A Guideline for Cross-Sector Coupling of Carbon Capture Technologies
Abstract
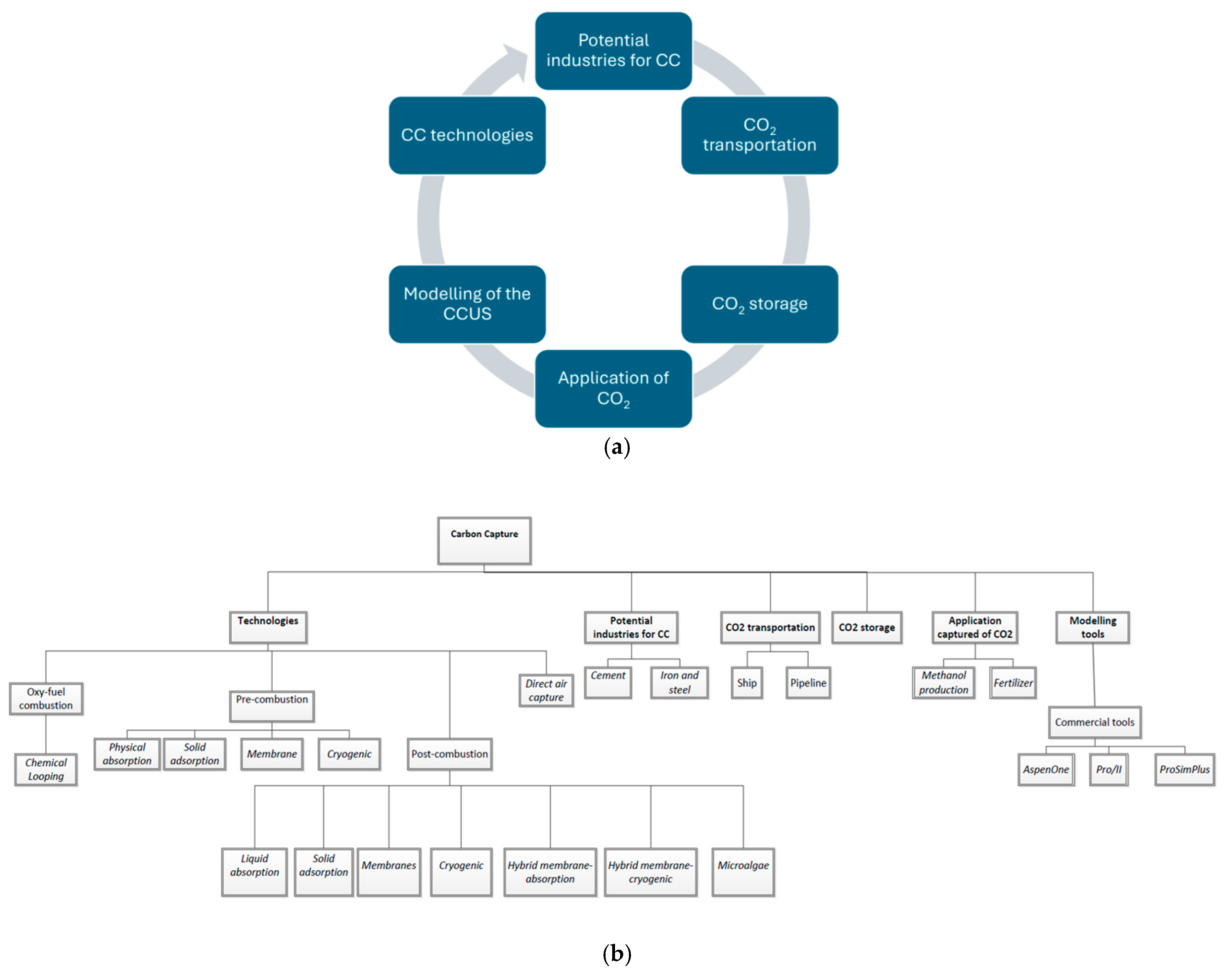
1. Introduction
2. Carbon Capture Technologies
2.1. Oxyfuel Combustion
2.1.1. Chemical Looping Combustion
2.1.2. Alternative O2 Separation Methods
2.2. Pre-Combustion Carbon Capture
2.2.1. CO2 Separation by Physical Absorption
2.2.2. CO2 Separation by Adsorption
2.2.3. CO2 Separation by Membrane
2.2.4. CO2 Separation by Cryogenic Technology
2.3. Post-Combustion Carbon Capture Approaches
2.3.1. CO2 Capture by Liquid Absorption
2.3.2. CO2 Separation by Membranes
2.3.3. CO2 Capture by Cryogenic Technology
2.3.4. CO2 Capture by Hybrid Membrane–Absorption Process
2.3.5. CO2 Capture by Hybrid Membrane–Cryogenic Process
2.3.6. CO2 Capture by Microalgae
2.4. Direct Air Capture
2.5. Summary of the Most Researched Carbon Capture Technologies
3. Main Potential Industries for CC
3.1. Iron and Steel
3.2. Cement Plants
3.3. Other Industries
4. Transportation of Captured CO2
4.1. Transportation via Pipelines
4.2. Transportation by Ships
4.3. Transportation Network Infrastructure
| Transportation Means | Pressure (bar) | Temperature (°C) | Water Content (in unit of ppm * or ppmv **) | |
|---|---|---|---|---|
| Pipeline | 90–150 [249,250] 150 [251] 100–150 [252] 85–150 [253] 110–300 [254] 75 [255] 152 [256] | 0–30 [257] 5–35 [250] 40 [251] 15–30 [252] 13–44 [253] 6–20 [254] 10 [255] 14 [256] | <50 * [246,258] 40–500 * [257] 500 ** [250] 20–257 ** [252] 50–630 * [259] <20 * [255] | 3–12 [260] 1–3 [261] 3.78 [251] 1.6 [254] 1.59 [256] |
| Ship | 6.5 [243,256,262] 7 [263,264] 15 [265] 31 [266] | −52 [243,256,262] −50 [263] −27 [265] −46 [264] −10 [266] | - | 1.33–2 [243] 2.2–3.2 [262] 1.7 [256] |
5. Storage of Captured CO2
5.1. Storage Purposes
5.2. Storage Characteristics and Effective Factors
6. Application of Captured CO2
6.1. Methanol Production
6.2. Fertiliser Industry
6.3. Other Applications
6.4. Sector Couplings
7. Computation Tools for Simulation of CC Processes
8. Conclusions and Future Directions
- The available literature lacks an extensive study on the influence of CC technologies on the national energy sectors. These studies are crucial, given the energy-intensive nature of the CC technologies and their significant influence on national energy landscapes.
- Furthermore, a comprehensive assessment of utilising the heat from flue gas in CO2 capture technologies remains largely unexplored. It is essential to evaluate the potential impacts of integrating this heat on lowering the energy penalties associated with these technologies.
- Despite the existence of numerous studies that have simulated and modelled CO2 capture technologies, the majority of them have been carried out under steady-state conditions. It is crucial to evaluate the dynamic behaviour of these technologies and investigate how these systems respond to fluctuations in power demand, given that the required power for these technologies is predominantly expected to be supplied by intermittent renewable sources.
- Novel CO2 capture technologies and materials, including metal–organic frameworks (MOFs) and covalent organic frameworks (COFs), have yet to be commercialised.
- The operating costs of the DAC remain significantly higher compared to CO2 capture technologies that capture emissions from point sources. As a result, DAC is still in its early stages and requires further research to become more cost-effective.
- For a truly circular economy, it is more beneficial to permanently sequester CO2 by converting it into long-lasting materials like carbonates, rather than using the captured CO2 for enhanced oil recovery, which ultimately emits CO2 when the oil is burned.
- Currently, there is no specific standard governing the purity of captured CO2 for pipeline transportation. It is imperative to thoroughly investigate CO2 transportation, particularly through pipelines, and establish a robust standard outlining the conditions for CO2 transportation. This standard significantly influences the infrastructure of CCUS, as well as the selection of the most suitable CC technology.
