The Modification of Dairy Cow Rations with Feed Additives Mitigates Methane Production and Reduces Nitrate Content During In Vitro Ruminal Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed Resources
2.2. Proximate Analysis
2.3. Inoculum Sources
2.4. In Vitro Gas Production
2.5. Methane and Nitrates Determinations
2.6. Statistical Analysis
3. Results
3.1. Feedstuff Composition Assessment
3.2. Methane Production and Nitrate Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, C.; Beauchemin, K.A. A review of feeding supplementary nitrate to ruminant animals: Nitrate toxicity, methane emissions, and production performance. Can. J. Anim. Sci. 2014, 94, 557–570. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Misselbrook, T.H.; Del Prado, A.; Chadwick, D.R. Opportunities for reducing environmental emissions from forage-based dairy farms. Agric. Food Sci. 2013, 22, 93–107. [Google Scholar] [CrossRef]
- Dijkstra, J.; Oenema, O.; Bannink, A. Dietary strategies to reducing N excretion from cattle: Implications for methane emissions. Curr. Opin. Environ. Sustain. 2011, 3, 414–422. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change Through Livestock–A Global Assessment of Emissions and Mitigation Opportunities; FAO: Rome, Italy, 2013; Available online: http://www.fao.org/docrep/018/i3437e/i3437e00.htm (accessed on 1 May 2025).
- Capelari, M.; Powers, W. The effect of nitrate and monensin on in vitro ruminal fermentation. Anim. Sci. J. 2017, 95, 5112–5123. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Global Dairy and GHG Emissions: FAO Analysis 2005–2015; FAO: Rome, Italy, 2019; Available online: https://www.fao.org/3/CA3165EN/ca3165en.pdf (accessed on 1 May 2025).
- Patra, A.K. Enteric methane mitigation technologies for ruminant livestock: A synthesis of current research and future directions. Environ. Monit. Assess. 2012, 184, 1929–1952. [Google Scholar] [CrossRef]
- Martin, G.B. Perspective: Science and the future of livestock industries. Front. Vet. Sci. 2024, 11, 1359247. [Google Scholar] [CrossRef]
- Fang, Q.; Ma, Y.; Zhang, X.; Wei, S.; Hou, Y. Mitigating nitrogen emissions from dairy farming systems in China. Front. Sustain. Food Syst. 2020, 4, 44. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; De Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Vilanueva, C.M.; Van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- Cellone, F.; Carol, E.; Pugliese, I.; Córdoba, J.; Butler, L.; Lamarche, L. Nitrate pollution in dairy farms and its impact on groundwater quality in a sector of the Pampas plain, Argentina. Environ. Earth Sci. 2020, 79, 1–7. [Google Scholar] [CrossRef]
- Mahmud, K.; Panday, D.; Mergoum, A.; Missaoui, A. Nitrogen losses and potential mitigation strategies for a sustainable agroecosystem. Sustainability 2021, 13, 2400. [Google Scholar] [CrossRef]
- Stafford, J. Site-specific nutrient management systems. In Precision Agriculture for Sustainability; Long, D.S., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 319–342. [Google Scholar]
- EEIG Alliance Environnement. Evaluation Study of the Impact of the CAP on Climate Change and Greenhouse Gas Emissions; European Commission, Directorate-General for Agriculture and Rural Development: Brussels, Belgium, 2019. [CrossRef]
- European Commission: Directorate-General for Energy. EU Methane Strategy; Publications Office of the European Union: Luxembourg, 2020. [CrossRef]
- Council Directive 91/676/EEC. The protection of waters against pollution caused by nitrates from agricultural sources. Off. J. Eur. Union 1991, L 375, 1–8. [Google Scholar]
- Elbasiouny, H.; Elbanna, B.A.; Al-Najoli, E.; Alsherief, A.; Negm, S.; Abou El-Nour, E.; Nofal, A.; Sharabash, S. Agricultural waste management for climate change mitigation: Some implications to Egypt. In Waste Management in MENA Regions; Springer: Cham, Switzerland, 2019; pp. 183–201. [Google Scholar] [CrossRef]
- National Statistics Office (NSO). Census of Agriculture; NSO: Valletta, Malta, 2022.
- Malta Resources Authority. Malta’s National Inventory Document of Greenhouse Gas Emissions & Removals; UNFCC: Rio de Janeiro, Brazil; New York, NY, USA, 2024; Available online: https://unfccc.int/sites/default/files/resource/Malta_GHGInv_1990-2022_NID_Sep2024.pdf (accessed on 1 May 2025).
