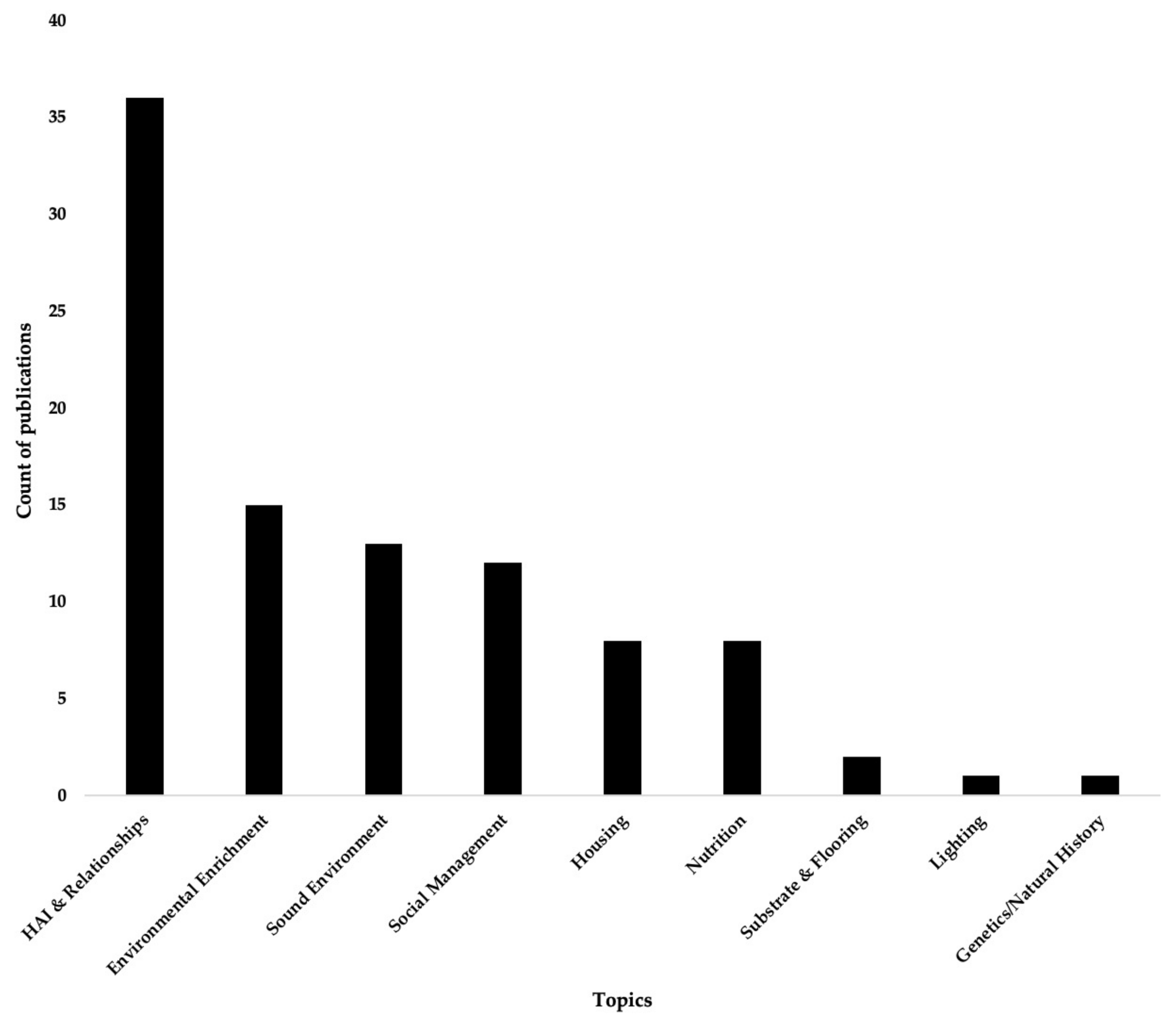
1. Introduction
2. Housing
3. Substrate and Flooring
4. Lighting
5. Sound Environment
6. Environmental Enrichment
7. Social Management
8. Human–Bird Interactions and Relationships
9. Genetics
10. Nutrition
11. Welfare Assessments
12. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, S.J.; Sherwen, S.; Clark, F.E. Advances in Applied Zoo Animal Welfare Science. J. Appl. Anim. Welf. Sci. 2018, 21, 23–33. [Google Scholar] [CrossRef]
- Miller, L.J.; Vicino, G.A.; Sheftel, J.; Lauderdale, L.K. Behavioral Diversity as a Potential Indicator of Positive Animal Welfare. Animals 2020, 10, 1211. [Google Scholar] [CrossRef]
- Melfi, V.A. There Are Big Gaps in Our Knowledge, and Thus Approach, to Zoo Animal Welfare: A Case for Evidence-Based Zoo Animal Management. Zoo Biol. 2009, 28, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Díez-León, M.; Mason, G. Animal Welfare Science: Recent Publication Trends and Future Research Priorities. Int. J. Consum. Stud. 2014, 27, 80–100. [Google Scholar] [CrossRef]
- Rose, P.E.; Brereton, J.E.; Rowden, L.J.; de Figueiredo, R.L.; Riley, L.M. What’s New from the Zoo? An Analysis of Ten Years of Zoo-Themed Research Output. Palgrave Commun. 2019, 5, 128. [Google Scholar] [CrossRef]
- Binding, S.; Farmer, H.; Krusin, L.; Cronin, K. Status of Animal Welfare Research in Zoos and Aquariums: Where Are We, Where to Next? J. Zoo Aquar. Res. 2020, 8, 166–174. [Google Scholar]
- Butterworth, A.; Mench, J.A.; Wielebnowski, N.; Olsson, I.A.S. Practical Strategies to Assess (and Improve) Welfare. In Animal Welfare; CAB International: Wallingford, UK, 2018; pp. 232–250. [Google Scholar]
- Fraser, D.; Weary, D.M.; Pajor, E.A.; Milligan, B.N. A Scientific Conception of Animal Welfare That Reflects Ethical Concerns. Anim. Welf. 1997, 6, 187–205. [Google Scholar]
- Fraser, D. Understanding Animal Welfare. Acta Vet. Scand. 2008, 50, S1. [Google Scholar] [CrossRef]
- Mellor, D. Updating Animal Welfare Thinking: Moving beyond the “Five Freedoms” towards “A Life Worth Living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef]
- Greggor, A.L.; Vicino, G.A.; Swaisgood, R.R.; Fidgett, A.; Brenner, D.; Kinney, M.E.; Farabaugh, S.; Masuda, B.; Lamberski, N. Animal Welfare in Conservation Breeding: Applications and Challenges. Front. Vet. Sci. 2018, 5, 323. [Google Scholar] [CrossRef]
- Souza, A.; Taconeli, C.; Plugge, N.; Molento, C. Broiler Chicken Meat Inspection Data in Brazil: A First Glimpse into an Animal Welfare Approach. Braz. J. Poult. Sci. 2018, 20, 547–554. [Google Scholar] [CrossRef]
- Tremolada, C.; Bielińska, H.; Minero, M.; Ferrante, V.; Canali, E.; Barbieri, S. Animal-Based Measures for the on-Farm Welfare Assessment of Geese. Animals 2020, 10, 890. [Google Scholar] [CrossRef]
- Ward, S.J.; Hosey, G. The Need for a Convergence of Agricultural/Laboratory and Zoo-Based Approaches to Animal Welfare. J. Appl. Anim. Welf. Sci. 2020, 23, 484–492. [Google Scholar] [CrossRef]
- Mendl, M.; Burman, O.H.P.; Paul, E.S. An Integrative and Functional Framework for the Study of Animal Emotion and Mood. Proc. R. Soc. B 2010, 277, 2895–2904. [Google Scholar] [CrossRef]
- Bessei, W. Impact of Animal Welfare on Worldwide Poultry Production. World’s Poult. Sci. J. 2018, 74, 211–224. [Google Scholar] [CrossRef]
- Powell, D.M.; Watters, J.V. The Evolution of the Animal Welfare Movement in U.S. Zoos and Aquariums. Der Zool. Gart. 2017, 86, 219–234. [Google Scholar] [CrossRef]
- De Azevedo, C.S.; Cipreste, C.F.; Young, R.J. Environmental Enrichment: A GAP Analysis. Appl. Anim. Behav. Sci. 2007, 102, 329–343. [Google Scholar] [CrossRef]
- Marshall, A.R.; Deere, N.J.; Little, H.A.; Snipp, R.; Goulder, J.; Mayer-Clarke, S. Husbandry and Enclosure Influences on Penguin Behavior and Conservation Breeding: Humboldt Penguin Enclosure Design. Zoo Biol. 2016, 35, 385–397. [Google Scholar] [CrossRef]
- McDonald Kinkaid, H. Species-Level Determinants of Stereotypic Behaviour, Reproductive Success, and Lifespan in Captive Parrots (Psittaciformes). Ph.D. Thesis, The University of Guelph, Guelph, ON, Canada, 2015. [Google Scholar]
- Meehan, C.L.; Mench, J.A. Captive Parrot Welfare. In Manual of Parrot Behavior; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 301–318. [Google Scholar]
- Bracke, M.B.M.; Hopster, H. Assessing the Importance of Natural Behavior for Animal Welfare. J. Agric. Environ. Ethics 2006, 19, 77–89. [Google Scholar] [CrossRef]
- Garner, J.P.; Meehan, C.L.; Mench, J.A. Stereotypies in Caged Parrots, Schizophrenia and Autism: Evidence for a Common Mechanism. Behav. Brain Res. 2003, 145, 125–134. [Google Scholar] [CrossRef]
- Garner, J.P.; Mason, G.J.; Smith, R. Stereotypic Route-Tracing in Experimentally Caged Songbirds Correlates with General Behavioural Disinhibition. Anim. Behav. 2003, 66, 711–727. [Google Scholar] [CrossRef]
- Mellor, E.; Brilot, B.; Collins, S. Abnormal Repetitive Behaviours in Captive Birds: A Tinbergian Review. Appl. Anim. Behav. Sci. 2018, 198, 109–120. [Google Scholar] [CrossRef]
- Rose, P.E.; Croft, D.P.; Lee, R. A Review of Captive Flamingo (Phoenicopteridae) Welfare: A Synthesis of Current Knowledge and Future Directions: Review: Flamingo Welfare: Zoological Institutions. Int. Zoo Yearb. 2014, 48, 139–155. [Google Scholar] [CrossRef]
- Spiezio, C.; Valsecchi, V.; Sandri, C.; Regaiolli, B. Investigating Individual and Social Behaviour of the Northern Bald Ibis (Geronticus eremita): Behavioural Variety and Welfare. PeerJ 2018, 6, e5436. [Google Scholar] [CrossRef]
- Costa, L.; Pereira, D.; Bueno, L.; Pandorfi, H. Some Aspects of Chicken Behavior and Welfare. Rev. Bras. Cienc. Avic. 2012, 14, 159–164. [Google Scholar] [CrossRef]
- Dawkins, M.S. Time Budgets in Red Junglefowl as a Baseline for the Assessment of Welfare in Domestic Fowl. Appl. Anim. Behav. Sci. 1989, 24, 77–80. [Google Scholar] [CrossRef]
- Whay, H.R.; Main, D.C.J.; Green, L.E.; Heaven, G.; Howell, H.; Morgan, M.; Pearson, A.; Webster, A.J.F. Assessment of the Behaviour and Welfare of Laying Hens on Free-Range Units. Vet. Rec. 2007, 161, 119–128. [Google Scholar] [CrossRef]
- Cronin, G.M.; Barnett, J.L.; Hemsworth, P.H. The Importance of Pre-Laying Behaviour and Nest Boxes for Laying Hen Welfare: A Review. Anim. Prod. Sci. 2012, 52, 398–405. [Google Scholar] [CrossRef]
- Rodenburg, T.B.; Bracke, M.B.M.; Berk, J.; Cooper, J.; Faure, J.M.; Guémené, D.; Guy, G.; Harlander, A.; Jones, T.; Knierim, U.; et al. Welfare of Ducks in European Duck Husbandry Systems. World’s Poult. Sci. J. 2005, 61, 633–646. [Google Scholar] [CrossRef]
- Klausen, B. A Mixed-Species Exhibit for African Water Birds (Including Pelicans, Flamingos, Spoonbills and Storks) at Odense Zoo, Denmark: Breeding Success, Animal Welfare and Education: Odense Zoo: Mixed-Species Exhibit for African Water Birds. Int. Zoo Yearb. 2014, 48, 61–68. [Google Scholar] [CrossRef]
- Hesterman, H.; Gregory, N.G.; Boardman, W.S.J. Deflighting Procedures and Their Welfare Implications in Captive Birds. Anim. Welf. 2001, 10, 405–419. [Google Scholar]
- Reese, L.; Ladwig-Wiegard, M.; von Fersen, L.; Haase, G.; Will, H.; Merle, R.; Encke, D.; Maegdefrau, H.; Baumgartner, K.; Thöne-Reineke, C. Deflighting Zoo Birds and Its Welfare Considerations. Anim. Welf. 2020, 29, 69–80. [Google Scholar] [CrossRef]
- Loeb, J.; Roberts, A. Do Birds Need to Fly? Vet. Rec. 2020, 186, 234–235. [Google Scholar] [CrossRef]
- Tyson, E. For an End to Pinioning: The Case against the Legal Mutilation of Birds in Captivity. J. Anim. Ethics 2014, 4, 4. [Google Scholar] [CrossRef]