Author Contributions
Funding
Conflicts of Interest
References
- Peters, G.P.; Andrew, R.M.; Canadell, J.G.; Friedlingstein, P.; Jackson, R.B.; Korsbakken, J.I.; Peregon, A. Carbon dioxide emissions continue to grow amidst slowly emerging climate policies A failure to recognize the factors behind continued emissions growth could limit the world’s ability. Nat. Clim. Chang. 2020, 10, 3–6. [Google Scholar] [CrossRef]
- Adopted, I.P.C.C. Climate Change 2014-Synthesis Report, Net Zero by 2050; IPCC: Geneva, Szwitzerland, 2014. [Google Scholar]
- Hange, I.P.O.C. Climate change 2007: The physical science basis. Agenda 2007, 6, 333. [Google Scholar]
- Yadav, R.M.; Li, Z.; Zhang, T.; Sahin, O.; Roy, S.; Gao, G.; Guo, H.; Vajtai, R.; Wang, L.; Ajayan, P.M.; et al. Amine-functionalized carbon nanodot electrocatalysts converting carbon dioxide to methane. Adv. Mater. 2022, 34. [Google Scholar] [CrossRef]
- Rubin, E.S.; Mantripragada, H.; Marks, A.; Versteeg, P.; Kitchin, J. The outlook for improved carbon capture technology. Prog. Energy Combust. Sci. 2012, 38, 630–671. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous inorganic membranes for CO2 capture: Present and prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef]
- Asgharian, H.; Iov, F.; Araya, S.S.; Pedersen, T.H.; Nielsen, M.P.; Baniasadi, E.; Liso, V. A Review on Process Modeling and Simulation of Cryogenic Carbon Capture for Post-Combustion Treatment. Energies 2023, 16, 1855. [Google Scholar] [CrossRef]
- Perrin, N.; Dubettier, R.; Lockwood, F.; Tranier, J.-P.; Bourhy-Weber, C.; Terrien, P. Oxycombustion for coal power plants: Advantages, solutions and projects. Appl. Therm. Eng. 2015, 74, 75–82. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Z.; Xiang, J.; Zhang, L.; Zhang, S.; Luo, C.; Zhao, Y. Fundamental and Technical Challenges for a Compatible Design Scheme of Oxyfuel Combustion Technology. Engineering 2015, 1, 139–149. [Google Scholar] [CrossRef]
- Meng, F.; Meng, Y.; Ju, T.; Han, S.; Lin, L.; Jiang, J. Research progress of aqueous amine solution for CO2 capture: A review. Renew. Sustain. Energy Rev. 2022, 168, 112902. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-Based CO2 Capture Technology Development from the Beginning of 2013—A Review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef]
- Shakerian, F.; Kim, K.-H.; Szulejko, J.E.; Park, J.-W. A comparative review between amines and ammonia as sorptive media for post-combustion CO2 capture. Appl. Energy 2015, 148, 10–22. [Google Scholar] [CrossRef]
- Beuttler, C.; Charles, L.; Wurzbacher, J. The Role of Direct Air Capture in Mitigation of Anthropogenic Greenhouse Gas Emissions. Front. Clim. 2019, 1. [Google Scholar] [CrossRef]
- Hanna, R.; Abdulla, A.; Xu, Y.; Victor, D.G. Emergency deployment of direct air capture as a response to the climate crisis. Nat. Commun. 2021, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, M.; Nayak, S.P.; Ruiz, A.D.; Jiang, W. iScience ll Current status and pillars of direct air capture technologies. Iscience 2022, 25, 103990. [Google Scholar] [CrossRef] [PubMed]
- Gür, T.M. Correction: Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 3055. [Google Scholar] [CrossRef]
- Sutherland, B.R. Pricing CO2 direct air capture. Joule 2019, 3, 1571–1573. [Google Scholar] [CrossRef]
- Scheffknecht, J.; Al-Makhadmeh, G.; Schnell, L.; Maier, U. Oxy-fuel coal combustion—A review of the current state-of-the-art. Int. J. Greenh. Gas Control 2011, 5, 16–35. [Google Scholar] [CrossRef]
- Chen, L.; Yong, S.Z.; Ghoniem, A.F. Oxy-fuel combustion of pulverized coal: Characterization, fundamentals, stabilization and CFD modeling. Prog. Energy Combust. Sci. 2012, 38, 156–214. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Luo, C.; Zhang, L.; Xu, Y.; Wu, Y.; Liu, J.; Duan, Y.; Zheng, C. Investigation on the thermodynamic calculation of a 35 MWth oxy-fuel combustion coal-fired boiler. Int. J. Greenh. Gas Control 2018, 71, 36–45. [Google Scholar] [CrossRef]
- Toftegaard, A.D.; Brix, M.B.; Jensen, J.; Glarborg, P.A.; Jensen, P. Oxy-fuel combustion of solid fuels. Prog. Energy Combust. Sci. 2010, 35, 581–625. [Google Scholar] [CrossRef]
- Wall, T.; Liu, Y.; Spero, C.; Elliott, L.; Khare, S.; Rathnam, R.; Zeenathal, F.; Moghtaderi, B.; Buhre, B.; Sheng, C.; et al. An overview on oxyfuel coal combustion—State of the art research and technology development. Chem. Eng. Res. Des. 2009, 87, 1003–1016. [Google Scholar] [CrossRef]
- Yin, C.; Yan, J. Oxy-fuel combustion of pulverized fuels: Combustion fundamentals and modeling. Appl. Energy 2016, 162, 742–762. [Google Scholar] [CrossRef]
- Stanger, T.; Wall, R. Sulphur impacts during pulverised coal combustion in oxy-fuel technology for carbon capture and storage. Prog. Energy Combust. Sci. 2011, 37, 69–88. [Google Scholar] [CrossRef]
- Yin, C.; Johansen, L.C.R.; Rosendahl, L.A.; Kær, S.K. New Weighted Sum of Gray Gases Model Applicable to Computational Fluid Dynamics (CFD) Modeling of Oxy−Fuel Combustion: Derivation, Validation, and Implementation. Energy Fuels 2010, 24, 6275–6282. [Google Scholar] [CrossRef]
- Yin, C.; Rosendahl, L.A.; Kær, S.K. Chemistry and radiation in oxy-fuel combustion: A computational fluid dynamics modeling study. Fuel 2011, 90, 2519–2529. [Google Scholar] [CrossRef]
- Yin, C. Prediction of air-fuel and oxy-fuel combustion through a generic gas radiation property model. Appl. Energy 2017, 189, 449–459. [Google Scholar] [CrossRef]
- Stanger, R.; Wall, T.; Spörl, R.; Paneru, M.; Grathwohl, S.; Weidmann, M.; Scheffknecht, G.; McDonald, D.; Myöhänen, K.; Ritvanen, J.; et al. Oxyfuel combustion for CO2 capture in power plants. Int. J. Greenh. Gas Control 2015, 40, 55–125. [Google Scholar] [CrossRef]
- Yadav, S.; Mondal, S.S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2022, 308, 122057. [Google Scholar] [CrossRef]
- White Rose Carbon Capture and Storage Project: Combined Heat and Power Assessment; Capture Power Ltd.: London, UK, 2014. Available online: https://assets.publishing.service.gov.uk/media/5a8055eae5274a2e87db93ad/Combined_Heat_and_Power_Assessment.pdf (accessed on 15 May 2024).
- Wu, F.; Argyle, M.D.; Dellenback, P.A.; Fan, M. Progress in O2 separation for oxy-fuel combustion–A promising way for cost-effective CO2 capture: A review. Prog. Energy Combust. Sci. 2018, 67, 188–205. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Process analysis of pressurized oxy-coal power cycle for carbon capture application integrated with liquid air power generation and binary cycle engines. Appl. Energe 2015, 154, 556–566. [Google Scholar] [CrossRef]
- Uchida, T.; Goto, T.; Yamada, T.; Kiga, T.; Spero, C. Oxyfuel combustion as CO2 capture technology advancing for practical use-Callide oxyfuel project. Energy Procedia 2013, 37, 1471–1479. [Google Scholar] [CrossRef]
- Hossain, H.I.; de Lasa, M.M. Chemical-looping combustion (CLC) for inherent CO2 separations—A review. Chem. Eng. Sci. 2008, 63, 4433–4451. [Google Scholar] [CrossRef]
- Luo, M.; Yi, Y.; Wang, C.; Liu, K.; Pan, J.; Wang, Q. Energy and Exergy Analysis of Power Generation Systems with Chemical Looping Combustion of Coal. Chem. Eng. Technol. 2018, 41, 776–787. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A.; Mendiara, T.; Gayán, P.; de Diego, L.F.; García-Labiano, F. Chemical looping combustion of solid fuels. Prog. Energy Combust. Sci. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A. Chemical-looping combustion: Status and research needs. Proc. Combust. Inst. 2019, 37, 4303–4317. [Google Scholar] [CrossRef]
- Czakiert, T.; Krzywanski, J.; Zylka, A.; Nowak, W. Chemical Looping Combustion: A Brief Overview. Energies 2022, 15, 1563. [Google Scholar] [CrossRef]
- Cao, Y.; He, B.; Ding, G.; Su, L.; Duan, Z. Performance Modeling of Integrated Chemical Looping Air Separation and IGCC with CO2 Capture. Energy Fuels 2016, 30, 9953–9961. [Google Scholar] [CrossRef]
- Chen, J.T.; Shih, C.C.; Fu, Y.J.; Huang, S.H.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Zeolite-Filled Porous Mixed Matrix Membranes for Air Separation. Ind. Eng. Chem. Res. 2014, 53, 2781–2789. [Google Scholar] [CrossRef]
- Sunarso, J.; Hashim, S.S.; Zhu, N.; Zhou, W. Perovskite oxides applications in high temperature oxygen separation, solid oxide fuel cell and membrane reactor: A review. Prog. Energy Combust. Sci. 2017, 61, 57–77. [Google Scholar] [CrossRef]
- Smith, A.; Klosek, J. A review of air separation technologies and their integration with energy conversion processes. Fuel Process. Technol. 2001, 70, 115–134. [Google Scholar] [CrossRef]
- Zhu, X.; Hoang, T.; Lobban, L.L.; Mallinson, R.G. Low CO content hydrogen production from bio-ethanol using a combined plasma reforming–catalytic water gas shift reactor. Appl. Catal. B Environ. 2010, 94, 311–317. [Google Scholar] [CrossRef]
- Jansen, D.; Gazzani, M.; Manzolini, G.; Van Dijk, E.; Carbo, M. Pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 40, 167–187. [Google Scholar] [CrossRef]
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663. [Google Scholar] [CrossRef]
- Available online: https://www.geos.ed.ac.uk/sccs/project-info/15 (accessed on 14 October 2024).