- Grizzetti, B.; Bouraoui, F.; Billen, G.; van Grinsven, H.; Cardoso, A.C.; Thieu, V.; Garnier, J.; Curtis, C.; Howarth, R.W.; Johnes, P. Nitrogen as a threat to European water quality. In European Nitrogen Assessment; Sutton, M.A., Howard, C.M., Erisman, J.W., Billen, G., Bleeker, A., Grennfelt, P., van Grinsven, H., Grizzetti, B., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 379–404. [Google Scholar]
- Feyissa, F.; Kitaw, G.; Assefa, G. Nutritional qualities of agro-industrial by-products and local supplementary feeds for dairy cattle feeding. Ethiop. J. Agric. Sci. 2015, 26, 13–26. [Google Scholar]
- Tuyen, D.V.; Phuong, H.N.; Cone, J.W.; Baars, J.J.P.; Sonnenberg, A.S.M.; Hendriks, W.H. Effect of fungal treatments of fibrous agricultural by-products on chemical composition and in vitro rumen fermentation and methane production. Bioresour. Technol. 2013, 129, 256–263. [Google Scholar] [CrossRef]
- Jayanegara, A.; Yantina, N.; Novandri, B.; Laconi, E.B.; Nahrowi, N.; Ridla, M. Evaluation of some insects as potential feed ingredients for ruminants: Chemical composition, in vitro rumen fermentation, and methane emissions. J. Indones. Trop. Anim. Agric. 2017, 42, 247–254. [Google Scholar] [CrossRef]
- Pastorelli, G.; Simeonidis, K.; Faustini, M.; Le Mura, A.; Cavalleri, M.; Serra, V.; Attard, E. Chemical characterization and in vitro gas production kinetics of alternative feed resources for small ruminants in the Maltese islands. Metabolites 2023, 13, 762. [Google Scholar] [CrossRef]
- Agius, A.; Pastorelli, G.; Attard, E. Cows fed hydroponic fodder and conventional diet: Effects on milk quality. Arch. Anim. Breed. 2019, 62, 517–525. [Google Scholar] [CrossRef]
- Alba, H.D.R.; Lima, V.G.O.; Carvalho, S.T.; Ítavo, L.C.V.; Pinto, L.F.B.; Carneiro, P.L.S.; Oliveira, R.L. Rumen content from slaughterhouse as an alternative inoculum source for in vitro analysis of feeds: A multivariate approach. Ruminants 2023, 3, 360–372. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Silva, M.F.; Bermejo de Lima, L.; de Camargo, C.; Telles Benatti, C. Usability of simplified UV–Vis spectrophotometric methods for the determination of nitrate in the presence of organic matter and chloride as interfering factors. Water Pract. Technol. 2024, 19, 1061–1070. [Google Scholar] [CrossRef]
- Hashimoto, A.G. Effect of inoculum/substrate ratio on methane yield and production rate from straw. Biol. Wastes 1989, 28, 247–255. [Google Scholar] [CrossRef]
- Hancock, D.W. Nitrate Toxicity; University of Georgia Cooperative Extension: Athens, GA, USA, 2013; Available online: https://extension.uga.edu/publications/detail.html?number=C915&title=Nitrate%20Toxicity (accessed on 2 March 2025).