- Haase, G.; Baumgartner, K.; von Fersen, L.; Merle, R.; Wiegard, M.; Will, H.; Reese, L.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M.; et al. Feather Corticosterone Measurements and Behavioral Observations in the Great White Pelican (Pelecanus onocrotalus) Living under Different Flight Restraint Conditions in German Zoos. Animals 2021, 11, 2522. [Google Scholar] [CrossRef] [PubMed]
- Reese, L.; Baumgartner, K.; von Fersen, L.; Merle, R.; Ladwig-Wiegard, M.; Will, H.; Haase, G.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M.; et al. Feather Corticosterone Measurements of Greater Flamingos Living under Different Forms of Flight Restraint. Animals 2020, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, S.M. Animal Welfare with and without Consciousness. J. Zool. 2017, 301, 1–10. [Google Scholar] [CrossRef]
- Barnett, J.; Hemsworth, P. Science and Its Application in Assessing the Welfare of Laying Hens in the Egg Industry. Aust. Vet. J. 2003, 81, 615–624. [Google Scholar] [CrossRef]
- Kajlich, A.S.; Shivaprasad, H.L.; Trampel, D.W.; Hill, A.E.; Parsons, R.L.; Millman, S.T.; Mench, J.A. Incidence, Severity, and Welfare Implications of Lesions Observed Postmortem in Laying Hens from Commercial Noncage Farms in California and Iowa. Avian Dis. 2016, 60, 8–15. [Google Scholar] [CrossRef]
- Lay, D.C.; Fulton, R.M.; Hester, P.Y.; Karcher, D.M.; Kjaer, J.B.; Mench, J.A.; Mullens, B.A.; Newberry, R.C.; Nicol, C.J.; O’Sullivan, N.P.; et al. Hen Welfare in Different Housing Systems. Poult. Sci. 2011, 90, 278–294. [Google Scholar] [CrossRef]
- Bračko, A.; King, C.E. Advantages of Aviaries and the Aviary Database Project: A New Approach to an Old Housing Option for Birds: Advantages of Aviaries and the Aviary Database Project. Int. Zoo Yearb. 2014, 48, 166–183. [Google Scholar] [CrossRef]
- Blay, N.; Côté, I.M. Optimal Conditions for Breeding of Captive Humboldt Penguins (Spheniscus humboldti): A Survey of British Zoos: Optimal Conditions for Humboldt Penguin Breeding. Zoo Biol. 2001, 20, 545–555. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.; Li, X.; Bao, J. Effects of Housing Systems on Behaviour, Performance and Welfare of Fast-Growing Broilers. Asian Australas. J. Anim. Sci. 2014, 27, 140–146. [Google Scholar] [CrossRef]
- Shimmura, T.; Bracke, M.B.M.; De Mol, R.M.; Hirahara, S.; Uetake, K.; Tanaka, T. Overall Welfare Assessment of Laying Hens: Comparing Science-Based, Environment-Based and Animal-Based Assessments. Anim. Sci. J. 2011, 82, 150–160. [Google Scholar] [CrossRef]
- Taylor, P.S.; Hemsworth, P.H.; Groves, P.J.; Gebhardt-Henrich, S.G.; Rault, J.-L. Frequent Range Visits Further from the Shed Relate Positively to Free-Range Broiler Chicken Welfare. Animal 2020, 14, 138–149. [Google Scholar] [CrossRef]
- Cronin, G.M.; Glatz, P.C. Causes of Feather Pecking and Subsequent Welfare Issues for the Laying Hen: A Review. Anim. Prod. Sci. 2020, 61, 990–1005. [Google Scholar] [CrossRef]
- Ferrante, V.; Lolli, S.; Vezzoli, G.; Cavalchini, L.G. Effects of Two Different Rearing Systems (Organic and Barn) on Production Performance, Animal Welfare Traits and Egg Quality Characteristics in Laying Hens. Ital. J. Anim. Sci. 2009, 8, 165–174. [Google Scholar] [CrossRef]
- Hinrichsen, L.K.; Riber, A.B.; Labouriau, R. Associations between and Development of Welfare Indicators in Organic Layers. Animal 2016, 10, 953–960. [Google Scholar] [CrossRef]
- Bestman, M.; Wagenaar, J.-P. Health and Welfare in Dutch Organic Laying Hens. Animals 2014, 4, 374–390. [Google Scholar] [CrossRef]
- Weeks, C.A.; Lambton, S.L.; Williams, A.G. Implications for Welfare, Productivity and Sustainability of the Variation in Reported Levels of Mortality for Laying Hen Flocks Kept in Different Housing Systems: A Meta-Analysis of Ten Studies. PLoS ONE 2016, 11, e0146394. [Google Scholar] [CrossRef]
- El-Deek, A.; El-Sabrout, K. Behaviour and Meat Quality of Chicken under Different Housing Systems. World’s Poult. Sci. J. 2019, 75, 105–114. [Google Scholar] [CrossRef]
- Aerts, J.-M.; Wathes, C.M.; Berckmans, D. Environmental Management for Laying Hens. In Welfare of the Laying Hen; CABI: Wallingford, UK, 2004; Volume 27, pp. 283–298. [Google Scholar]
- Taylor, P.; Hemsworth, P.; Groves, P.; Gebhardt-Henrich, S.; Rault, J.-L. Ranging Behaviour of Commercial Free-Range Broiler Chickens 1: Factors Related to Flock Variability. Animals 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Bestman, M.; Bikker-Ouwejan, J. Predation in Organic and Free-Range Egg Production. Animals 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.M.W.; Nielsen, S.S.; King, C.E.; Bertelsen, M.F. Classification and Prevalence of Foot Lesions in Captive Flamingos (Phoenicopteridae). J. Zoo Wildl. Med. 2010, 41, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Reidarson, T.H.; McBain, J.; Burch, L. A Novel Approach to the Treatment of Bumblefoot in Penguins. JAMS 1999, 13, 124–127. [Google Scholar]
- Stracke, J.; Klotz, D.; Wohlsein, P.; Döhring, S.; Volkmann, N.; Kemper, N.; Spindler, B. Scratch the Surface: Histopathology of Foot-Pad Dermatitis in Turkeys (Meleagris gallopavo). Anim. Welf. 2020, 29, 419–432. [Google Scholar] [CrossRef]
- Wolanin, J.A. A Critical Analysis of Bumblefoot: Care and Preventative Measures in Captive Penguins. Diploma Thesis, Grand Valley State University, Allendale, MI, USA, 2018. [Google Scholar]
- Erlacher-Reid, C.; Dunn, J.L.; Camp, T.; Macha, L.; Mazzaro, L.; Tuttle, A.D. Evaluation of Potential Variables Contributing to the Development and Duration of Plantar Lesions in a Population of Aquarium-Maintained African Penguins (Spheniscus demersus): Penguin Plantar Lesions. Zoo Biol. 2012, 31, 291–305. [Google Scholar] [CrossRef]
- Osório, L.G.; Xavier, M.O.; Ladeira, S.R.L.; Silva Filho, R.P.; Faria, R.O.; Vargas, G.D.; Cabana, A.L.; Mello, J.R.B.; Meireles, M.C.A. Study of Bacteria Isolated from the Foot Pad of Spheniscus magellanicus with and without Bumblefoot. Arq. Bras. Med. Vet. Zootec. 2013, 65, 47–54. [Google Scholar] [CrossRef]
- Robins, A.; Phillips, C.J.C. International Approaches to the Welfare of Meat Chickens. World’s Poult. Sci. J. 2011, 67, 351–369. [Google Scholar] [CrossRef]
- BenSassi, N.; Vas, J.; Vasdal, G.; Averós, X.; Estévez, I.; Newberry, R.C. On-Farm Broiler Chicken Welfare Assessment Using Transect Sampling Reflects Environmental Inputs and Production Outcomes. PLoS ONE 2019, 14, e0214070. [Google Scholar] [CrossRef]
- Bassler, A.W.; Arnould, C.; Butterworth, A.; Colin, L.; De Jong, I.C.; Ferrante, V.; Ferrari, P.; Haslam, S.; Wemelsfelder, F.; Blokhuis, H.J. Potential Risk Factors Associated with Contact Dermatitis, Lameness, Negative Emotional State, and Fear of Humans in Broiler Chicken Flocks. Poult. Sci. 2013, 92, 2811–2826. [Google Scholar] [CrossRef]
- Haslam, S.M.; Brown, S.N.; Wilkins, L.J.; Kestin, S.C.; Warriss, P.D.; Nicol, C.J. Preliminary Study to Examine the Utility of Using Foot Burn or Hock Burn to Assess Aspects of Housing Conditions for Broiler Chicken. Br. Poult. Sci. 2006, 47, 13–18. [Google Scholar] [CrossRef]
- Dawkins, M.S.; Donnelly, C.A.; Jones, T.A. Chicken Welfare Is Influenced More by Housing Conditions than by Stocking Density. Nature 2004, 427, 342–344. [Google Scholar] [CrossRef]
- Tauson, R. Management and Housing Systems for Layers—Effects on Welfare and Production. World’s Poult. Sci. J. 2005, 61, 477–490. [Google Scholar] [CrossRef]
- Freeman, N.; Tuyttens, F.A.M.; Johnson, A.; Marshall, V.; Garmyn, A.; Jacobs, L. Remedying Contact Dermatitis in Broiler Chickens with Novel Flooring Treatments. Animals 2020, 10, 1761. [Google Scholar] [CrossRef]
- Rogers, L.J.; Kaplan, G. Does Functional Lateralization in Birds Have Any Implications for Their Welfare? Symmetry 2019, 11, 1043. [Google Scholar] [CrossRef]
- Bessei, W. Welfare of Broilers: A Review. World’s Poult. Sci. J. 2006, 62, 455–466. [Google Scholar] [CrossRef]