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.J.; Lee, J.W.; Chun, S.N.; Bin Lee, J. The quantitative evaluation of two-stage pre-combustion CO2 capture processes using the physical solvents with various design parameters. Energy 2015, 81, 47–55. [Google Scholar] [CrossRef]
- Kapetaki, Z.; Brandani, P.; Brandani, S.; Ahn, H. Process simulation of a dual-stage Selexol process for 95% carbon capture efficiency at an integrated gasification combined cycle power plant. Int. J. Greenh. Gas Control 2015, 39, 17–26. [Google Scholar] [CrossRef]
- Sadegh-Vaziri, R.; Amovic, M.; Ljunggren, R.; Engvall, K. A Medium-Scale 50 MWfuel Biomass Gasification Based Bio-SNG Plant: A Developed Gas Cleaning Process. Energies 2015, 8, 5287–5302. [Google Scholar] [CrossRef]
- Olabi, A.G.; Obaideen, K.; Elsaid, K.; Wilberforce, T.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renew. Sustain. Energy Rev. 2022, 153, 111710. [Google Scholar] [CrossRef]
- Garcia, S.; Gil, M.V.; Martín, C.F.; Pis, J.J.; Rubiera, F.; Pevida, C. Breakthrough adsorption study of a commercial activated carbon for pre-combustion CO2 capture. Chem. Eng. J. 2011, 171, 549–556. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, L. Membranes for CO2 capture and separation: Progress in research and development for industrial applications. Sep. Purif. Technol. 2024, 335, 126022. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Anantharaman, R.; Berstad, D.; Roussanaly, S. Techno-economic Performance of a Hybrid Membrane–Liquefaction Process for Post-combustion CO2 Capture. Energy Procedia 2014, 61, 1244–1247. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential. Microporous Mesoporous Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Bryan, N.; Lasseuguette, E.; van Dalen, M.; Permogorov, N.; Amieiro, A.; Brandani, S.; Ferrari, M.C. Development of Mixed Matrix Membranes Containing Zeolites for Post-combustion Carbon Capture. Energy Procedia 2014, 63, 160–166. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Abu-Zahra, G.F.; Niederer, M.R.; Feron, J.P.; Versteeg, P.H. CO2 capture from power plants: Part II. A parametric study of the economical performance based on mono-ethanolamine. Int. J. Greenh. Gas Control 2007, 1, 135–142. [Google Scholar] [CrossRef]
- Nuchitprasittichai, A.; Cremaschi, S. Optimization of CO2 capture process with aqueous amines using response surface methodology. Comput. Chem. Eng. 2011, 35, 1521–1531. [Google Scholar] [CrossRef]
- Nielsen, C.J.; Herrmann, H.; Weller, C. Atmospheric chemistry and environmental impact of the use of amines in carbon capture and storage (CCS). Chem. Soc. Rev. 2012, 41, 6684. [Google Scholar] [CrossRef]
- da Silva, A.M.; Booth, E.F. Emissions from postcombustion CO2 capture plants. Environ. Technol. 2013, 47, 659–660. [Google Scholar] [CrossRef]
- Reynolds, P.; Verheyen, A.J.; Adeloju, T.V.; Meuleman, S.B.; Feron, E. Towards commercial scale postcombustion capture of CO2 with monoethanolamine solvent: Key considerations for solvent management and environmental impacts. Environ. Sci. Technol. 2012, 46, 3643–3654. [Google Scholar] [CrossRef]
- Giannaris, S.; Janowczyk, D.; Ruffini, J.; Hill, K.; Jacobs, B.; Bruce, C.; Feng, Y.; Srisang, W. SaskPower’s Boundary Dam Unit 3 Carbon Capture Facility-The Journey to Achieving Reliability. In Proceedings of the 15th Greenhouse Gas Control Technologies Conference, Abu Dhabi, UAE, 15–18 March 2021; pp. 15–18. [Google Scholar]
- Fujiwara, K. Petra Nova Carbon Capture, Utilization, and Storage Project: Capturing CO2 from the flue gas stream of a coal-fired power plant and increasing oil production from a legacy oil field. J. Jpn. Assoc. Pet. Technol. 2019, 84, 114–122. [Google Scholar] [CrossRef]
- Anderson, J.L.; Dixon, J.K.; Maginn, E.J.; Brennecke, J.F. Measurement of SO2 Solubility in Ionic Liquids. J. Phys. Chem. B 2006, 110, 15059–15062. [Google Scholar] [CrossRef]
- Habib, M.A.; Badr, H.M.; Ahmed, S.F.; Ben-Mansour, R.; Mezghani, K.; Imashuku, S.; Shao-Horn, Y.; Mancini, N.D.; Mitsos, A.; Ghoneim, A.F. A review of recent developments in carbon capture utilizing oxy-fuel combustion in conventional and ion transport membrane systems. Int. J. Energy Res. 2011, 35, 741–764. [Google Scholar] [CrossRef]
- Stadler, H.; Beggel, F.; Habermehl, M.; Persigehl, B.; Kneer, R.; Modigell, M.; Jeschke, P. Oxyfuel coal combustion by efficient integration of oxygen transport membranes. Int. J. Greenh. Gas Control 2011, 5, 7–15. [Google Scholar] [CrossRef]
- Lonsdale, H.K. The growth of membrane technology. J. Memb. Sci. 1982, 10, 81–181. [Google Scholar] [CrossRef]
- Gür, T.M. Permselectivity of zeolite filled polysulfone gas separation membranes. J. Membr. Sci. 1994, 93, 283–289. [Google Scholar] [CrossRef]
- Yegani, R.; Hirozawa, H.; Teramoto, M.; Himei, H.; Okada, O.; Takigawa, T.; Ohmura, N.; Matsumiya, N.; Matsuyama, H. Selective separation of CO2 by using novel facilitated transport membrane at elevated temperatures and pressures. J. Membr. Sci. 2007, 291, 157–164. [Google Scholar] [CrossRef]
- Verweij, H. Inorganic membranes. Curr. Opin. Chem. Eng. 2012, 1, 156–162. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Anderson, M.; Wang, H.; Lin, Y.S. Inorganic membranes for carbon dioxide and nitrogen separation. Rev. Chem. Eng. 2012, 28, 101–121. [Google Scholar] [CrossRef]
- Yang, H.-C.; Hou, J.; Chen, V.; Xu, Z.-K. Surface and interface engineering for organic–inorganic composite membranes. J. Mater. Chem. A 2016, 4, 9716–9729. [Google Scholar] [CrossRef]
- Ostwal, M.; Singh, R.P.; Dec, S.F.; Lusk, M.T.; Way, J.D. 3-Aminopropyltriethoxysilane functionalized inorganic membranes for high temperature CO2/N2 separation. J. Membr. Sci. 2011, 369, 139–147. [Google Scholar] [CrossRef]
- Jensen, M. Energy Process Enabled by Cryogenic Carbon Capture. Ph.D. Thesis, Brigham Young University, Provo, UT, USA, 2015. Available online: https://scholarsarchive.byu.edu/etd (accessed on 14 October 2024).
- Dong, N.; Fang, W.; Wang, M.; Liu, T.; Yi, F. CO2 capture by using a membrane-absorption hybrid process in the nature gas combined cycle power plants. Aerosol Air Qual. Res. 2021, 21, 200374. [Google Scholar] [CrossRef]
- Nasir, M.; Suleman, Q.; Elumalai, H. A hybrid membrane-absorption process for carbon dioxide capture: Effect of different alkanolamine on energy consumption. Iran. J. Chem. Chem. Eng. 2020, 39, 239–254. [Google Scholar]
- Belaissaoui, B.; Le Moullec, Y.; Willson, D.; Favre, E. Hybrid membrane cryogenic process for post-combustion CO2 capture. J. Membr. Sci. 2012, 415–416, 424–434. [Google Scholar] [CrossRef]
- Vale, M.A.; Ferreira, A.; Pires, J.C.M.; Gonçalves, A.L. CO2 capture using microalgae. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 381–405. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of Carbon Capture Technology: Microalgal Biorefinery Concept and State-of-the-Art. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization–A review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Sabatino, F.; Grimm, A.; Gallucci, F.; van Sint Annaland, M.; Kramer, G.J.; Gazzani, M. A comparative energy and costs assessment and optimization for direct air capture technologies. Joule 2021, 5, 2047–2076. [Google Scholar] [CrossRef]
- Creutzig, F.; Breyer, C.; Hilaire, J.; Minx, J.; Peters, G.P.; Socolow, R. The mutual dependence of negative emission technologies and energy systems. Energy Environ. Sci. 2019, 12, 1805–1817. [Google Scholar] [CrossRef]
- Galimova, T.; Ram, M.; Bogdanov, D.; Fasihi, M.; Khalili, S.; Gulagi, A.; Karjunen, H.; Mensah, T.N.O.; Breyer, C. Global demand analysis for carbon dioxide as raw material from key industrial sources and direct air capture to produce renewable electricity-based fuels and chemicals. J. Clean. Prod. 2022, 373, 133920. [Google Scholar] [CrossRef]
- Global Assessment of Direct Air Capture Costs; IEAGHG, Pure Offices: Cheltenham, UK, 2021; pp. 1–97. Available online: www.ieaghg.org (accessed on 14 October 2024).