- Chuang, S.T.; Li, K.Y.; Tu, P.W.; Ho, S.T.; Hsu, C.C.; Hsieh, J.C.; Chen, M.J. Investigating the Reciprocal Interrelationships among the Ruminal Microbiota, Metabolome, and Mastitis in Early Lactating Holstein Dairy Cows. Animals 2021, 11, 3108. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Terada, F. Factors affecting methane production and mitigation in ruminants. Anim. Sci. J. 2010, 81, 2–10. [Google Scholar] [CrossRef]
- Kim, E.T.; Lee, S.J.; Lee, S.M.; Lee, I.D.; Lee, S.K.; Lee, S.S. Effects of flavonoid-rich plant extracts on in vitro ruminal methanogenesis, microbial populations, and fermentation characteristics. Asian-Australas. J. Anim. Sci. 2015, 28, 530. [Google Scholar] [CrossRef]
- Duarte, A.C.; Holman, D.B.; Alexander, T.W.; Durmic, Z.; Vercoe, P.E.; Chaves, A.V. The type of forage substrate preparation included as substrate in a RUSITEC system affects the ruminal microbiota and fermentation characteristics. Front. Microbiol. 2017, 8, 704. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.J.; Kim, H.S.; Eom, J.S.; Jo, S.U.; Guan, L.L.; Seo, J.; Kim, H.; Lee, S.S. Effects of seaweed extracts on in vitro rumen fermentation characteristics, methane production, and microbial abundance. Sci. Rep. 2021, 11, 24092. [Google Scholar] [CrossRef]
- Moate, P.J.; Williams, S.R.O.; Deighton, M.H.; Hannah, M.C.; Ribaux, B.E.; Morris, G.L.; Jacobs, J.L.; Hill, J.; Wales, W.J. Effects of feeding wheat or corn and of rumen fistulation on milk production and methane emissions of dairy cows. Anim. Prod. Sci. 2018, 59, 891–905. [Google Scholar] [CrossRef]
- Gebreeyessus, G.D. Comparative evaluation of the biochemical methane potential of waste activated sludge acetic acid and cellulose substrates under mesophilic and thermophilic anaerobic digestion. SN Appl. Sci. 2020, 2, 1546. [Google Scholar] [CrossRef]
- Zhang, Y.; Kusch-Brandt, S.; Salter, A.M.; Heaven, S. Estimating the methane potential of energy crops: An overview on types of data sources and their limitations. Processes 2021, 9, 1565. [Google Scholar] [CrossRef]
- Varsha, S.S.V.; Soomro, A.F.; Baig, Z.T.; Vuppaladadiyam, A.K.; Murugavelh, S.; Antunes, E. Methane production from anaerobic mono- and co-digestion of kitchen waste and sewage sludge: Synergy study on cumulative methane production and biodegradability. Biomass Convers. Biorefin. 2020, 12, 3911–3919. [Google Scholar] [CrossRef]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Hornick, J.L.; Chentouf, M.; Cabaraux, J.F. Effects of sulla flexuosa hay as alternative feed resource on goat’s milk production and quality. Animals 2023, 13, 709. [Google Scholar] [CrossRef] [PubMed]
- Yasser, H.; Amal, J.; El Mejdoub, H.; Wafa, Y.; Fortina, R. Seagrass debris as a source of fiber and bioactive compounds in feed for dairy goats. Int. J. Agric. Biol. 2021, 26, 722–730. [Google Scholar] [CrossRef]