- Meluzzi, A.; Sirri, F. Welfare of Broiler Chickens. Ital. J. Anim. Sci. 2009, 8 (Suppl. 1), 161–173. [Google Scholar] [CrossRef]
- Taylor, P.E.; Coerse, N.C.A.; Haskell, M. The Effects of Operant Control over Food and Light on the Behaviour of Domestic Hens. Appl. Anim. Behav. Sci. 2001, 71, 319–333. [Google Scholar] [CrossRef]
- Greenwood, V.J.; Smith, E.L.; Goldsmith, A.R.; Cuthill, I.C.; Crisp, L.H.; Walter-Swan, M.B.; Bennett, A.T.D. Does the Flicker Frequency of Fluorescent Lighting Affect the Welfare of Captive European Starlings? Appl. Anim. Behav. Sci. 2004, 86, 145–159. [Google Scholar] [CrossRef]
- Bateson, M.; Feenders, G. The Use of Passerine Bird Species in Laboratory Research: Implications of Basic Biology for Husbandry and Welfare. ILAR J. 2010, 51, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.L. Making Sense of It All: The Importance of Taking into Account the Sensory Abilities of Animals in Their Housing and Management. Appl. Anim. Behav. Sci. 2018, 205, 175–180. [Google Scholar] [CrossRef]
- Ross, M.R.; Gillespie, K.L.; Hopper, L.M.; Bloomsmith, M.A.; Maple, T.L. Differential Preference for Ultraviolet Light among Captive Birds from Three Ecological Habitats. Appl. Anim. Behav. Sci. 2013, 147, 278–285. [Google Scholar] [CrossRef]
- Nelson, J.R.; Bray, J.L.; Delabbio, J.; Archer, G.S. Comparison of an Intermittent, Short-Dawn/Dusk Photoperiod with an Increasing, Long-Dawn/Dusk Photoperiod on Broiler Growth, Stress, and Welfare. Poult. Sci. 2020, 99, 3908–3913. [Google Scholar] [CrossRef]
- Hausmann, F.; Arnold, K.E.; Marshall, N.J.; Owens, I.P.F. Ultraviolet Signals in Birds Are Special. Proc. R. Soc. Lond. B 2003, 270, 61–67. [Google Scholar] [CrossRef]
- Maddocks, S.A.; Goldsmith, A.R.; Cuthill, I.C. Behavioural and Physiological Effects of Absence of Ultraviolet Wavelengths on European Starlings Sturnus vulgaris. J. Avian Biol. 2002, 33, 103–106. [Google Scholar] [CrossRef]
- James, C.; Asher, L.; Herborn, K.; Wiseman, J. The Effect of Supplementary Ultraviolet Wavelengths on Broiler Chicken Welfare Indicators. Appl. Anim. Behav. Sci. 2018, 209, 55–64. [Google Scholar] [CrossRef]
- Maddocks, S.A.; Cuthill, I.C.; Goldsmith, A.R.; Sherwin, C.M. Behavioural and Physiological Effects of Absence of Ultraviolet Wavelengths for Domestic Chicks. Anim. Behav. 2001, 62, 1013–1019. [Google Scholar] [CrossRef]
- Harding, H.R.; Gordon, T.A.C.; Eastcott, E.; Simpson, S.D.; Radford, A.N. Causes and Consequences of Intraspecific Variation in Animal Responses to Anthropogenic Noise. Behav. Ecol. 2019, 30, 1501–1511. [Google Scholar] [CrossRef]
- Morgan, K.N.; Tromborg, C.T. Sources of Stress in Captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Shepherdson, D.J.; Carlstead, K.C.; Wielebnowski, N. Cross-Institutional Assessment of Stress Responses in Zoo Animals Using Longitudinal Monitoring of Faecal Corticoids and Behaviour. Anim. Welf. 2004, 13, 105–113. [Google Scholar]
- Donofre, A.C.; da Silva, I.J.O.; Ferreira, I.E.P. Sound Exposure and Its Beneficial Effects on Embryonic Growth and Hatching of Broiler Chicks. Br. Poult. Sci. 2020, 61, 79–85. [Google Scholar] [CrossRef]
- Blanchett, M.; Finegan, E.; Atkinson, J. The Effects of Increasing Visitor and Noise Levels on Birds within a Free-Flight Aviary Examined Through Enclosure Use and Behavior. ABC 2020, 7, 49–69. [Google Scholar] [CrossRef]
- De Almeida, A.C.; Palme, R.; Moreira, N. How Environmental Enrichment Affects Behavioral and Glucocorticoid Responses in Captive Blue-and-Yellow Macaws (Ara ararauna). Appl. Anim. Behav. Sci. 2018, 201, 125–135. [Google Scholar] [CrossRef]
- Kalmar, I.D.; Janssens, G.P.J.; Moons, C.P.H. Guidelines and Ethical Considerations for Housing and Management of Psittacine Birds Used in Research. ILAR J. 2010, 51, 409–423. [Google Scholar] [CrossRef]
- Campo, J.L.; Gil, M.G.; Dávila, S.G. Effects of Specific Noise and Music Stimuli on Stress and Fear Levels of Laying Hens of Several Breeds. Appl. Anim. Behav. Sci. 2005, 91, 75–84. [Google Scholar] [CrossRef]
- Quadros, S.; Goulart, V.D.L.; Passos, L.; Vecci, M.A.M.; Young, R.J. Zoo Visitor Effect on Mammal Behaviour: Does Noise Matter? Appl. Anim. Behav. Sci. 2014, 156, 78–84. [Google Scholar] [CrossRef]
- Jakob-Hoff, R.; Kingan, M.; Fenemore, C.; Schmid, G.; Cockrem, J.F.; Crackle, A.; Van Bemmel, E.; Connor, R.; Descovich, K. Potential Impact of Construction Noise on Selected Zoo Animals. Animals 2019, 9, 504. [Google Scholar] [CrossRef]
- Fanning, L.; Larsen, H.; Taylor, P.S. A Preliminary Study Investigating the Impact of Musical Concerts on the Behavior of Captive Fiordland Penguins (Eudyptes pachyrhynchus) and Collared Peccaries (Pecari tajacu). Animals 2020, 10, 2035. [Google Scholar] [CrossRef]
- Brando, S.; Buchanan-Smith, H.M. The 24/7 Approach to Promoting Optimal Welfare for Captive Wild Animals. Behav. Processes 2018, 156, 83–95. [Google Scholar] [CrossRef]
- Orban, D.A.; Soltis, J.; Perkins, L.; Mellen, J.D. Sound at the Zoo: Using Animal Monitoring, Sound Measurement, and Noise Reduction in Zoo Animal Management. Zoo Biol. 2017, 36, 231–236. [Google Scholar] [CrossRef]
- Williams, I.; Hoppitt, W.; Grant, R. The Effect of Auditory Enrichment, Rearing Method and Social Environment on the Behavior of Zoo-Housed Psittacines (Aves: Psittaciformes); Implications for Welfare. Appl. Anim. Behav. Sci. 2017, 186, 85–92. [Google Scholar] [CrossRef]
- Robbins, L.; Margulis, S.W. Music for the Birds: Effects of Auditory Enrichment on Captive Bird Species: Music for the Birds. Zoo Biol. 2016, 35, 29–34. [Google Scholar] [CrossRef]
- Brumm, H.; Schmidt, R.; Schrader, L. Noise-Dependent Vocal Plasticity in Domestic Fowl. Anim. Behav. 2009, 78, 741–746. [Google Scholar] [CrossRef]
- Rose, P.; Badman-King, A.; Hurn, S.; Rice, T. Visitor Presence and a Changing Soundscape, alongside Environmental Parameters, Can Predict Enclosure Usage in Captive Flamingos. Zoo Biol. 2021, 40, 363–375. [Google Scholar] [CrossRef]
- Campbell, D.L.M.; de Haas, E.N.; Lee, C. A Review of Environmental Enrichment for Laying Hens during Rearing in Relation to Their Behavioral and Physiological Development. Poult. Sci. 2019, 98, 9–28. [Google Scholar] [CrossRef]
- Gilani, A.-M.; Knowles, T.G.; Nicol, C.J. The Effect of Rearing Environment on Feather Pecking in Young and Adult Laying Hens. Appl. Anim. Behav. Sci. 2013, 148, 54–63. [Google Scholar] [CrossRef]
- Parveen, Z.; Sidra, S.; Khan, B.N. Diet Preferences and General Behavior of Peafowls in Captive Environment. Punjab Univ. J. Zool. 2018, 33, 16–21. [Google Scholar] [CrossRef]
- Fiorucci, L.; Grande, F.; Macrelli, R.; Schnitzer, P.; Crosta, L. Hand-Rearing of Three Lesser Flamingo Chicks (Phoeniconaias minor). Animals 2020, 10, 1251. [Google Scholar] [CrossRef]
- Graham, J.; Wright, T.F.; Dooling, R.J.; Korbel, R. Sensory Capacities of Parrots. In Manual of Parrot Behavior; Luescher, A.U., Ed.; Blackwell Publishing Professional: Ames, IA, USA, 2006; pp. 33–41. [Google Scholar] [CrossRef]
- Pelletier, C.; Weladji, R.B.; Lazure, L.; Paré, P. Zoo Soundscape: Daily Variation of Low-to-high-frequency Sounds. Zoo Biol. 2020, 39, 374–381. [Google Scholar] [CrossRef]
- Heffner, R.; Cumming, J.F.; Koay, G.; Heffner, H.E. Hearing in Indian Peafowl (Pavo cristatus): Sensitivity to Infrasound. J. Comp. Physiol. A 2020, 206, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Alligood, C.; Leighty, K. Putting the “E” in SPIDER: Evolving Trends in the Evaluation of Environmental Enrichment Efficacy in Zoological Settings. ABC 2015, 2, 200–217. [Google Scholar] [CrossRef]
- Riber, A.B.; Casey-Trott, T.M.; Herskin, M.S. The Influence of Keel Bone Damage on Welfare of Laying Hens. Front. Vet. Sci. 2018, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.; Clubb, R.; Latham, N.; Vickery, S. Why and How Should We Use Environmental Enrichment to Tackle Stereotypic Behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef]