- Kownatzki, C.; Kather, S.; Günther, A. CO2 purity in coal fired oxyfuel processes. In Proceedings of the 2nd Oxyfuel Combustion Conference, Queensland, Australia, 12–16 September 2011; pp. 12–16. [Google Scholar]
- Borgert, K.J.; Rubin, E.S. Oxy-combustion carbon capture for pulverized coal in the integrated environmental control model. Energy Procedia 2017, 114, 522–529. [Google Scholar] [CrossRef]
- Borgert, K.J.; Rubin, E.S. Oxyfuel Combustion: Technical and Economic Considerations for the Development of Carbon Capture from Pulverized Coal Power Plants. Energy Procedia 2013, 37, 1291–1300. [Google Scholar] [CrossRef]
- Pipitone, G.; Bolland, O. Power generation with CO2 capture: Technology for CO2 purification. Int. J. Greenh. Gas Control 2009, 3, 528–534. [Google Scholar] [CrossRef]
- Kather, A.; Kownatzki, S. Assessment of the different parameters affecting the CO2 purity from coal fired oxyfuel process. Int. J. Greenh. Gas Control 2011, 5, S204–S209. [Google Scholar] [CrossRef]
- Li, H.; Yan, J.; Yan, J.; Anheden, M. Impurity impacts on the purification process in oxy-fuel combustion based CO2 capture and storage system. Appl. Energy 2009, 86, 202–213. [Google Scholar] [CrossRef]
- Xiong, J.; Zhao, H.; Chen, M.; Zheng, C. Simulation Study of an 800 MW e Oxy-combustion Pulverized-Coal-Fired Power Plant. Energy Fuels 2011, 25, 2405–2415. [Google Scholar] [CrossRef]
- Li, H.; Hu, Y.; Ditaranto, M.; Willson, D.; Yan, J. Optimization of Cryogenic CO2 Purification for Oxy-coal Combustion. Energy Procedia 2013, 37, 1341–1347. [Google Scholar] [CrossRef]
- Kazemifar, F. A review of technologies for carbon capture, sequestration, and utilization: Cost, capacity, and technology readiness. Greenh. Gases Sci. Technol. 2022, 12, 200–230. [Google Scholar] [CrossRef]
- Yan, K.; Wu, X.; Hoadley, A.; Xu, X.; Zhang, J.; Zhang, L. Sensitivity analysis of oxy-fuel power plant system. Energy Convers. Manag. 2015, 98, 138–150. [Google Scholar] [CrossRef]
- Ding, G.; He, B.; Cao, Y.; Wang, C.; Su, L.; Duan, Z.; Song, J.; Tong, W.; Li, X. Process simulation and optimization of municipal solid waste fired power plant with oxygen/carbon dioxide combustion for near zero carbon dioxide emission. Energy Convers. Manag. 2018, 157, 157–168. [Google Scholar] [CrossRef]
- Pettinau, A.; Ferrara, F.; Amorino, C. Combustion vs. gasification for a demonstration CCS (carbon capture and storage) project in Italy: A techno-economic analysis. Energy 2013, 50, 160–169. [Google Scholar] [CrossRef]
- Jin, B.; Zhao, H.; Zou, C.; Zheng, C. Comprehensive investigation of process characteristics for oxy-steam combustion power plants. Energy Convers. Manag. 2015, 99, 92–101. [Google Scholar] [CrossRef]
- Cau, G.; Tola, V.; Ferrara, F.; Porcu, A.; Pettinau, A. CO2-free coal-fired power generation by partial oxy-fuel and post-combustion CO2 capture: Techno-economic analysis. Fuel 2018, 214, 423–435. [Google Scholar] [CrossRef]
- Cormos, C.-C. Oxy-combustion of coal, lignite and biomass: A techno-economic analysis for a large scale Carbon Capture and Storage (CCS) project in Romania. Fuel 2016, 169, 50–57. [Google Scholar] [CrossRef]
- Garcia, J.A.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Technical analysis of CO2 capture pathways and technologies. J. Environ. Chem. Eng. 2022, 10, 108470. [Google Scholar] [CrossRef]
- Nemitallah, M.A.; Habib, M.A.; Badr, H.M.; Said, S.A.; Jamal, A.; Ben-Mansour, R.; Mokheimer, E.M.A.; Mezghani, K. Oxy-fuel combustion technology: Current status, applications, and trends. Int. J. Energy Res. 2017, 41, 1670–1708. [Google Scholar] [CrossRef]
- Yan, Y.; Clough, P.T.; Anthony, E.J. Investigation of the apparent kinetics of air and oxy-fuel biomass combustion in a spout fluidised-bed reactor. Chem. Eng. Res. Des. 2020, 153, 276–283. [Google Scholar] [CrossRef]
- Baqain, M.; Neshumayev, D.; Konist, A. Oxyfuel Conversion of Ca-rich fuel in a 60 kWth Circulating Fluidized Bed. In Proceedings of the 16th International Conference on Greenhouse Gas Control Technologies, Lyon, France, 23–27 October 2022. [Google Scholar]
- Energy Technology Perspectives 2020-Special Report on Carbon Capture Utilisation and Storage. In Energy Technology Perspectives 2020-Special Report on Carbon Capture Utilisation and Storage; International Energy Agency: Paris, France, 2020. [CrossRef]
- Hanak, D.P.; Powell, D.; Manovic, V. Techno-economic analysis of oxy-combustion coal-fired power plant with cryogenic oxygen storage. Appl. Energy 2017, 191, 193–203. [Google Scholar] [CrossRef]
- López, R.; Fernández, C.; Martínez, O.; Sánchez, M.E. Techno-economic analysis of a 15MW corn-rape oxy-combustion power plant. Fuel Process. Technol. 2016, 142, 296–304. [Google Scholar] [CrossRef]
- Goto, K.; Kazama, S.; Furukawa, A.; Serizawa, M.; Aramaki, S.; Shoji, K. Effect of CO2 Purity on Energy Requirement of CO2 Capture Processes. Energy Procedia 2013, 37, 806–812. [Google Scholar] [CrossRef]
- Skorek-Osikowska, A.; Kotowicz, J.; Janusz-Szymańska, K. Comparison of the Energy Intensity of the Selected CO2-Capture Methods Applied in the Ultra-supercritical Coal Power Plants. Energy Fuels 2012, 26, 6509–6517. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.; Ditaranto, M.; Yan, J.; Yu, Z. Capturing CO2 from Biogas Plants. Energy Procedia 2017, 114, 6030–6035. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Hassan, E.; Douglas, S.N.; Croiset, P.L. Techno-economic study of CO2 capture from an existing cement plant using MEA scrubbing. Int. J. Green Energy 2007, 4, 197–220. [Google Scholar] [CrossRef]
- Singh, D.; Croiset, E.; Douglas, P.; Douglas, M. Techno-economic study of CO2 capture from an existing coal-fired power plant: MEA scrubbing vs. O2/CO2 recycle combustion. Energy Convers. Manag. 2003, 44, 3073–3091. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Liu, X.; Li, H.; Xiao, W.; Gao, M.; Liang, H. Reduction of energy requirement of CO2 desorption from a rich CO2-loaded MEA solution by using solid acid catalysts. Appl. Energy 2017, 202, 673–684. [Google Scholar] [CrossRef]
- Abu-Zahra, G.F.; Schneiders, M.R.; Niederer, L.H.; Feron, J.P.; Versteeg, P.H. CO2 capture from power plants: Part I. A parametric study of the technical performance based on monoethanolamine. Int. J. Greenh. Gas Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- Mores, S.; Scenna, P.; Mussati, N. CO2 capture using monoethanolamine (MEA) aqueous solution: Modeling and optimization of the solvent regeneration and CO2 desorption process. Energy 2012, 45, 1042–1058. [Google Scholar] [CrossRef]
- Nwaoha, C.; Saiwan, C.; Tontiwachwuthikul, P.; Supap, T.; Rongwong, W.; Idem, R.; AL-Marri, M.J.; Benamor, A. Carbon dioxide (CO2) capture: Absorption-desorption capabilities of 2-amino-2-methyl-1-propanol (AMP), piperazine (PZ) and monoethanolamine (MEA) tri-solvent blends. J. Nat. Gas Sci. Eng. 2016, 33, 742–750. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, T.; Liu, W.; Fang, F.; Farooq, M.; Luo, M. Enhanced CO2 absorption and desorption by monoethanolamine (MEA)-based nanoparticle suspensions. Ind. Eng. Chem. Res. 2016, 55, 7830–7838. [Google Scholar] [CrossRef]
- Van Wagener, D.H.; Rochelle, G.T. Stripper configurations for CO2 capture by aqueous monoethanolamine and piperazine. Energy Procedia 2011, 4, 1323–1330. [Google Scholar] [CrossRef]
- Kuparinen, K.; Vakkilainen, E.; Tynjälä, T. Biomass-based carbon capture and utilization in kraft pulp mills. Mitig. Adapt. Strateg. Glob. Chang. 2019, 24, 1213–1230. [Google Scholar] [CrossRef]
- Alie, C.; Backham, L.; Croiset, E.; Douglas, P.L. Simulation of CO2 capture using MEA scrubbing: A flowsheet decomposition method. Energy Convers. Manag. 2005, 46, 475–487. [Google Scholar] [CrossRef]
- Kundu, X.; Chakma, P.K.; Feng, A. Effectiveness of membranes and hybrid membrane processes in comparison with absorption using amines for post-combustion CO2 capture. Int. J. Greenh. Gas Control 2014, 28, 248–256. [Google Scholar] [CrossRef]
- Hasan, M.M.F.; Baliban, R.C.; Elia, J.A.; Floudas, C.A. Modeling, Simulation, and Optimization of Postcombustion CO2 Capture for Variable Feed Concentration and Flow Rate. 1. Chemical Absorption and Membrane Processes. Ind. Eng. Chem. Res. 2012, 51, 15642–15664. [Google Scholar] [CrossRef]
- Rao, E.S.; Rubin, A.B. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef]
- Rao, E.S.; Rubin, A.B. Identifying cost-effective CO2 control levels for amine-based CO2 capture systems. Ind. Eng. Chem. Res. 2006, 45, 2421–2429. [Google Scholar] [CrossRef]
- Hoeger, C.; Burt, S.; Baxter, L. Cryogenic Carbon CaptureTM Technoeconomic Analysis. In Proceedings of the 15th Greenhouse Gas Control Technologies Conference, Abu Dhabi, UAE, 15–18 March 2021; pp. 1–11. [Google Scholar] [CrossRef]