- Singh, S. Non-Conventional Fodder Resources for Feeding Livestock. Recent Approaches in Crop Residue Management and Value Addition for Entrepreneurship Development; ICAR-Indian Grassland and Fodder Research Institute: Jhansi, Indian, 2016; pp. 7–12. [Google Scholar]
- Kaal, J.; Serrano, O.; del Río, J.C.; Rencoret, J. Radically different lignin composition in Posidonia species may link to differences in organic carbon sequestration capacity. Org. Geochem. 2018, 124, 247–256. [Google Scholar] [CrossRef]
- Kamra, D.N.; Agarwal, N.; Chaudhary, L.C. Nitrate/nitrite toxicity and possibilities of their use in ruminant diet. In Rumen Microbiology; Springer: New Delhi, India, 2015; pp. 343–353. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, Z.; Meng, Q. Effects of nitrate on methane production, fermentation, and microbial populations in in vitro ruminal cultures. Bioresour. Technol. 2012, 103, 173–179. [Google Scholar] [CrossRef]
- Yang, C.; Rooke, J.A.; Cabeza, I.; Wallace, R.J. Nitrate and inhibition of ruminal methanogenesis: Microbial ecology, obstacles, and opportunities for lowering methane emissions from ruminant livestock. Front. Microbiol. 2016, 7, 132. [Google Scholar] [CrossRef]
- Fernandez-Bayo, J.D.; Simmons, C.W.; VanderGheynst, J.S. Characterization of digestate microbial community structure following thermophilic anaerobic digestion with varying levels of green and food wastes. J. Ind. Microbiol. Biotechnol. 2020, 47, 1031–1044. [Google Scholar] [CrossRef]
- Su, R.; Zhou, L.; Ding, L.; Fu, B.; Fu, H.; Shuang, Y.; Ye, L.; Hu, H.; Ma, H.; Ren, H. How anaerobic sludge microbiome respond to different concentrations of nitrite, nitrate, and ammonium ions: A comparative analysis. Environ. Sci. Pollut. Res. 2023, 30, 49026–49037. [Google Scholar] [CrossRef]
- Bartocci, P.; Massoli, S.; Zampilli, M.; Liberti, F.; Yunjun, Y.; Yang, Q.; Yang, H.; Zhou, H.; Gul, E.; Bidini, G.; et al. Substrate characterization in the anaerobic digestion process. In Bioenergy Research; Springer: Singapore, 2021; pp. 307–342. [Google Scholar] [CrossRef]
- Van Zijderveld, S.M.; Gerrits, W.J.J.; Apajalahti, J.A.; Newbold, J.R.; Dijkstra, J.; Leng, R.A.; Perdok, H.B. Nitrate and sulfate: Effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. J. Dairy Sci. 2010, 93, 5856–5866. [Google Scholar] [CrossRef]
- Jayanegara, A.; Ridla, M.; Nahrowi Laconi, E.B. Estimation and validation of total digestible nutrient values of forage and concentrate feedstuffs. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042016. [Google Scholar] [CrossRef]