- Riley, L.M.; Rose, P.E. Concepts, Applications, Uses and Evaluation of Environmental Enrichment: Perceptions of Zoo Professionals. J. Zoo Aquar. Res. 2020, 8, 18–28. [Google Scholar]
- Tahamtani, F.M.; Pedersen, I.J.; Riber, A.B. Effects of Environmental Complexity on Welfare Indicators of Fast-Growing Broiler Chickens. Poult. Sci. 2020, 99, 21–29. [Google Scholar] [CrossRef]
- Reisfeld, L.; Barbirato, M.; Ippolito, L.; Cardoso, R.C.; Nichi, M.; Sgai, M.G.F.G.; Pizzutto, C.S. Reducing Bumblefoot Lesions in a Group of Captive Magellanic Penguins (Spheniscus magellanicus) with the Use of Environmental Enrichment. Pesq. Vet. Bras. 2013, 33, 791–795. [Google Scholar] [CrossRef][Green Version]
- Reimer, J.; Maia, C.M.; Santos, E.F. Environmental Enrichments for a Group of Captive Macaws: Low Interaction Does Not Mean Low Behavioral Changes. J. Appl. Anim. Welf. Sci. 2016, 19, 385–395. [Google Scholar] [CrossRef]
- Ross, M.; Rausch, Q.; Vandenberg, B.; Mason, G. Hens with Benefits: Can Environmental Enrichment Make Chickens More Resilient to Stress? Physiol. Behav. 2020, 226, 113077. [Google Scholar] [CrossRef]
- Ross, M.; Garland, A.; Harlander-Matauschek, A.; Kitchenham, L.; Mason, G. Welfare-Improving Enrichments Greatly Reduce Hens’ Startle Responses, despite Little Change in Judgment Bias. Sci. Rep. 2019, 9, 11881. [Google Scholar] [CrossRef]
- Collins, C.; Quirke, T.; Overy, L.; Flannery, K.; O’Riordan, R. The Effect of the Zoo Setting on the Behavioural Diversity of Captive Gentoo Penguins and the Implications for Their Educational Potential. J. Zoo Aquar. Res. 2016, 4, 85–90. [Google Scholar]
- Averós, X.; Estevez, I. Meta-Analysis of the Effects of Intensive Rearing Environments on the Performance and Welfare of Broiler Chickens. Poult. Sci. 2018, 97, 3767–3785. [Google Scholar] [CrossRef]
- Riber, A.B.; van de Weerd, H.A.; de Jong, I.C.; Steenfeldt, S. Review of Environmental Enrichment for Broiler Chickens. Poult. Sci. J. 2018, 97, 378–396. [Google Scholar] [CrossRef]
- Dixon, L.; Duncan, I.; Mason, G. The Effects of Four Types of Enrichment on Feather-Pecking Behaviour in Laying Hens Housed in Barren Environments. Anim. Welf. 2010, 19, 429–435. [Google Scholar]
- Ali, B.A.; Toscano, M.; Siegford, J.M. Later Exposure to Perches and Nests Reduces Individual Hens’ Occupancy of Vertical Space in an Aviary and Increases Force of Falls at Night. Poult. Sci. 2019, 98, 6251–6262. [Google Scholar] [CrossRef]
- Karaarslan, S.; Nazlıgül, A. Effects of Lighting, Stocking Density, and Access to Perches on Leg Health Variables as Welfare Indicators in Broiler Chickens. Livest. Sci. 2018, 218, 31–36. [Google Scholar] [CrossRef]
- Jones, T.A.; Waitt, C.D.; Dawkins, M.S. Water off a Duck’s Back: Showers and Troughs Match Ponds for Improving Duck Welfare. Appl. Anim. Behav. Sci. 2009, 116, 52–57. [Google Scholar] [CrossRef]
- Murphy, S.M.; Braun, J.V.; Millam, J.R. Bathing Behavior of Captive Orange-Winged Amazon Parrots (Amazona amazonica). Appl. Anim. Behav. Sci. 2011, 132, 200–210. [Google Scholar] [CrossRef]
- Checon, C.T.; Rosenfield, D.A.; Jorge-Neto, P.N.; Pizzutto, C.S. Influence of Environmental Enrichment on the Behavioral Variables of Caged Hyacinth Macaws (Anodorhynchus hyacinthinus). Ornithol. Res. 2020, 28, 125–132. [Google Scholar] [CrossRef]
- Fangmeier, M.L.; Burns, A.L.; Melfi, V.A.; Meade, J. Foraging Enrichment Alleviates Oral Repetitive Behaviors in Captive Red-Tailed Black Cockatoos (Calyptorhynchus banksii). Zoo Biol. 2020, 39, 3–12. [Google Scholar] [CrossRef]
- Daigle, C.L.; Rodenburg, T.B.; Bolhuis, J.E.; Swanson, J.C.; Siegford, J.M. Use of Dynamic and Rewarding Environmental Enrichment to Alleviate Feather Pecking in Non-Cage Laying Hens. Appl. Anim. Behav. Sci. 2014, 161, 75–85. [Google Scholar] [CrossRef]
- Lumeij, J.T.; Hommers, C.J. Foraging ‘Enrichment’ as Treatment for Pterotillomania. Appl. Anim. Behav. Sci. 2008, 111, 85–94. [Google Scholar] [CrossRef]
- Garner, M.M.; Clubb, S.L.; Mitchell, M.A.; Brown, L. Feather-Picking Psittacines: Histopathology and Species Trends. Vet. Pathol. 2008, 45, 401–408. [Google Scholar] [CrossRef]
- Van Hoek, C.S.; Cate, C.T. Abnormal Behavior in Caged Birds Kept as Pets. J. Appl. Anim. Welf. Sci. 1998, 1, 51–64. [Google Scholar] [CrossRef]
- Van Zeeland, Y.R.A.; Spruit, B.M.; Rodenburg, T.B.; Riedstra, B.; van Hierden, Y.M.; Buitenhuis, B.; Korte, S.M.; Lumeij, J.T. Feather Damaging Behaviour in Parrots: A Review with Consideration of Comparative Aspects. Appl. Anim. Behav. Sci. 2009, 121, 75–95. [Google Scholar] [CrossRef]
- Rodenburg, T.B.; Van Krimpen, M.M.; De Jong, I.C.; De Haas, E.N.; Kops, M.S.; Riedstra, B.J.; Nordquist, R.E.; Wagenaar, J.P.; Bestman, M.; Nicol, C.J. The Prevention and Control of Feather Pecking in Laying Hens: Identifying the Underlying Principles. World’s Poult. Sci. J. 2013, 69, 361–374. [Google Scholar] [CrossRef]
- Butler, D.A.; Davis, C. Effects of Plastic Bits on the Condition and Behavior of Captive-Reared Pheasants. Vet. Rec. 2010, 166, 398–401. [Google Scholar] [CrossRef]
- Dalton, H.A. The Relationships between the Performance of Injurious Pecking and Behavioural and Physical Traits in Domestic Turkeys. Ph.D. Thesis, The University of Guelph, Guelph, ON, Canada, 2017. [Google Scholar]
- Fossum, O.; Jansson, D.S.; Etterlin, P.E.; Vågsholm, I. Causes of Mortality in Laying Hens in Different Housing Systems in 2001 to 2004. Acta Vet. Scand. 2009, 51, 3. [Google Scholar] [CrossRef]
- Meehan, C.L.; Millam, J.R.; Mench, J.A. Foraging Opportunity and Increased Physical Complexity Both Prevent and Reduce Psychogenic Feather Picking by Young Amazon Parrots. Appl. Anim. Behav. Sci. 2003, 80, 71–85. [Google Scholar] [CrossRef]
- Meehan, C.L.; Garner, J.P.; Mench, J.A. Environmental Enrichment and Development of Cage Stereotypy in Orange-Winged Amazon Parrots (Amazona amazonica). Dev. Psychobiol. 2004, 44, 209–218. [Google Scholar] [CrossRef]
- Rodríguez-López, R. Environmental Enrichment for Parrot Species: Are We Squawking up the Wrong Tree? Appl. Anim. Behav. Sci. 2016, 180, 1–10. [Google Scholar] [CrossRef]
- Vargas-Ashby, H.; Pankhurst, S. Effects of Feeding Enrichment on the Behaviour and Welfare of Captive Waldrapps (Northern Bald Ibis) (Geronticus Eremita). Anim. Welf. 2007, 16, 369–374. [Google Scholar]
- Slocum, S.K.; Morris, K.L. Assessing Preference in a Paired-Stimulus Arrangement with Captive Vultures (Aegypius Monachus). J. Appl. Anim. Welf. Sci. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brereton, J.E.; Myhill, M.N.G.; Shora, J.A. Investigating the Effect of Enrichment on the Behavior of Zoo-Housed Southern Ground Hornbills. JZBG 2021, 2, 600–609. [Google Scholar] [CrossRef]
- Gaengler, H.; Clum, N. Investigating the Impact of Large Carcass Feeding on the Behavior of Captive Andean Condors (Vultur Gryphus) and Its Perception by Zoo Visitors: Carcass Feeding for Captive Vultures. Zoo Biol. 2015, 34, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Rozek, J.C.; Millam, J.R. Preference and Motivation for Different Diet Forms and Their Effect on Motivation for a Foraging Enrichment in Captive Orange-Winged Amazon Parrots (Amazona amazonica). Appl. Anim. Behav. Sci. 2011, 129, 153–161. [Google Scholar] [CrossRef]
- Mason, G.J. Species Differences in Responses to Captivity: Stress, Welfare and the Comparative Method. Trends Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef]
- Rault, J.-L. Friends with Benefits: Social Support and Its Relevance for Farm Animal Welfare. Appl. Anim. Behav. Sci. 2012, 136, 1–14. [Google Scholar] [CrossRef]