- Ho, M.T.; Allinson, G.W.; Wiley, D.E. Reducing the Cost of CO2 Capture from Flue Gases Using Pressure Swing Adsorption. Ind. Eng. Chem. Res. 2008, 47, 4883–4890. [Google Scholar] [CrossRef]
- Hanifa, M.; Agarwal, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. A review on CO2 capture and sequestration in the construction industry: Emerging approaches and commercialised technologies. J. CO2 Util. 2023, 67, 102292. [Google Scholar] [CrossRef]
- Versteeg, P.; Rubin, E.S. A technical and economic assessment of ammonia-based post-combustion CO2 capture at coal-fired power plants. Int. J. Greenh. Gas Control 2011, 5, 1596–1605. [Google Scholar] [CrossRef]
- Kearns, C.; Liu, D.; Consoli, H. Technology Readiness and Costs of CCS; Global CCS Institute: Melbourne, Australia, 2021. [Google Scholar]
- Rezaei, S.; Liu, A.; Hovington, P. Emerging technologies in post-combustion carbon dioxide capture & removal. Catal. Today 2023, 423, 114286. [Google Scholar] [CrossRef]
- Hou, R.; Fong, C.; Freeman, B.D.; Hill, M.R.; Xie, Z. Current status and advances in membrane technology for carbon capture. Sep. Purif. Technol. 2022, 300, 121863. [Google Scholar] [CrossRef]
- Peh, S.B.; Farooq, S.; Zhao, D. Techno-economic analysis of MOF-based adsorption cycles for postcombustion CO2 capture from wet flue gas. Chem. Eng. Sci. 2023, 268, 118390. [Google Scholar] [CrossRef]
- Yang, M.W.; Chen, N.C.; Huang, C.H.; Shen, Y.T.; Yang, H.S.; Chou, C.T. Temperature swing adsorption process for CO2 capture using polyaniline solid sorbent. Energy Procedia 2014, 63, 2351–2358. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoudi, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations–A review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- An, X.; Yang, J.; Qi, G.; Li, T.; Zhao, K.; Fu, D. Functional molecular engineering hierarchical pore-interface based on thermodynamic-kinetic synergy strategy for efficient CO2 capture and separation. Fuel 2023, 332, 126243. [Google Scholar] [CrossRef]
- Al-Absi, A.A.; Domin, A.; Mohamedali, M.; Benneker, A.M.; Mahinpey, N. CO2 capture using in-situ polymerized amines into pore-expanded-SBA-15: Performance evaluation, kinetics, and adsorption isotherms. Fuel 2023, 333, 126401. [Google Scholar] [CrossRef]
- Helmi, M.; Moazami, F.; Ghaemi, A.; Hemmati, A. Synthesis, characterization and performance evaluation of NaOH@Chitosan-Fe3O4 as an adsorbent for CO2 capture. Fuel 2023, 338, 127300. [Google Scholar] [CrossRef]
- Samaddoost, L.; Soltani, M.; Fatehifar, E.; Asl, E.A. Design of amine-functionalized resin via a facial method with efficient CO2 capture from air. Process Saf. Environ. Prot. 2023, 171, 18–27. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Guillerm, V.; Eddaoudi, M. Low concentration CO2 capture using physical adsorbents: Are metal–organic frameworks becoming the new benchmark materials? Chem. Eng. J. 2016, 296, 386–397. [Google Scholar] [CrossRef]
- Jelen, D. Advanced Structured Adsorbent Architectures for Transformative Carbon Dioxide Capture Performance (Final Report); Electricore Inc.: Pittsburgh, PA, USA; Morgantown, WV, USA, 2023. [Google Scholar] [CrossRef]
- Bounaceur, E.; Lape, R.; Roizard, N.; Vallieres, D.; Favre, C. Membrane processes for post-combustion carbon dioxide capture: A parametric study. Energy 2006, 31, 2556–2570. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Sheng, M.; Wang, Z.; Zhao, S.; Wang, J.; Mao, S.; Wang, D.; Guo, B.; Ye, N.; et al. Membrane technology for CO2 capture: From pilot-scale investigation of two-stage plant to actual system design. J. Membr. Sci. 2021, 624, 119137. [Google Scholar] [CrossRef]
- Favre, E.; Bounaceur, R.; Roizard, D. Biogas, membranes and carbon dioxide capture. J. Membr. Sci. 2009, 328, 11–14. [Google Scholar] [CrossRef]
- Hussain, A.; Hägg, M.-B. A feasibility study of CO2 capture from flue gas by a facilitated transport membrane. J. Membr. Sci. 2010, 359, 140–148. [Google Scholar] [CrossRef]
- He, X. Polyvinylamine-Based Facilitated Transport Membranes for Post-Combustion CO2 Capture: Challenges and Perspectives from Materials to Processes. Engineering 2021, 7, 124–131. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Esfilar, R.; Moosavian, S.M.A. Introducing a novel air separation process based on cold energy recovery of LNG integrated with coal gasification, transcritical carbon dioxide power cycle and cryogenic CO2 capture. J. Clean. Prod. 2017, 142, 1749–1764. [Google Scholar] [CrossRef]
- Tan, J.; Nookuea, Y.; Li, W.; Thorin, H.; Yan, E. Cryogenic technology for biogas upgrading combined with carbon capture-a review of systems and property impacts. In Proceedings of the 9th International Conference on Applied Energy, Cardiff, UK, 21–24 August 2017. [Google Scholar]
- Knapik, E.; Kosowski, P.; Stopa, J. Cryogenic liquefaction and separation of CO2 using nitrogen removal unit cold energy. Chem. Eng. Res. Des. 2018, 131, 66–79. [Google Scholar] [CrossRef]
- Xu, G.; Liang, F.; Yang, Y.; Hu, Y.; Zhang, K.; Liu, W. An Improved CO2 Separation and Purification System Based on Cryogenic Separation and Distillation Theory. Energies 2014, 7, 3484–3502. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Pan, X.; Clodic, D.; Toubassy, J. CO2 capture by antisublimation process and its technical economic analysis. Greenh. Gases Sci. Technol. 2013, 3, 8–20. [Google Scholar] [CrossRef]
- Font-Palma, C.; Cann, D.; Udemu, C. Review of Cryogenic Carbon Capture Innovations and Their Potential Applications. C 2021, 7, 58. [Google Scholar] [CrossRef]
- Wilson, S.M.W. High purity CO2 from direct air capture using a single TVSA cycle with Na-X zeolites. Sep. Purif. Technol. 2022, 294, 121186. [Google Scholar] [CrossRef]
- Ozkan, M. Direct air capture of CO2: A response to meet the global climate targets. MRS Energy Sustain. 2021, 8, 51–56. [Google Scholar] [CrossRef]
- Shu, Q.; Legrand, L.; Kuntke, P.; Tedesco, M.; Hamelers, H.V. Electrochemical regeneration of spent alkaline absorbent from direct air capture. Environ. Sci. Technol. 2020, 54, 8990–8998. [Google Scholar]
- Kulkarni, A.R.; Sholl, D.S. Analysis of Equilibrium-Based TSA Processes for Direct Capture of CO2 from Air. Ind. Eng. Chem. Res. 2012, 51, 8631–8645. [Google Scholar] [CrossRef]
- Zeman, F. Reducing the Cost of Ca-Based Direct Air Capture of CO2. Environ. Sci. Technol. 2014, 48, 11730–11735. [Google Scholar] [CrossRef]
- Deutz, S.; Bardow, A. Life-cycle assessment of an industrial direct air capture process based on temperature–vacuum swing adsorption. Nat. Energy 2021, 6, 203–213. [Google Scholar] [CrossRef]
- McQueen, N.; Gomes, K.V.; McCormick, C.; Blumanthal, K.; Pisciotta, M.; Wilcox, J. A review of direct air capture (DAC): Scaling up commercial technologies and innovating for the future. Prog. Energy 2021, 3, 032001. [Google Scholar] [CrossRef]
- Gutknecht, V.; Snæbjörnsdóttir, S.Ó.; Sigfússon, B.; Aradóttir, E.S.; Charles, L. Creating a carbon dioxide removal solution by combining rapid mineralization of CO2 with direct air capture. Energy Procedia 2018, 146, 129–134. [Google Scholar] [CrossRef]
- Rim, G.; Kong, F.; Song, M.; Rosu, C.; Priyadarshini, P.; Lively, R.P.; Jones, C.W. Sub-Ambient Temperature Direct Air Capture of CO2 using Amine-Impregnated MIL-101(Cr) Enables Ambient Temperature CO2 Recovery. JACS Au 2022, 2, 380–393. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Farooqui, A.; McCoy, S.T. The impact of climate on solvent-based direct air capture systems. Appl. Energy 2022, 325, 119895. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Soeherman, J.K.; Jones, A.J.; Dauenhauer, P.J. Overcoming the Entropy Penalty of Direct Air Capture for Efficient Gigatonne Removal of Carbon Dioxide. ACS Eng. Au 2023, 3, 114–127. [Google Scholar] [CrossRef]
- McQueen, N.; Psarras, P.; Pilorgé, H.; Liguori, S.; He, J.; Yuan, M.; Woodball, C.B.; Kian, K.; Pierpoint, L.; Jurewicz, K.; et al. Cost Analysis of Direct Air Capture and Sequestration Coupled to Low-Carbon Thermal Energy in the United States. Environ. Sci. Technol. 2020, 54, 7542–7551. [Google Scholar] [CrossRef]
- Möllersten, K.; Naqvi, R. Technology Readiness Assessment, Costs, and Limitations of five shortlisted NETs; Climate Strategies 2022. Available online: https://www.researchgate.net/profile/Kenneth-Moellersten-2/publication/359427009_Technology_Readiness_Assessment_Costs_and_Limitations_of_five_shortlisted_NETs_Accelerated_mineralisation_Biochar_as_soil_additive_BECCS_DACCS_Wetland_restoration/links/623ba (accessed on 14 October 2024).