- Brask, M.; Weisbjerg, M.R.; Hellwing, A.L.F.; Bannink, A.; Lund, P. Methane production and diurnal variation measured in dairy cows and predicted from fermentation pattern and nutrient or carbon flow. Animal 2015, 9, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Weersink, A.; Wagner-Riddle, C.; Fox, G. Optimizing ration formulation as a strategy for greenhouse gas mitigation in intensive dairy production systems. Agric. Syst. 2015, 137, 1–11. [Google Scholar] [CrossRef]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Louvieaux, J.; Hornick, J.L.; Cabaraux, J.F.; Chentouf, M. Growth performance, carcass characteristics, fatty acid profile, and meat quality of male goat kids supplemented by alternative feed resources: Bitter vetch and sorghum grains. Arch. Anim. Breed. 2024, 67, 481–492. [Google Scholar] [CrossRef]
- Weiss, W.P. Estimating the available energy content of feeds for dairy cattle. J. Dairy Sci. 1998, 81, 830–839. [Google Scholar] [CrossRef]
- Pastorelli, G.; Serra, V.; Vannuccini, C.; Attard, E. Opuntia spp. as alternative fodder for sustainable livestock production. Animals 2022, 12, 1597. [Google Scholar] [CrossRef]
| Basal Rations | Opuntia ficus indica | Posidonia oceanica | Brassica rapa | |||||
|---|---|---|---|---|---|---|---|---|
| Ration 1 | Ration 2 | Ration 1 | Ration 2 | Ration 1 | Ration 2 | Ration 1 | Ration 2 | |
| Special Dairy 1 | 18.8 | 14.6 | 18.5 | 14.6 | ||||
| Dairy B 2 | 22.9 | 18.8 | 22.6 | 18.8 | ||||
| Summer Dairy 3 | 45.8 | 45.3 | 45.4 | 41.2 | ||||
| Ground maize | 8.3 | 6.3 | 8.3 | 6.2 | 8.0 | 5.9 | 4.2 | 1.3 |
| Sugar beet | 8.3 | 8.3 | 8.0 | 4.2 | ||||
| Alfalfa Hay | 18.8 | 8.3 | 14.6 | 8.2 | 18.5 | 8.0 | 14.6 | 3.4 |
| Alfalfa | 14.6 | 8.3 | 10.4 | 8.2 | 14.3 | 8.0 | 10.4 | 3.4 |
| Corn Silage | 22.9 | 6.2 | 22.6 | 18.1 | ||||
| Straw | 8.3 | 8.3 | 8.3 | 8.2 | 8.0 | 8.0 | 4.2 | 3.4 |
| OFI 4 | 16.7 | 17.7 | ||||||
| PO 5 | 2.1 | 2.1 | ||||||
| BR 6 | 29.2 | 29.4 | ||||||
| DM % | Ash % | CP % | EE % | NDF % | ADF % | NFC % | Energy (kcal/100 g) | |
|---|---|---|---|---|---|---|---|---|
| Special Dairy | 88.17 ± 1.01 | 10.48 ± 0.08 | 19.54 ± 0.33 | 2.42 ± 0.03 | 38.89 ± 0.67 | 16.23 ± 0.14 | 28.67 ± 1.05 | 255.50 |
| Dairy B | 84.79 ± 0.73 | 9.87 ± 0.08 | 15.86 ± 0.18 | 1.62 ± 0.01 | 45.71 ± 0.39 | 18.54 ± 0.16 | 26.94 ± 0.81 | 260.86 |
| Summer Dairy | 83.91 ± 1.40 | 9.47 ± 0.15 | 16.45 ± 0.27 | 1.65 ± 0.01 | 44.6 ± 0.381 | 14.94 ± 0.25 | 27.83 ± 0.90 | 259.05 |
| Ground Maize | 85.92 ± 0.74 | 5.45 ± 0.04 | 11.78 ± 0.19 | 2.16 ± 0.01 | 45.84 ± 0.39 | 5.473 ± 0.04 | 34.77 ± 0.79 | 249.92 |
| Sugar Beet | 87.07 ± 1.00 | 8.22 ± 0.06 | 17.55 ± 0.14 | 0.49 ± 0.01 | 43.18 ± 0.36 | 34.19 ± 0.29 | 30.56 ± 0.75 | 247.33 |
| Hay1 | 87.66 ± 0.75 | 15.34 ± 0.13 | 25.09 ± 0.21 | 0.86 ± 0.00 | 34.88 ± 0.29 | 27.45 ± 0.23 | 23.83 ± 0.79 | 247.62 |
| Hay2 | 87.46 ± 1.00 | 12.68 ± 0.14 | 21.68 ± 0.24 | 0.91 ± 0.09 | 40.15 ± 0.34 | 30.66 ± 0.26 | 24.58 ± 0.9 | 255.51 |
| Alfalfa | 87.55 ± 0.75 | 12.54 ± 0.10 | 22.43 ± 0.19 | 0.83 ± 0.09 | 38.72 ± 0.33 | 30.86 ± 0.26 | 25.48 ± 0.84 | 252.07 |
| Straw | 88.12 ± 1.52 | 10.22 ± 0.08 | 12.74 ± 0.10 | 0.19 ± 0.09 | 65.47 ± 0.75 | 43.07 ± 0.37 | 11.38 ± 1.00 | 314.55 |