- Buijs, S.; Keeling, L.; Rettenbacher, S.; Van Poucke, E.; Tuyttens, F.A.M. Stocking Density Effects on Broiler Welfare: Identifying Sensitive Ranges for Different Indicators. Poult. Sci. 2009, 88, 1536–1543. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Samara, E.M.; Hussein, E.O.S.; Al-Ghadi, M.Q.; Al-Atiyat, R.M. Impacts of Stocking Density on the Performance and Welfare of Broiler Chickens. Ital. J. Anim. Sci. 2013, 12, e11. [Google Scholar] [CrossRef]
- Bailie, C.L.; Ijichi, C.; O’Connell, N.E. Effects of Stocking Density and String Provision on Welfare-Related Measures in Commercial Broiler Chickens in Windowed Houses. Poult. Sci. 2018, 97, 1503–1510. [Google Scholar] [CrossRef]
- Geng, A.L.; Liu, H.G.; Zhang, Y.; Zhang, J.; Wang, H.H.; Chu, Q.; Yan, Z.X. Effects of Indoor Stocking Density on Performance, Egg Quality, and Welfare Status of a Native Chicken during 22 to 38 Weeks. Poult. Sci. 2020, 99, 163–171. [Google Scholar] [CrossRef]
- Rose, P.E.; Croft, D.P. Evaluating the Social Networks of Four Flocks of Captive Flamingos over a Five-Year Period: Temporal, Environmental, Group and Health Influences on Assortment. Behav. Processes 2020, 175, 104118. [Google Scholar] [CrossRef]
- Rose, P.; O’Brien, M. Welfare Assessment for Captive Anseriformes: A Guide for Practitioners and Animal Keepers. Animals 2020, 10, 1132. [Google Scholar] [CrossRef]
- Meehan, C.L.; Garner, J.P.; Mench, J.A. Isosexual Pair Housing Improves the Welfare of Young Amazon Parrots. Appl. Anim. Behav. Sci. 2003, 81, 73–88. [Google Scholar] [CrossRef]
- Edgar, J.; Held, S.; Jones, C.; Troisi, C. Influences of Maternal Care on Chicken Welfare. Animals 2016, 6, 2. [Google Scholar] [CrossRef]
- Schmid, R.; Doherr, M.G.; Steiger, A. The Influence of the Breeding Method on the Behaviour of Adult African Grey Parrots (Psittacus erithacus). Appl. Anim. Behav. Sci. 2006, 98, 293–307. [Google Scholar] [CrossRef]
- Engebretson, M. The Welfare and Suitability of Parrots as Companion Animals: A Review. Anim. Welf. 2006, 15, 263–276. [Google Scholar]
- Flanagan, A.M.; Rutz, C.; Farabaugh, S.; Greggor, A.L.; Masuda, B.; Swaisgood, R.R. Inter-Aviary Distance and Visual Access Influence Conservation Breeding Outcomes in a Territorial, Endangered Bird. Biol. Conserv. 2020, 242, 108429. [Google Scholar] [CrossRef]
- Kozlowski, C.P.; Bauman, K.L.; Asa, C.S. Reproductive Behavior of the Great Hornbill (Buceros bicornis): Hornbill Reproductive Behavior. Zoo Biol. 2015, 34, 328–334. [Google Scholar] [CrossRef]
- Monckton, V.; van Staaversen, N.; Baes, C.F.; Balzani, A.; Kwon, I.Y.; McBride, P.; Harlander-Matauschek, A. Are Turkeys (Meleagris gallopavo) Motivated to Avoid Excreta-Soiled Substrate? Animals 2020, 10, 2015. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Hemsworth, P.H. The Visitor Effect on Zoo Animals: Implications and Opportunities for Zoo Animal Welfare. Animals 2019, 9, 366. [Google Scholar] [CrossRef]
- Edes, A.N.; Baskir, E.; Bauman, K.L.; Chandrasekharan, N.; Macek, M.; Tieber, A. Effects of Crowd Size, Composition, and Noise Level On Pool Use in a Mixed-Species Penguin Colony. ABC 2021, 8, 507–520. [Google Scholar] [CrossRef]
- Zhang, J.; Quirke, T.; Wu, S.; Li, S.; Butler, F. Impact of Weather Changes and Human Visitation on the Behavior and Activity Level of Captive Humboldt Penguins. Pak. J. Zool. 2021, 53, 1–12. [Google Scholar] [CrossRef]
- Chiew, S.J.; Butler, K.L.; Sherwen, S.L.; Coleman, G.J.; Fanson, K.V.; Hemsworth, P.H. Effects of Regulating Visitor Viewing Proximity and the Intensity of Visitor Behaviour on Little Penguin (Eudyptula minor) Behaviour and Welfare. Animals 2019, 9, 285. [Google Scholar] [CrossRef]
- Ozella, L.; Favaro, L.; Carnovale, I.; Pessani, D. Pond Use by Captive African Penguins (Spheniscus demersus) in an Immersive Exhibit Adjacent to Human Bathers. J. Appl. Anim. Welf. Sci. 2015, 18, 303–309. [Google Scholar] [CrossRef]
- Chiew, S.J.; Butler, K.L.; Sherwen, S.L.; Coleman, G.J.; Melfi, V.; Burns, A.; Hemsworth, P.H. Effect of Covering a Visitor Viewing Area Window on the Behaviour of Zoo-Housed Little Penguins (Eudyptula minor). Animals 2020, 10, 1224. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Magrath, M.J.L.; Butler, K.L.; Hemsworth, P.H. Little Penguins, Eudyptula minor, Show Increased Avoidance, Aggression and Vigilance in Response to Zoo Visitors. Appl. Anim. Behav. Sci. 2015, 168, 71–76. [Google Scholar] [CrossRef]
- Collins, C.K.; Marples, N.M. Zoo Playgrounds: A Source of Enrichment or Stress for a Group of Nearby Cockatoos? A Case Study. J. Appl. Anim. Welf. Sci. 2015, 18, 375–387. [Google Scholar] [CrossRef]
- Downes, K. Is There a Visitor Effect on Behaviour and Enclosure Use of Mixed Bird Species in a Zoo Enclosure? Plymouth Stud. Sci. 2012, 5, 38–60. [Google Scholar]
- Hartell-DeNardo, J. Establishment and Application of a Fecal Glucocorticoid Metabolite Assay for 4.1 Magellanic Penguins (Spheniscus magellanicus) for the Assessment of Adrenal Activity in Conjunction with Behavioral Observations to Understand the Potential Impact Associated with Variables of Behind the Scenes Tours at a Zoological Facility. Master’s Thesis, George Mason University, Fairfax, VA, USA, 2015. [Google Scholar]
- Scheun, J.; Gulson, J.; Ganswindt, A. Monitoring Glucocorticoid Metabolite Concentrations as a Proxy of Environmental Stress across Important Life-History Stages in Captive African Penguins. Gen. Comp. Endocrinol. 2020, 296, 113539. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, C.S.; Lima, M.F.F.; da Silva, V.C.A.; Young, R.J.; Rodrigues, M. Visitor Influence on the Behavior of Captive Greater Rheas (Rhea americana, Rheidae aves). J. Appl. Anim. Welf. Sci. 2012, 15, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.E.; Brereton, J.E.; Croft, D.P. Measuring Welfare in Captive Flamingos: Activity Patterns and Exhibit Usage in Zoo-Housed Birds. Appl. Anim. Behav. Sci. 2018, 205, 115–125. [Google Scholar] [CrossRef]
- Saiyed, S.T.; Hopper, L.M.; Cronin, K.A. Evaluating the Behavior and Temperament of African Penguins in a Non-Contact Animal Encounter Program. Animals 2019, 9, 326. [Google Scholar] [CrossRef]
- Williams, E.; Carter, A.; Rendle, J.; Ward, S.J. Understanding Impacts of Zoo Visitors: Quantifying Behavioural Changes of Two Popular Zoo Species during COVID-19 Closures. Appl. Anim. Behav. Sci. 2021, 236, 105253. [Google Scholar] [CrossRef]
- Wascher, C.A.F.; Baur, N.; Hengl, M.; Köck, C.; Pegger, T.; Schindlbauer, J.; Wemer, L. Behavioral Responses of Captive Corvids to the Presence of Visitors. ABC 2021, 8, 481–492. [Google Scholar] [CrossRef]
- Rose, P.E.; Scales, J.S.; Brereton, J.E. Why the “Visitor Effect” Is Complicated. Unraveling Individual Animal, Visitor Number, and Climatic Influences on Behavior, Space Use and Interactions With Keepers—A Case Study on Captive Hornbills. Front. Vet. Sci. 2020, 7, 236. [Google Scholar] [CrossRef]
- Ozella, L.; Anfossi, L.; Di Nardo, F.; Pessani, D. Effect of Weather Conditions and Presence of Visitors on Adrenocortical Activity in Captive African Penguins (Spheniscus demersus). Gen. Comp. Endocrinol. 2015, 242, 49–58. [Google Scholar] [CrossRef]
- Levey, D.J.; Londono, G.A.; Ungvari-Martin, J.; Hiersoux, M.R.; Jankowski, J.E.; Poulsen, J.R.; Stracey, C.M.; Robinson, S.K. Urban Mockingbirds Quickly Learn to Identify Individual Humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8959–8962. [Google Scholar] [CrossRef]
- Homberger, D.G.; de Silva, K.N. The Role of Mechanical Forces on the Patterning of the Avian Feather-Bearing Skin: A Biomechanical Analysis of the Integumentary Musculature in Birds. J. Exp. Zool. 2003, 298, 123–139. [Google Scholar] [CrossRef]
- Leblanc, F.; Pothet, G.; Saint Jalme, M.; Dorval, M.; Bovet, D. Training Large Macaws for Artificial Insemination Procedures. J. Appl. Anim. Welf. Sci. 2011, 14, 187–210. [Google Scholar] [CrossRef]