- McQueen, N.; Desmond, M.J.; Socolow, R.H.; Psarras, P.; Wilcox, J. Natural Gas vs. Electricity for Solvent-Based Direct Air Capture. Front. Clim. 2021, 2. [Google Scholar] [CrossRef]
- Zolfaghari, Z.; Aslani, A.; Moshari, A.; Malekli, M. Direct air capture from demonstration to commercialization stage: A bibliometric analysis. Int. J. Energy Res. 2022, 46, 383–396. [Google Scholar] [CrossRef]
- Viebahn, P.; Scholz, A.; Zelt, O. The Potential Role of Direct Air Capture in the German Energy Research Program—Results of a Multi-Dimensional Analysis. Energies 2019, 12, 3443. [Google Scholar] [CrossRef]
- Baskaran, D.; Saravanan, P.; Nagarajan, L.; Byun, H.S. An overview of technologies for Capturing, Storing, and utilizing carbon Dioxide: Technology Readiness, large-scale Demonstration, and cost. Chem. Eng. J. 2024, 491, 151998. [Google Scholar] [CrossRef]
- Hong, W.Y. A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future. Carbon Capture Sci. Technol. 2022, 491, 151998. [Google Scholar]
- Yeh, J.T.; Pennline, H.W.; Resnik, K.P. Study of CO2 Absorption and Desorption in a Packed Column. Energy Fuels 2001, 15, 274–278. [Google Scholar] [CrossRef]
- Hüser, N.; Schmitz, O.; Kenig, E.Y. A comparative study of different amine-based solvents for CO2-capture using the rate-based approach. Chem. Eng. Sci. 2017, 157, 221–231. [Google Scholar] [CrossRef]
- Amrollahi, Z.; Ystad, P.A.M.; Ertesvåg, I.S.; Bolland, O. Optimized process configurations of post-combustion CO2 capture for natural-gas-fired power plant-Power plant efficiency analysis. Int. J. Greenh. Gas Control 2012, 8, 1–11. [Google Scholar] [CrossRef]
- Arachchige, U.S.P.R.; Kawan, D.; Tokheim, L.-A.; Melaaen, M.C. Model Development for CO2 Capture in the Cement Industry. Int. J. Model. Optim. 2013, 3, 535–540. [Google Scholar] [CrossRef]
- Ntiamoah, A.; Ling, J.; Xiao, P.; Webley, P.A.; Zhai, Y. CO2 Capture by Temperature Swing Adsorption: Use of Hot CO2 -Rich Gas for Regeneration. Ind. Eng. Chem. Res. 2016, 55, 703–713. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Shen, W.; Kong, X.; Li, P.; Yu, J.; Rodrigues, A.E. Experimental evaluation of adsorption technology for CO2 capture from flue gas in an existing coal-fired power plant. Chem. Eng. Sci. 2013, 101, 615–619. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Xu, G.; Zhang, K.; Zhang, D. CO2 capture by chemical absorption in coal-fired power plants: Energy-saving mechanism, proposed methods, and performance analysis. Int. J. Greenh. Gas Control 2015, 39, 449–462. [Google Scholar] [CrossRef]
- Duan, L.; Zhao, M.; Yang, Y. Integration and optimization study on the coal-fired power plant with CO2 capture using MEA. Energy 2012, 45, 107–116. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, J.; Xu, L.; Zhang, Q. The CO2 emission reduction path towards carbon neutrality in the Chinese steel industry: A review. Environ. Impact Assess. Rev. 2023, 99, 107017. [Google Scholar] [CrossRef]
- Bouckaert, S.; Pales, A.F.; McGlade, C.; Remme, U.; Wanner, B.; Varro, L.; D’Ambrosio, D.; Spencer, T. Net Zero by 2050 A Roadmap for the Global Energy Sector; OECD Publishing: Boulogne-Billancourt, France; Paris, France, 2021. [Google Scholar]
- Tan, C.; Yu, X.; Guan, Y. A technology-driven pathway to net-zero carbon emissions for China’s cement industry. Appl. Energy 2022, 325, 119804. [Google Scholar] [CrossRef]
- Amran, M.; Makul, N.; Fediuk, R.; Lee, Y.H.; Vatin, N.I.; Lee, Y.Y.; Mohammed, K. Global carbon recoverability experiences from the cement industry. Case Stud. Constr. Mater. 2022, 17. [Google Scholar] [CrossRef]
- Selosse, S.; Ricci, O. Achieving negative emissions with BECCS (bioenergy with carbon capture and storage) in the power sector: New insights from the TIAM-FR (TIMES Integrated Assessment Model France) model. Energy 2014, 76, 967–975. [Google Scholar] [CrossRef]
- Pires, J.C.M. Negative emissions technologies: A complementary solution for climate change mitigation. Sci. Total Environ. 2019, 672, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Koytsoumpa, E.I.; Magiri, D.; Karellas, S.; Kakaras, E. Bioenergy with carbon capture and utilization: A review on the potential deployment towards a European circular bioeconomy. Renew. Sustain. Energy Rev. 2021, 152, 111641. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Large scale application of carbon capture to process industries—A review. J. Clean. Prod. 2022, 362. [Google Scholar] [CrossRef]
- Holz, F.; Scherwath, T.; del Granado, P.C.; Skar, C.; Olmos, L.; Ploussard, Q.; Ramos, A.; Herbest, A. A 2050 perspective on the role for carbon capture and storage in the European power system and industry sector. Energy Econ. 2021, 104. [Google Scholar] [CrossRef]
- van Straelen, J.; Geuzebroek, F.; Goodchild, N.; Protopapas, G.; Mahony, L. CO2 capture for refineries, a practical approach. Int. J. Greenh. Gas Control 2010, 4, 316–320. [Google Scholar] [CrossRef]
- Kuramochi, T.; Ramírez, A.; Turkenburg, W.; Faaij, A. Comparative assessment of CO2 capture technologies for carbon-intensive industrial processes. Prog. Energy Combust. Sci. 2012, 38, 87–112. [Google Scholar] [CrossRef]
- Fennell, A.; Davis, P.S.; Mohammed, S.J. Decarbonizing cement production. Joule 2021, 5, 1305–1311. [Google Scholar] [CrossRef]
- Farfan, J.; Fasihi, M.; Breyer, C. Trends in the global cement industry and opportunities for long-term sustainable CCU potential for Power-to-X. J. Clean. Prod. 2019, 217, 821–835. [Google Scholar] [CrossRef]
- Rad, E.A.; Mohammadi, S. Energetic and exergetic optimized Rankine cycle for waste heat recovery in a cement factory. Appl. Therm. Eng. 2018, 132, 410–422. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Hashim, H. A novel design for green and economical cement manufacturing. J. Clean. Prod. 2012, 22, 60–66. [Google Scholar] [CrossRef]
- Oss, H.G.; Padovani, A.C. Cement Manufacture and the Environment Part II: Environmental Challenges and Opportunities. J. Ind. Ecol. 2003, 7, 93–126. [Google Scholar] [CrossRef]
- Skagestad, R.; Normann, F.; Garðarsdóttir, S.Ó.; Sundqvist, M.; Anheden, M.; Eldrup, N.H.; Ali, H.; Haugen, H.A.; Mathisen, A. CO2stCap-Cutting Cost of CO2 Capture in Process Industry. Energy Procedia 2017, 114, 6303–6315. [Google Scholar] [CrossRef]
- Garðarsdóttir, S.Ó.; Normann, F.; Skagestad, R.; Johnsson, F. Investment costs and CO2 reduction potential of carbon capture from industrial plants–A Swedish case study. Int. J. Greenh. Gas Control 2018, 76, 111–124. [Google Scholar] [CrossRef]
- Hägg, M.-B.; Lindbråthen, A.; He, X.; Nodeland, S.G.; Cantero, T. Pilot Demonstration-reporting on CO2 Capture from a Cement Plant Using Hollow Fiber Process. Energy Procedia 2017, 114, 6150–6165. [Google Scholar] [CrossRef]
- Quader, M.A.; Ahmed, S.; Ghazilla, R.A.R.; Ahmed, S.; Dahari, M. A comprehensive review on energy efficient CO2 breakthrough technologies for sustainable green iron and steel manufacturing. Renew. Sustain. Energy Rev. 2015, 50, 594–614. [Google Scholar] [CrossRef]
- Gielen, D. CO2 removal in the iron and steel industry. Energy Convers. Manag. 2003, 44, 1027–1037. [Google Scholar] [CrossRef]
- Bains, P.; Psarras, P.; Wilcox, J. CO2 capture from the industry sector. Prog. Energy Combust. Sci. 2017, 63, 146–172. [Google Scholar] [CrossRef]
- Farahani, S.; Worrell, E.; Bryntse, G. CO2-free paper? Resour. Conserv. Recycl. 2004, 42, 317–336. [Google Scholar] [CrossRef]
- Onarheim, K.; Santos, S.; Kangas, P.; Hankalin, V. Performance and costs of CCS in the pulp and paper industry part 1: Performance of amine-based post-combustion CO2 capture. Int. J. Greenh. Gas Control 2017, 59, 58–73. [Google Scholar] [CrossRef]
- Svensson, E.; Wiertzema, H.; Harvey, S. Potential for Negative Emissions by Carbon Capture and Storage From a Novel Electric Plasma Calcination Process for Pulp and Paper Mills. Front. Clim. 2021, 3. [Google Scholar] [CrossRef]
- Santos, M.P.S.; Manovic, V.; Hanak, D.P. Unlocking the potential of pulp and paper industry to achieve carbon-negative emissions via calcium looping retrofit. J. Clean. Prod. 2021, 280, 124431. [Google Scholar] [CrossRef]
- Kong, L.; Hasanbeigi, A.; Price, L. Emerging Energy-Efficiency and Greenhouse Gas Mitigation Technologies for the Pulp and Paper Industry; USDOE Office of Science: Berkeley, CA, USA, 2012. [Google Scholar] [CrossRef]
- Roussanaly, S.; Anantharaman, R.; Lindqvist, K.; Zhai, H.; Rubin, E. Membrane properties required for post-combustion CO2 capture at coal-fired power plants. J. Membr. Sci. 2016, 511, 250–264. [Google Scholar] [CrossRef]
- Strube, R.; Manfrida, G. CO2 capture in coal-fired power plants—Impact on plant performance. Int. J. Greenh. Gas Control 2011, 5, 710–726. [Google Scholar] [CrossRef]
- Lawal, A.; Wang, M.; Stephenson, P.; Yeung, H. Dynamic modelling of CO2 absorption for post combustion capture in coal-fired power plants. Fuel 2009, 88, 2455–2462. [Google Scholar] [CrossRef]
- Hu, G.; Li, X.; Liu, X.; Hu, J.; Otitoju, O.; Wang, M.; Du, W.; Ye, Z.; Long, J.; Qian, F. Techno-economic evaluation of post-combustion carbon capture based on chemical absorption for the thermal cracking furnace in ethylene manufacturing. Fuel 2023, 331, 125604. [Google Scholar] [CrossRef]
- Shamsi, M.; Naeiji, E.; Rooeentan, S.; Shahandashty, B.F.; Namegoshayfard, P.; Bonyadi, M. Proposal and investigation of CO2 capture from fired heater flue gases to increase methanol production: A case study. Energy 2023, 274, 127375. [Google Scholar] [CrossRef]
- Aspelund, A.; Tveit, S.P.; Gundersen, T. A liquefied energy chain for transport and utilization of natural gas for power production with CO2 capture and storage–Part 3: The combined carrier and onshore storage. Appl. Energy 2009, 86, 805–814. [Google Scholar] [CrossRef]
- Suzuki, T.; Toriumi, M.; Sakemi, T.; Masui, N.; Yano, S.; Fujita, H.; Furukawa, H. Conceptual Design of CO2 Transportation System for CCS. Energy Procedia 2013, 37, 2989–2996. [Google Scholar] [CrossRef]
- Wang, J.; Ryan, D.; Anthony, E.J.; Wildgust, N.; Aiken, T. Effects of impurities on CO2 transport, injection and storage. Energy Procedia 2011, 4, 3071–3078. [Google Scholar] [CrossRef]
- Simonsen, K.R.; Hansen, D.S.; Pedersen, S. Challenges in CO2 transportation: Trends and perspectives. Renew. Sustain. Energy Rev. 2024, 191, 114149. [Google Scholar] [CrossRef]
- Lewicki, J.L.; Birkholzer, J.; Tsang, C.-F. Natural and industrial analogues for leakage of CO2 from storage reservoirs: Identification of features, events, and processes and lessons learned. Environ. Geol. 2007, 52, 457–467. [Google Scholar] [CrossRef]
- Onyebuchi, V.E.; Kolios, A.; Hanak, D.P.; Biliyok, C.; Manovic, V. A systematic review of key challenges of CO2 transport via pipelines. Renew. Sustain. Energy Rev. 2018, 81, 2563–2583. [Google Scholar] [CrossRef]
- Carter, L.D. Capture and Storage of CO2 with Other Air Pollutants; IEA Clean Coal Centre: London, UK, 2010. [Google Scholar]
- Steyn, A.M.; Oglesby, J.; Turan, G.; Zapantis, R.G. Global Status of CCS 2022; Global CCS Institute: Sydney, Australia, 2022; Available online: https://www.globalccsinstitute.com/resources/global-status-of-ccs-2022/ (accessed on 14 October 2024).