| Silage | 37.83 ± 0.64 | 9.89 ± 0.16 | 8.22 ± 0.06 | 2.17 ± 0.01 | 60.5 ± 0.51 | 39.04 ± 0.34 | 19.22 ± 0.86 | 294.41 |
| OFI | 86.14 ± 0.74 | 18.88 ± 0.15 | 12.23 ± 0.20 | 0.71 ± 0.06 | 30.16 ± 0.25 | 20.58 ± 0.35 | 38.02 ± 0.81 | 175.95 |
| PO | 70.55 ± 0.60 | 12.82 ± 0.10 | 19.88 ± 0.16 | 0.55 ± 0.01 | 49.27 ± 0.84 | 40.23 ± 0.34 | 17.48 ± 1.05 | 281.55 |
| BR | 90.32 ± 1.55 | 13.65 ± 0.11 | 28.28 ± 0.24 | 1.6 ± 0.01 | 24.63 ± 0.20 | 21.81 ± 0.25 | 31.84 ± 0.75 | 226.04 |
| Ration 1 | Ration 2 | OFI-Supplemented | BR-Supplemented | PO-Supplemented | ||||
|---|---|---|---|---|---|---|---|---|
| Ration 1 | Ration 2 | Ration 1 | Ration 2 | Ration 1 | Ration 2 | |||
| DM (%) | 86.24 ± 0.29 | 86.16 ± 0.04 | 86.16 ± 0.14 | 83.74 ± 0.06 | 86.17 ± 0.06 | 85 ± 0.12 | 85.35 ± 0.16 | 84.09 ± 0.21 |
| Ash (%) | 11.71 ± 0.06 | 11.02 ± 0.25 | 12.12 ± 0.16 | 12.21 ± 0.44 | 11.78 ± 0.37 | 12.2 ± 0.21 | 12.29 ± 0.16 | 12.47 ± 0.36 |
| CP (%) | 19.89 ± 0.07 | 17.03 ± 0.09 | 14.83 ± 0.15 | 13.41 ± 0.23 | 17.4 ± 0.29 | 16.89 ± 0.18 | 17.92 ± 0.12 | 15.37 ± 0.22 |
| Fat (%) | 1.19 ± 0.06 | 1.587 ± 0.01 | 1.533 ± 0.03 | 1.497 ± 0.04 | 1.78 ± 0.06 | 1.787 ± 0.04 | 1.383 ± 0.14 | 1.603 ± 0.13 |
| NDF (%) | 43.33 ± 0.03 | 46.65 ± 0.27 | 43.84 ± 0.72 | 47.69 ± 0.82 | 41.57 ± 1.01 | 42.34 ± 0.65 | 44.58 ± 0.86 | 49.41 ± 1.08 |
| ADF (%) | 25.93 ± 0.35 | 25.71 ± 0.94 | 24.16 ± 0.75 | 22.17 ± 0.89 | 21.22 ± 1.26 | 18.61 ± 0.91 | 26.55 ± 1.79 | 29.59 ± 2.38 |
| NFC (%) | 23.89 ± 0.06 | 23.72 ± 0.57 | 27.68 ± 0.58 | 25.2 ± 0.67 | 27.47 ± 0.68 | 26.77 ± 0.67 | 23.83 ± 0.98 | 21.15 ± 1.35 |
| Energy (kcal/100 g) | 263.59 | 269.03 | 248.45 | 257.9 | 251.9 | 253.03 | 262.42 | 273.52 |
| Methane Production (L CH4. kg−1) | Nitrate Content (mg L−1) | |||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | Bo | k | ||
| Special Dairy | 104.23 ± 24.28 | 181.86 ± 31.25 | 37.04 ± 51.93 | 31.51 | 0.170 | 344.50 ± 56.07 |
| Dairy B | 180.37 ± 29.96 | 37.04 ± 56.84 | 117.65 ± 22.65 | 32.47 | 0.996 | 236.10 ± 54.13 |
| Dairy Summer | 177.05 ± 25.58 | 60.66 ± 18.89 | 102.86 ± 32.07 | 33.00 | 0.997 | 80.07 ± 5.87 |
| Ground Maize | 142.07 ± 8.11 | 97.93 ± 64.06 | 76.48 ± 40.42 | 30.67 | 0.988 | 326.80 ± 80.43 |
| Sugar Beet | 68.23 ± 11.63 | 110.08 ± 28.65 | 41.97 ± 20.52 | 21.69 | 0.128 | 66.5 ± 21.50 |
| Hay1 | 149.29 ± 48.16 | 172.11 ± 77.59 | 83.13 ± 37.67 | 39.20 | 0.995 | 405.50 ± 5.67 |
| Hay2 | 81.64 ± 29.75 | 118.79 ± 15.95 | 130.15 ± 55.24 | 41.03 | 0.034 | 306.70 ± 43.73 |
| Alfalfa | 76.83 ± 19.17 | 114.55 ± 19.80 | 80.84 ± 50.17 | 28.09 | 0.078 | 54.60 ± 3.15 |
| Straw | 7.57 ± 11.83 | 19.03 ± 5.18 | 35.55 ± 47.84 | 100.00 | 0.001 | 203.50 ± 88.57 |
| Silage | 43.00 ± 13.07 | 124.18 ± 10.58 | 217.41 ± 54.96 | 100.00 | 0.011 | 227.20 ± 14.30 |