- Lèche, A.; Della Costa, N.S.; Hansen, C.; Navarro, J.L.; Marin, R.H.; Martella, M.B. Corticosterone Stress Response of Greater Rhea (Rhea americana) during Short-Term Road Transport. Poult. Sci. 2013, 92, 60–63. [Google Scholar] [CrossRef]
- Barrows, M.; Killick, R.; Saunders, R.; Tahas, S.; Day, C.; Wyatt, K.; Horspool, T.; Lackey, B.; Cook, J. Retrospective Analysis of Elective Health Examinations as Preventative Medicine Interventions at a Zoological Collection. J. Zoo Aquar. Res. 2017, 5, 25–32. [Google Scholar]
- Baird, B.A.; Kuhar, C.W.; Lukas, K.E.; Amendolagine, L.A.; Fuller, G.A.; Nemet, J.; Willis, M.A.; Schook, M.W. Program Animal Welfare: Using Behavioral and Physiological Measures to Assess the Well-Being of Animals Used for Education Programs in Zoos. Appl. Anim. Behav. Sci. 2016, 176, 150–162. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Coleman, G.J. Managing Poultry: Human-Bird Interactions and Their Implications. In The Welfare of Domestic Fowl and Other Captive Birds; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; Volume 9, pp. 219–235. [Google Scholar]
- Mota-Rojas, D.; Broom, D.M.; Orihuela, A.; Velarde, A.; Napolitano, F.; Alonso-Spilsbury, M. Effects of Human-Animal Relationship on Animal Productivity and Welfare. JABB 2020, 8, 196–205. [Google Scholar] [CrossRef]
- Waiblinger, S.; Boivin, X.; Pedersen, V.; Tosi, M.-V.; Janczak, A.M.; Visser, E.K.; Jones, R.B. Assessing the Human–Animal Relationship in Farmed Species: A Critical Review. Appl. Anim. Behav. Sci. 2006, 101, 185–242. [Google Scholar] [CrossRef]
- Ramont, M.; Leahy, M.; Cronin, K. Domestic Animal Welfare at the Zoo: The Impact of an Animal Visitor Interaction Program on Chickens. Anim. Behav. Cogn. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Henley, E. A Bird’s Eye View of Breakdown in Parrot–Caregiver Relations. Companion Anim. 2018, 23, 104–108. [Google Scholar] [CrossRef]
- Hess, L.; Clubb, S.L.; Echols, M.S.; Forbes, N.; Hernandez, S.; Hooimeijer, J.; Joyner, L.; Klaphake, E.; Orosz, S.; Rich, G.; et al. Parrots: Appropriate Pets or Best Not Bred? J. Avian Med. Surg. 2016, 30, 286–297. [Google Scholar] [CrossRef]
- Theule, J.; Rimlinger, D. Artificial Incubation and Hand-Rearing of Superb Bird-of-Paradise (Lophorina superba). Zoo Biol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Gage, L.; Duerr, R. Hand-Rearing Birds; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Anderson, P.K. Human-Bird Interactions. In The Welfare of Domestic Fowl and Other Captive Birds; Spinger Science & Business Media: Berlin/Heidelberg, Germany, 2009; Volume 9, pp. 17–51. [Google Scholar]
- Wigham, E.; Grist, A.; Mullan, S.; Wotton, S.; Butterworth, A. The Influence of Welfare Training on Bird Welfare and Carcass Quality in Two Commercial Poultry Primary Processing Plants. Animals 2019, 9, 584. [Google Scholar] [CrossRef]
- Edwards, L.E.; Coleman, G.J.; Butler, K.L.; Hemsworth, P.H. The Human-Animal Relationship in Australian Caged Laying Hens. Animals 2019, 9, 211. [Google Scholar] [CrossRef]
- Brassó, D.L.; Béri, B.; Komlósi, I. Studies on Ostrich (Struthio camelus)—Review. Acta Agrar. Debr. 2020, 1, 15–22. [Google Scholar] [CrossRef]
- Hanselmann, R.; Hallager, S.; Murray, S.; Mazet, J. Causes of morbidity and mortality in captive KORI bustards (Ardeotis kori) in the united states. J. Zoo Wildl. Med. 2013, 44, 348–363. [Google Scholar] [CrossRef]
- Tuyttens, F.A.M.; Federici, J.F.; Vanderhasselt, R.F.; Goethals, K.; Duchateau, L.; Sans, E.C.O.; Molento, C.F.M. Assessment of Welfare of Brazilian and Belgian Broiler Flocks Using the Welfare Quality Protocol. Poult. Sci. 2015, 94, 1758–1766. [Google Scholar] [CrossRef]
- Girling, S.J. The Welfare of Captive Birds in the Future. In The Welfare of Domestic Fowl and Other Captive Birds; Duncan, I.J.H., Hawkins, P., Eds.; Animal Welfare; Springer: Dordrecht, The Netherlands, 2010; Volume 9, pp. 115–133. [Google Scholar] [CrossRef]
- Dawkins, M.S.; Layton, R. Breeding for Better Welfare: Genetic Goals for Broiler Chickens and Their Parents. Anim. Welf.-UFAW J. 2012, 21, 147–155. [Google Scholar] [CrossRef]
- Ellen, E.; van der Sluis, M.; Siegford, J.; Guzhva, O.; Toscano, M.; Bennewitz, J.; van der Zande, L.; van der Eijk, J.; de Haas, E.; Norton, T.; et al. Review of Sensor Technologies in Animal Breeding: Phenotyping Behaviors of Laying Hens to Select Against Feather Pecking. Animals 2019, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, T.; Bijma, P.; Ellen, E.; Bergsma, R.; de Vries, S.; Bolhuis, J.; Kemp, B.; van Arendonk, J. Breeding Amiable Animals? Improving Farm Animal Welfare by Including Social Effects in Breeding Programmes. Anim. Welf. 2010, 19, 77–82. [Google Scholar]
- Wang, S.; Ni, Y.; Guo, F.; Fu, W.; Grossmann, R.; Zhao, R. Effect of Corticosterone on Growth and Welfare of Broiler Chickens Showing Long or Short Tonic Immobility. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.; Mench, J.; Millman, S. Farm Animals and Their Welfare in 2000. In The State of the Animals 2001; Humane Society Press: Washington, DC, USA, 2001; pp. 87–99. [Google Scholar]
- Williams, S.E.; Hoffman, E.A. Minimizing Genetic Adaptation in Captive Breeding Programs: A Review. Biol. Conserv. 2009, 142, 2388–2400. [Google Scholar] [CrossRef]
- Frankham, R. Genetic Adaptation to Captivity in Species Conservation Programs. Mol. Ecol. 2008, 17, 325–333. [Google Scholar] [CrossRef]
- Mellor, E.L. Does Natural Foraging Niche Influence Captive Animal Health and Welfare? Ph.D. Thesis, University of Bristol, Bristol, UK, 2020. [Google Scholar]
- Mellor, E.L.; McDonald Kinkaid, H.K.; Mendl, M.T.; Cuthill, I.C.; van Zeeland, Y.R.A.; Mason, G.J. Nature Calls: Intelligence and Natural Foraging Style Predict Poor Welfare in Captive Parrots. Proc. R. Soc. B 2021, 288, 20211952. [Google Scholar] [CrossRef]
- Ali, A.B.A.; Campbell, D.L.M.; Siegford, J.M. A Risk Assessment of Health, Production, and Resource Occupancy for 4 Laying Hen Strains across the Lay Cycle in a Commercial-Style Aviary System. Poult. Sci. 2020, 99, 4672–4684. [Google Scholar] [CrossRef]
- Castellini, C.; Mugnai, C.; Moscati, L.; Mattioli, S.; Guarino Amato, M.; Cartoni Mancinelli, A.; Dal Bosco, A. Adaptation to Organic Rearing System of Eight Different Chicken Genotypes: Behaviour, Welfare and Performance. Ital. J. Anim. Sci. 2016, 15, 37–46. [Google Scholar] [CrossRef]
- Bosco, A.D.; Mugnai, C.; Amato, M.G.; Piottoli, L.; Cartoni, A.; Castellini, C. Effect of Slaughtering Age in Different Commercial Chicken Genotypes Reared According to the Organic System: 1. Welfare, Carcass and Meat Traits. Ital. J. Anim. Sci. 2014, 13, 3308. [Google Scholar] [CrossRef]
- Humphrey, S.; Chaloner, G.; Kemmett, K.; Davidson, N.; Williams, N.; Kipar, A.; Humphrey, T.; Wigley, P. Campylobacter Jejuni Is Not Merely a Commensal in Commercial Broiler Chickens and Affects Bird Welfare. mBio 2014, 5, e01364-14. [Google Scholar] [CrossRef]
- Dauncey, M.J. Nutrition, Environment and Gene Expression: Impact on Health, Welfare and Production. In Proceedings of the FACTA Avian Nutrigenomics Course, Campinas, Brazil, 27–28 May 2014; p. 11. [Google Scholar]
- Gonçalves, S.A.; Ferreira, R.A.; Pereira, I.G.; de Oliveira, C.C.; Amaral, P.I.S.; Garbossa, C.A.P.; da Silva Fonseca, L. Behavioral and Physiological Responses of Different Genetic Lines of Free-Range Broiler Raised on a Semi-Intensive System. JABB 2017, 5, 112–117. [Google Scholar] [CrossRef]
- Muir, W.M.; Cheng, H.-W.; Croney, C. Methods to Address Poultry Robustness and Welfare Issues through Breeding and Associated Ethical Considerations. Front. Genet. 2014, 5, 407. [Google Scholar] [CrossRef]