- Uilenreef, J.; Kombrink, M. Flow Assurance & Control Philosophy ROAD Special Report for the Global Carbon Capture and Storage Institute; ROAD Maasvlakte CCS Project CV: Schiedam, The Netherlands, 2013. [Google Scholar]
- Stang, H.G.J.; Løvseth, S.W.; Størset, S.Ø.; Malvik, B.; Rekstad, H. Accurate Measurements of CO2 Rich Mixture Phase Equilibria Relevant for CCS Transport and Conditioning. Energy Procedia 2013, 37, 2897–2903. [Google Scholar] [CrossRef]
- Global CCS. Technical Guidance on Hazard Analysis for Onshore Carbon Capture Installations and Onshore Pipelines, 1st ed.; Energy Institute: London, UK, 2010. [Google Scholar]
- Aspelund, A.; Jordal, K. Gas conditioning—The interface between CO2 capture and transport. Int. J. Greenh. Gas Control 2007, 1, 343–354. [Google Scholar] [CrossRef]
- Demofonti, G.; Di Biagio, M.; Fonzo, A.; Lucci, A.; Spinelli, C.M. Definition of Requirements for Safe and Reliable CO2 Transportation Network Through an Integrated Laboratory, Computer Modelling and Full Scale Methodology. In Proceedings of the ISOPE International Ocean and Polar Engineering Conference, Anchorage, AK, USA, 30 June–5 July 2013; ISOPE: Mountain View, CA, USA, 2013. [Google Scholar]
- Clausen, S.; Munkejord, S.T. Depressurization of CO2—A Numerical Benchmark Study. Energy Procedia 2012, 23, 266–273. [Google Scholar] [CrossRef]
- Brown, S.; Beck, J.; Mahgerefteh, H.; Fraga, E.S. Global sensitivity analysis of the impact of impurities on CO2 pipeline failure. Reliab. Eng. Syst. Saf. 2013, 115, 43–54. [Google Scholar] [CrossRef]
- Thomas, D.C.; Benson, S.M. (Eds.) Carbon Dioxide Capture for Storage in Deep Geologic Formations-Results from the CO2 Capture Project; Vol 1-capture and separation of carbon dioxide from combustion; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Roussanaly, E.S.; Brunsvold, S.; Hognes, A.L. Benchmarking of CO2 transport technologies: Part II–Offshore pipeline and shipping to an offshore site. Int. J. Greenh. Gas Control 2014, 28, 283–299. [Google Scholar] [CrossRef]
- Roussanaly, S.; Jakobsen, J.P.; Hognes, E.H.; Brunsvold, A.L. Benchmarking of CO2 transport technologies: Part I—Onshore pipeline and shipping between two onshore areas. Int. J. Greenh. Gas Control 2013, 19, 584–594. [Google Scholar] [CrossRef]
- Bjerketvedt, V.S.; Tomasgard, A.; Roussanaly, S. Optimal design and cost of ship-based CO2 transport under uncertainties and fluctuations. Int. J. Greenh. Gas Control 2020, 103, 103190. [Google Scholar] [CrossRef]
- Aspelund, A.; Mølnvik, M.J.; De Koeijer, G. Ship transport of CO2: Technical solutions and analysis of costs, energy utilization, exergy efficiency and CO2 emissions. Chem. Eng. Res. Des. 2006, 84(9), 847–855. [Google Scholar] [CrossRef]
- Jakobsen, J.; Roussanaly, S.; Anantharaman, R. A techno-economic case study of CO2 capture, transport and storage chain from a cement plant in Norway. J. Clean. Prod. 2017, 144, 523–539. [Google Scholar] [CrossRef]
- Roussanaly, S.; Bureau-Cauchois, G.; Husebye, J. Costs benchmark of CO2 transport technologies for a group of various size industries. Int. J. Greenh. Gas Control 2013, 12, 341–350. [Google Scholar] [CrossRef]
- Knoope, M.M.J.; Ramírez, A.; Faaij, A.P.C. The influence of uncertainty in the development of a CO2 infrastructure network. Appl. Energy 2015, 158, 332–347. [Google Scholar] [CrossRef]
- Weihs, G.A.F.; Kumar, K.; Wiley, D.E. Understanding the Economic Feasibility of Ship Transport of CO2 within the CCS Chain. Energy Procedia 2014, 63, 2630–2637. [Google Scholar] [CrossRef]
- Lu, H.; Ma, X.; Huang, K.; Fu, L.; Azimi, M. Carbon dioxide transport via pipelines: A systematic review. J. Clean. Prod. 2020, 266, 121994. [Google Scholar] [CrossRef]
- Wang, Z.; Weihs, G.A.F.; Cardenas, G.I.; Wiley, D.E. Optimal pipeline design for CCS projects with anticipated increasing CO2 flow rates. Int. J. Greenh. Gas Control 2014, 31, 165–174. [Google Scholar] [CrossRef]
- Awoyomi, A.; Patchigolla, K.; Anthony, E.J. CO2/SO2 emission reduction in CO2 shipping infrastructure. Int. J. Greenh. Gas Control 2019, 88, 57–70. [Google Scholar]
- White, E.; Allam, V.; Miller, R. Purification of oxyfuel-derived CO2 for sequestration or EOR. In Proceedings of the 8th International Conference on Greenhouse Gas Control Technologies, Trondheim, Norway, 19–22 June 2006; pp. 19–22. [Google Scholar]
- Abbas, Z.; Mezher, T.; Abu-Zahra, M.R.M. Evaluation of CO2 Purification Requirements and the Selection of Processes for Impurities Deep Removal from the CO2 Product Stream. Energy Procedia 2013, 37, 2389–2396. [Google Scholar] [CrossRef]
- Dugstad, A.; Morland, B.; Clausen, S. Corrosion of transport pipelines for CO2–Effect of water ingress. Energy Procedia 2011, 4, 3063–3070. [Google Scholar] [CrossRef]
- Wetenhall, B.; Race, J.M.; Downie, M.J. The Effect of CO2 Purity on the Development of Pipeline Networks for Carbon Capture and Storage Schemes. Int. J. Greenh. Gas Control 2014, 30, 197–211. [Google Scholar] [CrossRef]
- Martynov, S.B.; Daud, N.K.; Mahgerefteh, H.; Brown, S.; Porter, R.T.J. Impact of stream impurities on compressor power requirements for CO2 pipeline transportation. Int. J. Greenh. Gas Control 2016, 54, 652–661. [Google Scholar] [CrossRef]
- Skaugen, G.; Roussanaly, S.; Jakobsen, J.; Brunsvold, A. Techno-economic evaluation of the effects of impurities on conditioning and transport of CO2 by pipeline. Int. J. Greenh. Gas Control 2016, 54, 627–639. [Google Scholar] [CrossRef]
- Vitali, M.; Corvaro, F.; Marchetti, B.; Terenzi, A. Thermodynamic challenges for CO2 pipelines design: A critical review on the effects of impurities, water content, and low temperature. Int. J. Greenh. Gas Control 2022, 114, 103605. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wang, G.X.; Massarotto, P.; Rudolph, V. Optimization of pipeline transport for CO2 sequestration. Energy Convers. Manag. 2006, 47, 702–715. [Google Scholar] [CrossRef]
- Patchigolla, K.; Oakey, J.E. Design Overview of High Pressure Dense Phase CO2 Pipeline Transport in Flow Mode. Energy Procedia 2013, 37, 3123–3130. [Google Scholar] [CrossRef]
- Kang, K.; Huh, C.; Kang, S.-G.; Baek, J.-H.; Noh, H.J. Estimation of CO2 Pipeline Transport Cost in South Korea Based on the Scenarios. Energy Procedia 2014, 63, 2475–2480. [Google Scholar] [CrossRef]
- Skovholt, O. CO2 transportation system. Energy Convers. Manag. 1993, 34, 1095–1103. [Google Scholar] [CrossRef]
- Vandeginste, V.; Piessens, K. Pipeline design for a least-cost router application for CO2 transport in the CO2 sequestration cycle. Int. J. Greenh. Gas Control 2008, 2, 571–581. [Google Scholar] [CrossRef]
- Gao, L.; Fang, M.; Li, H.; Hetland, J. Cost analysis of CO2 transportation: Case study in China. Energy Procedia 2011, 4, 5974–5981. [Google Scholar] [CrossRef]
- Buit, F.; Ahmad, L.; Mallon, M.; Hage, W. CO2 EuroPipe study of the occurrence of free water in dense phase CO2 transport. Energy Procedia 2011, 3056–3062. [Google Scholar] [CrossRef]
- Forbes, S.M.; Verma, P.; Curry, T.E.; Friedmann, S.J.; Wade, S.M. Guidelines for Carbon Dioxide Capture, Transport, and Storage; World Resource Sinstitute: Washington, DC, USA, 2008. [Google Scholar]