| OFI | 106.41 ± 13.15 | 116.73 ± 14.41 | 141.38 ± 36.42 | 39.57 | 0.058 | 353.10 ± 22.27 |
| PO | 92.19 ± 6.54 | 100.45 ± 28.10 | 80.84 ± 41.56 | 26.50 | 1.000 | 198.60 ± 6.29 |
| BR | 10.55 ± 2.30 | 78.09 ± 40.20 | 128.31 ± 50.58 | 100.00 | 0.005 | 264.50 ± 70.76 |
| Supplementation | Time | Methane Production (L CH4. kg−1) | Bo | k | Nitrate Content (mg L−1) | ||
|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||||
| None | Ration 1 | 150.10 ± 19.17 | 146.89 ± 33.44 | 184.61 ± 6.78 | 49.451 | 0.080 | 211.40 ± 39.80 |
| Ration 2 | 53.43 ± 15.69 | 153.08 ± 33.23 | 157.55 ± 28.16 | 80.747 | 0.013 | 97.09 ± 71.03 | |
| OFI-supplemented | Ration 1 | 146.54 ± 22.70 | 97.52 ± 47.64 | 161.68 ± 52.29 | 39.333 | 0.990 | 120.90 ± 14.72 |
| Ration 2 | 181.98 ± 45.75 | 158.24 ± 44.72 | 112.83 ± 2.75 | 43.900 | 1.000 | 49.40 ± 6.77 | |
| BR-supplemented | Ration 1 | 148.95 ± 14.10 | 202.10 ± 0.86 | 168.90 ± 30.27 | 54.048 | 0.074 | 21.55 ± 0.80 |
| Ration 2 | 127.45 ± 51.43 | 142.76 ± 49.19 | 146.72 ± 45.58 | 42.536 | 0.085 | 23.25 ± 5.14 | |
| PO-supplemented | Ration 1 | 107.16 ± 37.67 | 135.19 ± 62.95 | 116.44 ± 54.87 | 36.602 | 0.086 | 44.40 ± 10.46 |
| Ration 2 | 90.47 ± 1.72 | 80.49 ± 17.89 | 54.35 ± 5.50 | 21.833 | 0.977 | 74.60 ± 9.229 | |
| Substrates (Feedstuffs) | Supplemented Rations | |
|---|---|---|
| High B0 and Low k | PO and Straw have a very high methane production potential but degrade very slowly, making them suitable for systems with longer retention times | Ration 2: has a high methane production potential but degrades very slowly, making it suitable for systems with longer retention times to achieve full methane yield. |
| High B0 and High k | Hay 1, despite a high potential, degrades very quickly, providing rapid methane production | BR-supplemented ration 1 offers high methane production potential with a moderate degradation rate, suitable for systems balancing both yield and degradation rate. |
| Moderate B0 and High k | Substrates like Dairy B Mash, Maize Ground, and Ground Sugar Beet have moderate methane potential but degrade rapidly, suitable for fast biogas generation. | OFI-supplemented rations and PO-supplemented ration 2. These rations provide moderate methane production but degrade very quickly, making them suitable for systems requiring rapid methane production. |
| Moderate B0 and Moderate/Low k | Substrates like Dairy Summer Mash and PO provide moderate methane production at a slower rate, balancing between yield and degradation speed. | Ration 1, BR-supplemented ration 2, PO-supplemented ration 1: These rations offer a balanced approach with moderate methane production and a steady degradation rate. |
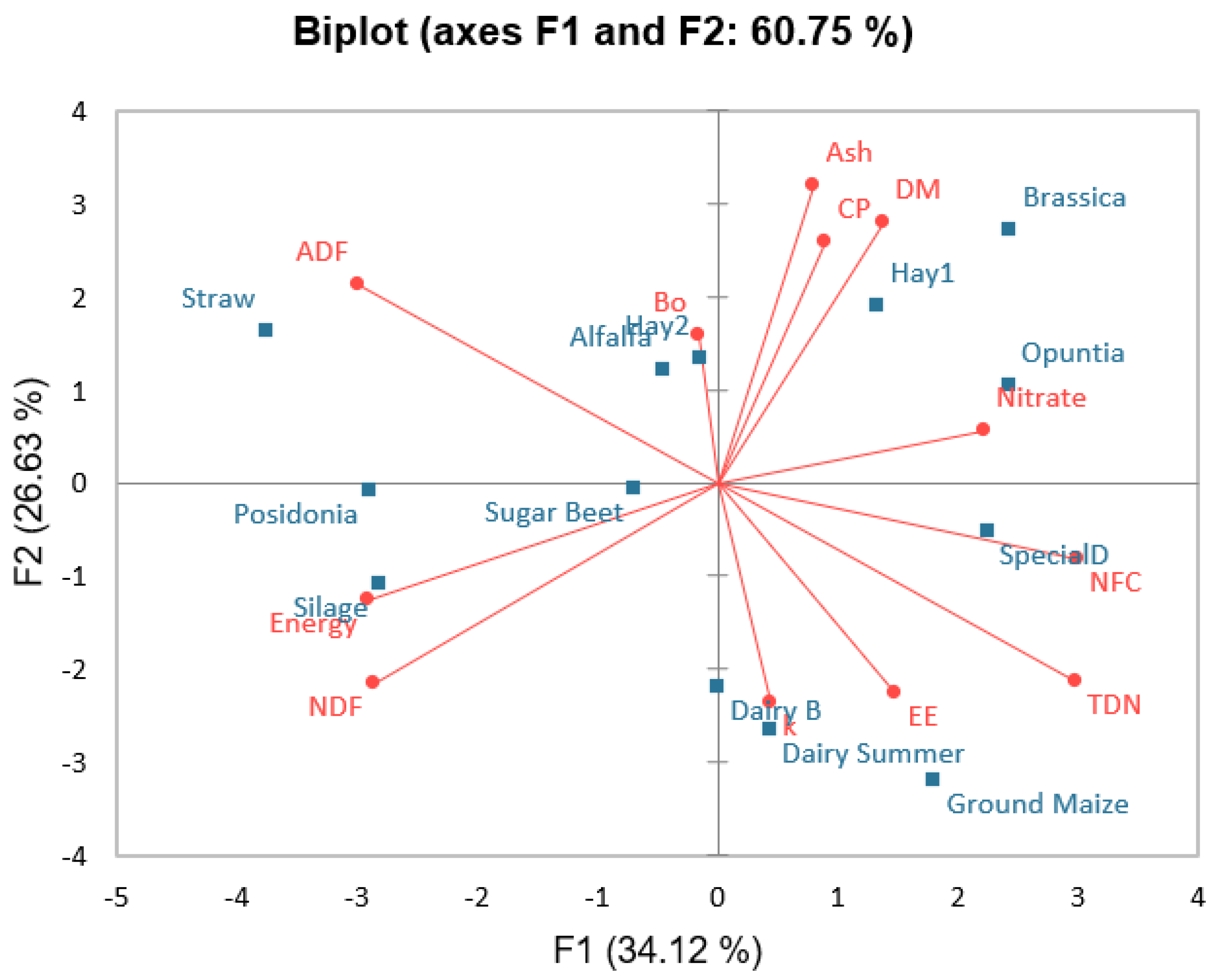
| Variables | Ash | CP | EE | NDF | ADF | NFC | Bo | k | Nitrate | TDN | Energy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | 0.368 | 0.577 | −0.154 | −0.560 | −0.016 | 0.143 | 0.221 | −0.465 | 0.286 | 0.016 | −0.401 |