- Crissey, S. The Complexity of Formulating Diets for Zoo Animals: A Matrix. Int. Zoo Yearb. 2005, 39, 36–43. [Google Scholar] [CrossRef]
- Fidgett, A.L.; Gardner, L. Advancing Avian Nutrition through Best Feeding Practice: Advancing Avian Nutrition. Int. Zoo Yearb. 2014, 48, 116–127. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Hunt, K.A.; Rose, P.E. A Multi-Zoo Investigation of Nutrient Provision for Captive Red-Crested Turacos. Zoo Biol. 2017, 36, 152–160. [Google Scholar] [CrossRef]
- Wyss, F.; Wolf, P.; Wenker, C.; Hoby, S.; Schumacher, V.; Béchet, A.; Robert, N.; Liesegang, A. Comparison of Plasma Vitamin A and E, Copper and Zinc Levels in Free-Ranging and Captive Greater Flamingos (Phoenicopterus roseus) and Their Relation to Pododermatitis. J. Anim. Physiol. Anim. Nutr. 2014, 98, 1102–1109. [Google Scholar] [CrossRef]
- Cabana, F.; Lee, J.G. Feeding Cluster Preferences in Four Genera of Lories and Lorikeets (Loriinae) That Should Be Considered in the Diet of Nectarivorous Psittacine Species in Captivity. J. Anim. Physiol. Anim. Nutr. 2019, 103, 354–362. [Google Scholar] [CrossRef]
- Edwards, M.S. Nutrition of Zoo Animals. Recent Adv. Anim. Nutr. Aust. 2003, 14, 10. [Google Scholar]
- Koutsos, E.A.; Matson, K.D.; Klasing, K.C. Nutrition of Birds in the Order Psittaciformes: A Review. J. Avian Med. Surg. 2001, 15, 257–275. [Google Scholar] [CrossRef]
- Livingston, S.; Valdes, E.V. Growth Rates and Nutrient Intake of Hand-Reared Punk-Backed Pelicans (Pelecanus rufescens) at Disney’s Animal Kingdom. In Proceedings of the Seventh Conference on Zoo and Wildlife Nutrition, Knoxville, TN, USA, 20–26 October 2007; p. 10. [Google Scholar]
- MacLeod, M.G. Nutrition, Feedstuffs and Feeding. In Welfare of the Laying Hen; CABI: Wallingford, UK, 2004; Volume 27. [Google Scholar]
- Brereton, J.E. Challenges and Directions in Zoo and Aquarium Food Presentation Research: A Review. JZBG 2020, 1, 13–23. [Google Scholar] [CrossRef]
- Schlegel, M.L.; Howenstein, S. Case Report: Enhancing the Vitamin E Status and Coloration of a Southern Lanner Falcon (Falco biarmicus biarmicus). In Proceedings of the Tenth Conference on Zoo and Wildlife Nutrition, Salt Lake City, UT, USA, 29 September–2 October 2013; p. 3. [Google Scholar]
- James, C.W.; Nicholls, A.J.; Freeman, M.S.; Hunt, K.A.; Brereton, J.E. Should Zoo Foods Be Chopped: Macaws for Consideration. J. Zoo Aquar. Res. 2021, 9, 200–207. [Google Scholar]
- Toddes, B.; Luu, P.; Baltz, A. Training, Enrichment and Sound Animal Nutrition Can Coexist. The Development of a Multifaceted Bird Feeding Program at the Philadelphia Zoo. In Proceedings of the Fifth Conference on Zoo and Wildlife Nutrition, Minneapolis, MN, USA, 5–8 October 2003; p. 5. [Google Scholar]
- Schlegel, M.L.; Howenstein, S. Hornbill Diets at San Diego Zoo Global: A Review. In Proceedings of the Tenth Conference on Zoo and Wildlife Nutrition, Salt Lake City, UT, USA, 29 September–2 October 2013. [Google Scholar]
- Bozakova, N.; Popova-Ralcheva, S.; Sredkova, V.; Gerzilov, V.; Atanasova, S.; Atanasov, A.; Sotirov, L.; Georgieva, N. Mathematical Welfare Assessment Model of Chicken Breeder Flocks. Bulg. J. Agric. Sci. 2012, 18, 278–287. [Google Scholar]
- Nagaraj, M.; Wilson, C.A.P.; Hess, J.B.; Bilgili, S.F. Effect of High-Protein and All-Vegetable Diets on the Incidence and Severity of Pododermatitis in Broiler Chickens. J. Appl. Poult. Res. 2007, 16, 304–312. [Google Scholar] [CrossRef]
- Jacobs, L.; Persia, M.E.; Siman-Tov, N.; McCoy, J.; Ahmad, M.; Lyman, J.; Good, L. Impact of Water Sanitation on Broiler Chicken Production and Welfare Parameters. J. Appl. Poult. Res. 2020, 29, 258–268. [Google Scholar] [CrossRef]
- Abd El-Wahab, A.; Radko, D.; Kamphues, J. High Dietary Levels of Biotin and Zinc to Improve Health of Foot Pads in Broilers Exposed Experimentally to Litter with Critical Moisture Content. Poult. Sci. 2013, 92, 1774–1782. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Jacobs, J.A.; Murugesan, G.R.; Cheng, H.W. Effect of Dietary Synbiotic Supplement on Behavioral Patterns and Growth Performance of Broiler Chickens Reared under Heat Stress. Poult. Sci. 2018, 97, 1101–1108. [Google Scholar] [CrossRef]
- Freeman, H.D.; Valuska, A.J.; Taylor, R.R.; Ferrie, G.M.; Grand, A.P.; Leighty, K.A. Plumage Variation and Social Partner Choice in the Greater Flamingo (Phoenicopterus roseus): Plumage Variation in Greater Flamingos. Zoo Biol. 2016, 35, 409–414. [Google Scholar] [CrossRef]
- Tovar, G.; Cornejo, J.; Macek, M.; Dierenfeld, E.S. Intake and Digestion of Horned Guan Oreophasis derbianus Diets Measured in Three Mexican Zoos. Zoo Biol. 2009, 28, 319–330. [Google Scholar] [CrossRef]
- Ferguson, T.L.; Rude, B.J.; King, D.T. Nutrient Utilization by and Diet Preference of American White Pelicans (Pelecanus erythrorhynchos) When Offered Diets of Channel Catfish and(or) Grass Carp. In Proceedings of the Eighth Conference on Zoo and Wildlife Nutrition, Tulsa, OK, USA, 4–8 October 2009; p. 5. [Google Scholar]
- Squadrone, S.; Abete, M.C.; Brizio, P.; Pessani, D.; Favaro, L. Metals in Feathers of African Penguins (Spheniscus demersus): Considerations for the Welfare and Management of Seabirds Under Human Care. Bull. Environ. Contam. Toxicol. 2018, 100, 465–471. [Google Scholar] [CrossRef]
- Frederick, H.; Dierenfeld, E.; Irlbeck, N.; Ward, A.; Brooks, M.; Maslanka, M. Analysis of Nectar Replacement Products and a Case of Iron Toxicosis in Hummingbirds. In Proceedings of the Fifth Conference on Zoo and Wildlife Nutrition, Minneapolis, MN, USA, 5–8 October 2003; p. 6. [Google Scholar]
- Sheppard, C.; Dierenfeld, E. Iron Storage Disease in Birds: Speculation on Etiology and Implications for Captive Husbandry. J. Avian Med. Surg. 2002, 16, 192–197. [Google Scholar] [CrossRef]
- Olds, J.E.; Ensley, S.; Horst, R.; Burrough, E.; Madson, D.; Schwartz, K.; Stevenson, G.W.; Gauger, P.; Janke, B. Feed-Related Hypervitaminosis D in a Captive Flock of Budgerigars (Melopsittacus undulatas): Morbidity, Mortalities, and Pathologic Lesions. In Proceedings of the Tenth Conference on Zoo and Wildlife Nutrition, Salt Lake City, UT, USA, 29 September–2 October 2013. [Google Scholar]
- Sherwen, S.; Hemsworth, L.; Beausoleil, N.; Embury, A.; Mellor, D. An Animal Welfare Risk Assessment Process for Zoos. Animals 2018, 8, 130. [Google Scholar] [CrossRef]
- Bertin, A.; Beraud, A.; Lansade, L.; Mulot, B.; Arnould, C. Bill Covering and Nape Feather Ruffling as Indicators of Calm States in the Sulphur-Crested Cockatoo (Cacatua galerita). Behav. Processes 2020, 178, 104188. [Google Scholar] [CrossRef]
- Vieira Rios, H.; Waquil, P.D.; Soster de Carvalho, P.; Norton, T. How Are Information Technologies Addressing Broiler Welfare? A Systematic Review Based on the Welfare Quality® Assessment. Sustainability 2020, 12, 1413. [Google Scholar] [CrossRef]
- Hill, S.P.; Broom, D.M. Measuring Zoo Animal Welfare: Theory and Practice. Zoo Biol. 2009, 28, 531–544. [Google Scholar] [CrossRef]
- Rose, P. Evidence for Aviculture: Identifying Research Needs to Advance the Role of Ex Situ Bird Populations in Conservation Initiatives and Collection Planning. Birds 2021, 2, 77–95. [Google Scholar] [CrossRef]
- Hafez, H.M.; Attia, Y.A. Challenges to the Poultry Industry: Current Perspectives and Strategic Future After the COVID-19 Outbreak. Front. Vet. Sci. 2020, 7, 516. [Google Scholar] [CrossRef] [PubMed]
- Plaza, P.I.; Blanco, G.; Barbar, F.; Wiemeyer, G.; Alarcón, P.; Donázar, J.A.; Hiraldo, F.; Lambertucci, S.A. Protein Electrophoresis in Andean Condors (Vultur gryphus): Reference Values and Differences between Wild and Rehabilitating Individuals. Zoo Biol. 2019, 38, 508–515. [Google Scholar] [CrossRef]
- Krebs, B.; Marrin, D.; Phelps, A.; Krol, L.; Watters, J. Managing Aged Animals in Zoos to Promote Positive Welfare: A Review and Future Directions. Animals 2018, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Schwean-Lardner, K.; Crowe, T.G.; Fancher, B.I.; Classen, H.L. Effect of Light Intensity on Broiler Production, Processing Characteristics, and Welfare. Poult. Sci. 2010, 89, 2326–2333. [Google Scholar] [CrossRef]
- Rose, P.E.; Nash, S.M.; Riley, L.M. To Pace or Not to Pace? A Review of What Abnormal Repetitive Behavior Tells Us about Zoo Animal Management. J. Vet. Behav. 2017, 20, 11–21. [Google Scholar] [CrossRef]
- Siegford, J.M. Multidisciplinary Approaches and Assessment Techniques to Better Understand and Enhance Zoo Nonhuman Animal Welfare. J. Appl. Anim. Welf. Sci. 2013, 16, 300–318. [Google Scholar] [CrossRef]
- Mollenhorst, H.; Rodenburg, T.B.; Bokkers, E.A.M.; Koene, P.; de Boer, I.J.M. On-Farm Assessment of Laying Hen Welfare: A Comparison of One Environment-Based and Two Animal-Based Methods. Appl. Anim. Behav. Sci. 2005, 90, 277–291. [Google Scholar] [CrossRef]
- Bertin, A.; Beraud, A.; Lansade, L.; Blache, M.-C.; Diot, A.; Mulot, B.; Arnould, C. Facial Display and Blushing: Means of Visual Communication in Blue-and-Yellow Macaws (Ara ararauna)? PLoS ONE 2018, 13, 12. [Google Scholar] [CrossRef]
- Berckmans, D. Precision Livestock Farming Technologies for Welfare Management in Intensive Livestock Systems. Rev. Sci. Tech. OIE 2014, 33, 189–196. [Google Scholar] [CrossRef]
- Herborn, K.A.; McElligott, A.G.; Mitchell, M.A.; Sandilands, V.; Bradshaw, B.; Asher, L. Spectral Entropy of Early-Life Distress Calls as an Iceberg Indicator of Chicken Welfare. J. R. Soc. Interface 2020, 17, 20200086. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of Positive Emotions in Animals to Improve Their Welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Humphrey, T. Are Happy Chickens Safer Chickens? Poultry Welfare and Disease Susceptibility 1. Br. Poult. Sci. 2006, 47, 379–391. [Google Scholar] [CrossRef]
- Wurtz, K.; Camerlink, I.; D’Eath, R.B.; Fernández, A.P.; Norton, T.; Steibel, J.; Siegford, J. Recording Behaviour of Indoor-Housed Farm Animals Automatically Using Machine Vision Technology: A Systematic Review. PLoS ONE 2019, 14, e0226669. [Google Scholar] [CrossRef]
- Brilot, B.O.; Asher, L.; Feenders, G.; Bateson, M. Quantification of Abnormal Repetitive Behaviour in Captive European Starlings (Sturnus vulgaris). Behav. Processes 2009, 82, 256–264. [Google Scholar] [CrossRef][Green Version]
- Rose, P.E.; Lloyd, I.; Brereton, J.E.; Croft, D.P. Patterns of Nocturnal Activity in Captive Greater Flamingos. Zoo Biol. 2018, 37, 290–299. [Google Scholar] [CrossRef]
- Okinda, C.; Nyalala, I.; Korohou, T.; Okinda, C.; Wang, J.; Achieng, T.; Wamalwa, P.; Mang, T.; Shen, M. A Review on Computer Vision Systems in Monitoring of Poultry: A Welfare Perspective. Artif. Intell. Agric. 2020, 4, 184–208. [Google Scholar] [CrossRef]
- Church, J.S.; Cook, N.J.; Schaefer, A.L. Recent Applications of Infrared Thermography for Animal Welfare and Veterinary Research: Everything from Chicks to Elephants. Proc. Inframation 2009, 10, 215–224. [Google Scholar]
- Aydin, A.; Bahr, C.; Viazzi, S.; Exadaktylos, V.; Buyse, J.; Berckmans, D. A Novel Method to Automatically Measure the Feed Intake of Broiler Chickens by Sound Technology. Comput. Electron. Agric. 2014, 101, 17–23. [Google Scholar] [CrossRef]
- Dawkins, M.S.; Lee, H.; Waitt, C.D.; Roberts, S.J. Optical FLow Patterns in Broiler Chicken FLocks as Automated Measures of Behaviour and Gait. Appl. Anim. Behav. Sci. 2009, 119, 203–209. [Google Scholar] [CrossRef]
- Dawkins, M.S. Optical Flow, Flock Behaviour and Chicken Welfare. Anim. Behav. 2012, 84, 219–223. [Google Scholar] [CrossRef]
- Dawkins, M.S.; Cain, R.; Merelie, K.; Roberts, S.J. In Search of the Behavioural Correlates of Optical Flow Patterns in the Automated Assessment of Broiler Chicken Welfare. Appl. Anim. Behav. Sci. 2013, 145, 44–50. [Google Scholar] [CrossRef]
- Dawkins, M.S.; Roberts, S.J.; Cain, R.J.; Nickson, T.; Donnelly, C.A. Early Warning of Footpad Dermatitis and Hockburn in Broiler Chicken Flocks Using Optical Flow, Bodyweight and Water Consumption. Vet. Rec. 2017, 180, 499. [Google Scholar] [CrossRef]
- Gonzalez, J.J.; Nasirahmadi, A.; Knierim, U. Automatically Detected Pecking Activity in Group-Housed Turkeys. Animals 2020, 10, 2034. [Google Scholar] [CrossRef]
- Ferrie, G.M.; Sky, C.; Schutz, P.J.; Quinones, G.; Breeding, S.; Plasse, C.; Leighty, K.A.; Bettinger, T.L. Application of Video Recording Technology to Improve Husbandry and Reproduction in the Carmine Bee-Eater (Merops n. nubicus): Applying Video Recording to Improve Husbandry. Zoo Biol. 2016, 35, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ren, Z.; Li, D.; Zeng, L. Review: Automated Techniques for Monitoring the Behaviour and Welfare of Broilers and Laying Hens: Towards the Goal of Precision Livestock Farming. Animal 2020, 14, 617–625. [Google Scholar] [CrossRef]
- Fuller, G.; Heintz, M.R.; Allard, S. Validation and Welfare Assessment of Flipper-Mounted Time-Depth Recorders for Monitoring Penguins in Zoos and Aquariums. Appl. Anim. Behav. Sci. 2019, 212, 114–122. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Marchant, T.A.; Blas, J.; German, T. Corticosterone in Feathers Is a Long-Term, Integrated Measure of Avian Stress Physiology. Funct. Ecol. 2008, 22, 494–500. [Google Scholar] [CrossRef]
- Staley, M.; Conners, M.G.; Hall, K.; Miller, L.J. Linking Stress and Immunity: Immunoglobulin A as a Non-Invasive Physiological Biomarker in Animal Welfare Studies. Horm. Behav. 2018, 102, 55–68. [Google Scholar] [CrossRef]
- Cockrem, J.F. Stress, Corticosterone Responses and Avian Personalities. J. Ornithol. 2007, 148, 169–178. [Google Scholar] [CrossRef]
- Alm, M.; Tauson, R.; Holm, L.; Wichman, A.; Kalliokoski, O.; Wall, H. Welfare Indicators in Laying Hens in Relation to Nest Exclusion. Poult. Sci. 2016, 95, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Bienboire-Frosini, C.; Alnot-Perronin, M.; Chabaud, C.; Asproni, P.; Lafont-Lecuelle, C.; Cozzi, A.; Pageat, P. Assessment of Commercially Available Immunoassays to Measure Glucocorticoid Metabolites in African Grey Parrot (Psittacus erithacus) Droppings: A Ready Tool for Non-Invasive Monitoring of Stress. Animals 2018, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.P.; Fujihara, C.J.; Fruhvald, E.; Trevisol, E.; Destro, F.C.; Teixeira, C.R.; Pantoja, J.C.F.; Schmidt, E.M.S.; Palme, R. Non-Invasive Measurement of Adrenocortical Activity in Blue-Fronted Parrots (Amazona aestiva, Linnaeus, 1758). PLoS ONE 2015, 10, e0145909. [Google Scholar] [CrossRef] [PubMed]
- Nicol, C.; Caplen, G.; Edgar, J.; Richards, G.; Browne, W. Relationships between Multiple Welfare Indicators Measured in Individual Chickens across Different Time Periods and Environments. Anim. Welf. 2011, 20, 133. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woods, J.M.; Eyer, A.; Miller, L.J. Bird Welfare in Zoos and Aquariums: General Insights across Industries. J. Zool. Bot. Gard. 2022, 3, 198-222. https://doi.org/10.3390/jzbg3020017
Woods JM, Eyer A, Miller LJ. Bird Welfare in Zoos and Aquariums: General Insights across Industries. Journal of Zoological and Botanical Gardens. 2022; 3(2):198-222. https://doi.org/10.3390/jzbg3020017
Chicago/Turabian StyleWoods, Jocelyn M., Adrienne Eyer, and Lance J. Miller. 2022. "Bird Welfare in Zoos and Aquariums: General Insights across Industries" Journal of Zoological and Botanical Gardens 3, no. 2: 198-222. https://doi.org/10.3390/jzbg3020017
APA StyleWoods, J. M., Eyer, A., & Miller, L. J. (2022). Bird Welfare in Zoos and Aquariums: General Insights across Industries. Journal of Zoological and Botanical Gardens, 3(2), 198-222. https://doi.org/10.3390/jzbg3020017