| Ash | 0.527 | −0.297 | −0.610 | 0.214 | −0.099 | 0.331 | −0.195 | 0.440 | −0.214 | −0.324 | |
| CP | −0.198 | −0.621 | 0.099 | −0.093 | −0.110 | 0.063 | −0.016 | −0.099 | −0.330 | ||
| EE | −0.005 | −0.654 | 0.258 | 0.138 | 0.157 | 0.330 | 0.654 | 0.082 | |||
| NDF | 0.352 | −0.555 | −0.039 | 0.107 | −0.390 | −0.352 | 0.841 | ||||
| ADF | −0.720 | 0.122 | −0.385 | −0.445 | −1.000 | 0.401 | |||||
| NFC | −0.193 | 0.025 | 0.291 | 0.720 | −0.791 | ||||||
| Bo | −0.683 | 0.354 | −0.122 | 0.160 | |||||||
| k | −0.039 | 0.385 | 0.069 | ||||||||
| Nitrate | 0.445 | −0.374 | |||||||||
| TDN | −0.401 |
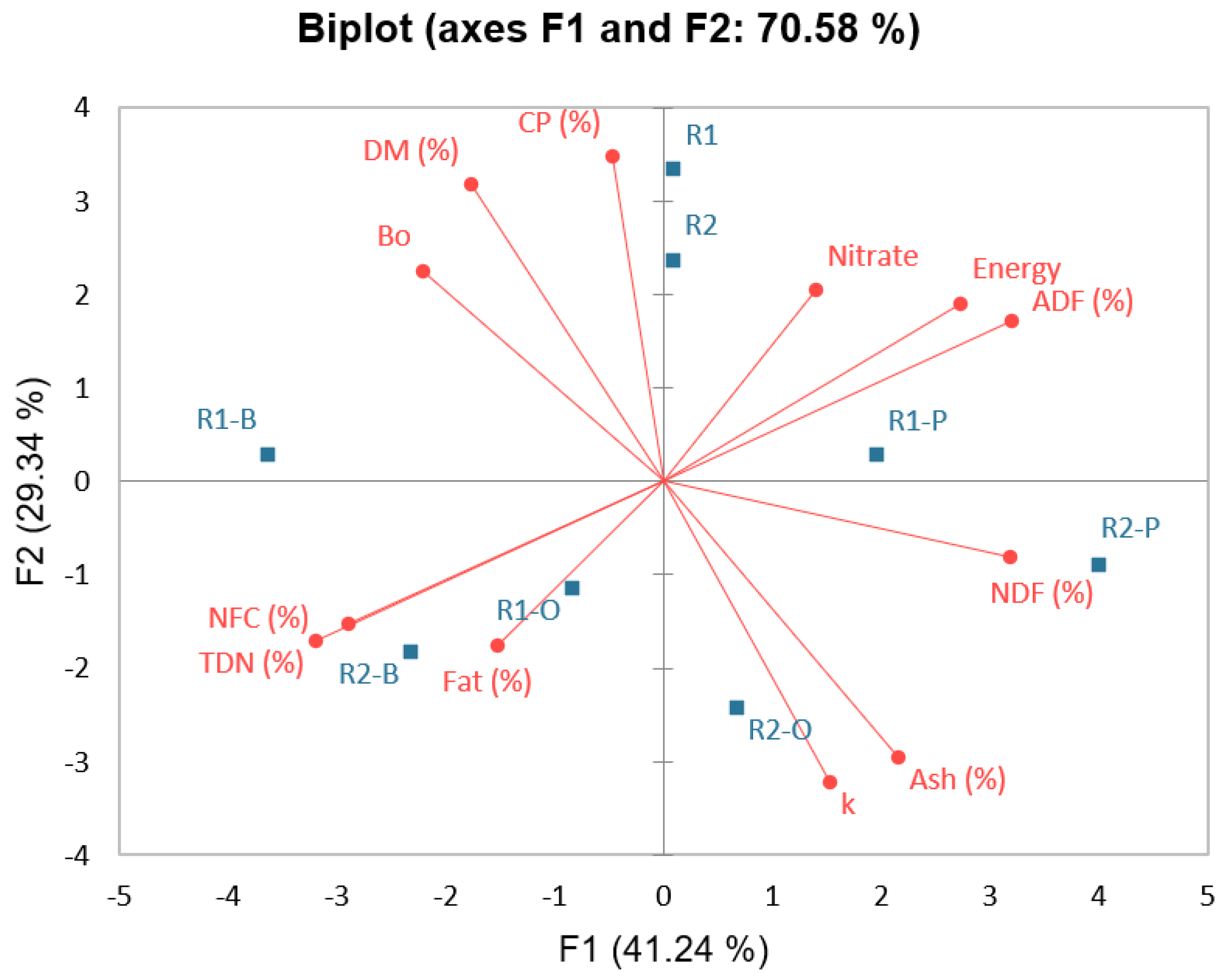
| Variables | Ash (%) | CP (%) | Fat (%) | NDF (%) | ADF (%) | NFC (%) | Energy | TDN (%) | Bo | k | Nitrate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DM (%) | −0.738 | 0.667 | −0.214 | −0.667 | −0.024 | 0.333 | −0.238 | 0.024 | 0.476 | −0.595 | 0.310 |
| Ash (%) | −0.381 | 0.048 | 0.476 | 0.310 | −0.238 | 0.119 | −0.310 | −0.881 | 0.714 | −0.333 | |
| CP (%) | −0.286 | −0.476 | 0.262 | −0.214 | 0.238 | −0.262 | 0.286 | −0.714 | 0.000 | ||
| Fat (%) | −0.262 | −0.524 | 0.190 | −0.214 | 0.524 | 0.048 | −0.238 | −0.595 | |||
| NDF (%) | 0.619 | −0.690 | 0.643 | −0.619 | −0.381 | 0.476 | 0.333 | ||||
| ADF (%) | −0.762 | 0.738 | −1.000 | −0.452 | 0.095 | 0.500 | |||||
| NFC (%) | −0.976 | 0.762 | 0.167 | 0.167 | −0.214 | ||||||
| Energy | −0.738 | −0.071 | −0.214 | 0.333 | |||||||
| TDN (%) | 0.452 | −0.095 | −0.500 | ||||||||
| Bo | −0.690 | 0.024 | |||||||||
| k | 0.095 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attard, E.; Buttigieg, J.; Simeonidis, K.; Pastorelli, G. The Modification of Dairy Cow Rations with Feed Additives Mitigates Methane Production and Reduces Nitrate Content During In Vitro Ruminal Fermentation. Gases 2025, 5, 12. https://doi.org/10.3390/gases5030012
Attard E, Buttigieg J, Simeonidis K, Pastorelli G. The Modification of Dairy Cow Rations with Feed Additives Mitigates Methane Production and Reduces Nitrate Content During In Vitro Ruminal Fermentation. Gases. 2025; 5(3):12. https://doi.org/10.3390/gases5030012
Chicago/Turabian StyleAttard, Everaldo, Jamie Buttigieg, Kalliroi Simeonidis, and Grazia Pastorelli. 2025. "The Modification of Dairy Cow Rations with Feed Additives Mitigates Methane Production and Reduces Nitrate Content During In Vitro Ruminal Fermentation" Gases 5, no. 3: 12. https://doi.org/10.3390/gases5030012
APA StyleAttard, E., Buttigieg, J., Simeonidis, K., & Pastorelli, G. (2025). The Modification of Dairy Cow Rations with Feed Additives Mitigates Methane Production and Reduces Nitrate Content During In Vitro Ruminal Fermentation. Gases, 5(3), 12. https://doi.org/10.3390/gases5030012