- Munkejord, S.T.; Hammer, M.; Løvseth, S.W. CO2 transport: Data and models—A review. Appl. Energy 2016, 169, 499–523. [Google Scholar] [CrossRef]
- Turkenburg, W.C. Sustainable development, climate change, and carbon dioxide removal (CDR). Energy Convers. Manag. 1997, 38, S3–S12. [Google Scholar] [CrossRef]
- Freund, P. Making deep reductions in CO2 emissions from coal-fired power plant using capture and storage of CO2. Proc. Inst. Mech. Eng. Part A J. Power Energy 2003, 217, 1–7. [Google Scholar] [CrossRef]
- Decarre, S.; Berthiaud, J.; Butin, N.; Guillaume-Combecave, J.-L. CO2 maritime transportation. Int. J. Greenh. Gas Control 2010, 4, 857–864. [Google Scholar] [CrossRef]
- Kjärstad, J.; Skagestad, R.; Eldrup, N.H.; Johnsson, F. Ship transport—A low cost and low risk CO2 transport option in the Nordic countries. Int. J. Greenh. Gas Control 2016, 54, 168–184. [Google Scholar] [CrossRef]
- Roussanaly, S.; Deng, H.; Skaugen, G.; Gundersen, T. At what Pressure Shall CO2 Be Transported by Ship? An in-Depth Cost Comparison of 7 and 15 Barg Shipping. Energies 2021, 14, 5635. [Google Scholar] [CrossRef]
- Seo, Y.; Huh, C.; Lee, S.; Chang, D. Comparison of CO2 liquefaction pressures for ship-based carbon capture and storage (CCS) chain. Int. J. Greenh. Gas Control 2016, 52, 1–12. [Google Scholar] [CrossRef]
- Kokubun, N.; Ko, K.; Ozaki, M. Cargo Conditions of CO2 in Shuttle Transport by Ship. Energy Procedia 2013, 37, 3160–3167. [Google Scholar] [CrossRef]
- Ozotta, F.; Ostadhassan, O.; Liu, M.; Liu, K.; Kolawole, B.; Hadavimoghaddam, O. Reassessment of CO2 sequestration in tight reservoirs and associated formations. J. Pet. Sci. Eng. 2021, 206. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Wang, F.; Sun, H.; Wang, H.; Yao, Z. A state-of-the-art review of CO2 enhanced oil recovery as a promising technology to achieve carbon neutrality in China. Environ. Res. 2022, 210, 112986. [Google Scholar] [CrossRef]
- Farhat, K.; Brandt, A.; Benson, S.M. CO2 Interim storage: Technical characteristics and potential role in CO2 market development. Energy Procedia 2011, 4, 2628–2636. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rezaee, R.; Bing, C.H.; Nagarajan, R.; Hamid, M.A. CO2 storage in depleted gas reservoirs: A study on the effect of residual gas saturation. Petroleum 2018, 4, 95–107. [Google Scholar] [CrossRef]
- Li, Z.; Dong, M.; Li, S.; Huang, S. CO2 sequestration in depleted oil and gas reservoirs—Caprock characterization and storage capacity. Energy Convers. Manag. 2006, 47, 1372–1382. [Google Scholar] [CrossRef]
- Hamouda, A.; Chughtai, S. Miscible CO2 Flooding for EOR in the Presence of Natural Gas Components in Displacing and Displaced Fluids. Energies 2018, 11, 391. [Google Scholar] [CrossRef]
- Nordbotten, J.M.; Celia, M.A.; Bachu, S. Injection and storage of CO2 in deep saline aquifers: Analytical solution for CO2 plume evolution during injection. Transp. Porous Media 2005, 58, 339–360. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.-S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Shi, J.Q.; Durucan, S. CO2 Storage in Deep Unminable Coal Seams. Oil Gas Sci. Technol. 2005, 60, 547–558. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Y.; Ye, L.; Chen, Z.; Yuan, L.; Chen, N.; Yang, Y. Experimental Investigation of Supercritical CO2–Rock–Water Interactions in a Tight Formation with the Pore Scale during CO2–EOR and Sequestration. ACS omega 2022, 7, 27291–27299. [Google Scholar] [CrossRef]
- Müller, N. Supercritical CO2-Brine Relative Permeability Experiments in Reservoir Rocks—Literature Review and Recommendations. Transp. Porous Media 2011, 87, 367–383. [Google Scholar] [CrossRef]
- Yin, G.; Zhou, H.; Xian, J.; Jiang, X.; Lu, Y.; Tan, Z.; Liu, J. Experimental study of the effects of sub-and super-critical CO2 saturation on the mechanical characteristics of organic-rich shales. Energy 2017, 132, 84–95. [Google Scholar] [CrossRef]
- Ott, H.; de Kloe, K.; Van Bakel, M.; Vos, F.; Van Pelt, A.; Legerstee, P.; Bauer, A.; Eide, K.; van der Linden, A.; Berg, S.; et al. Core-flood experiment for transport of reactive fluids in rocks. Rev. Sci. Instrum. 2012, 83. [Google Scholar] [CrossRef] [PubMed]
- Ojala, I.O. The effect of CO2 on the mechanical properties of reservoir and cap rock. Energy Procedia 2011, 4, 5392–5397. [Google Scholar] [CrossRef]
- Major, J.R.; Eichhubl, P.; Dewers, T.A.; Olson, J.E. Effect of CO2–brine–rock interaction on fracture mechanical properties of CO2 reservoirs and seals. Earth Planet. Sci. Lett. 2018, 499, 37–47. [Google Scholar] [CrossRef]
- Zhang, S.; Xian, X.; Zhou, J.; Zhang, L. Mechanical behaviour of Longmaxi black shale saturated with different fluids: An experimental study. RSC Adv. 2017, 7, 42946–42955. [Google Scholar] [CrossRef]
- Sorensen, J.A.; Braunberger, J.R.; Liu, G.; Smith, S.A.; Klenner, R.C.; Steadman, E.N.; Harju, J.A. CO2 Storage and Utilization in Tight Hydrocarbon-bearing Formations: A Case Study of the Bakken Formation in the Williston Basin. Energy Procedia 2014, 63, 7852–7860. [Google Scholar] [CrossRef]
- Akai, T.; Saito, N.; Hiyama, M.; Okabe, H. Numerical Modelling on CO2 Storage Capacity in Depleted Gas Reservoirs. Energies 2021, 14, 3978. [Google Scholar] [CrossRef]
- Peres, C.B.; Resende, P.M.R.; Nunes, L.J.R.; Morais, L.C.D. Advances in Carbon Capture and Use (CCU) Technologies: A Comprehensive Review and CO2 Mitigation Potential Analysis. Clean Technol. 2022, 4, 1193–1207. [Google Scholar] [CrossRef]
- Ramirez, A.; Sarathy, S.M.; Gascon, J. CO2 Derived E-Fuels: Research Trends, Misconceptions, and Future Directions. Trends Chem. 2020, 2, 785–795. [Google Scholar] [CrossRef]
- Rothbart, M. e-Fuel Production via Renewables and the Impact on the In-Use CO2 Performance; SAE: Warrendale, PA, USA, 2020. [Google Scholar] [CrossRef]
- Meunier, N.; Chauvy, R.; Mouhoubi, S.; Thomas, D.; De Weireld, G. Alternative production of methanol from industrial CO2. Renew. Energy 2020, 146, 1192–1203. [Google Scholar] [CrossRef]
- Kim, C.; Yoo, C.-J.; Oh, H.-S.; Min, B.K.; Lee, U. Review of carbon dioxide utilization technologies and their potential for industrial application. J. CO2 Util. 2022, 65, 102239. [Google Scholar] [CrossRef]
- John, J.M.; Alwi, S.R.W.; Omoregbe, D.I. Techno-economic analysis of carbon dioxide capture and utilisation analysis for an industrial site with fuel cell integration. J. Clean. Prod. 2021, 281, 124920. [Google Scholar] [CrossRef]
- Devkota, S.; Pokhrel, R.; Rayamajhi, B.; Uprety, B. Design and cost estimation of a CO2 capture plant from cement flue gas for urea production in Nepal. Int. J. Greenh. Gas Control 2021, 111, 103484. [Google Scholar] [CrossRef]
- Chai, S.Y.W.; Ngu, L.H.; How, B.S.; Chin, M.Y.; Abdouka, K.; Adini, M.J.B.A.; Kassim, A.M. Review of CO2 capture in construction-related industry and their utilization. Int. J. Greenh. Gas Control 2022, 119, 103727. [Google Scholar] [CrossRef]
- Woodall, C.M.; McQueen, N.; Pilorgé, H.; Wilcox, J. Utilization of mineral carbonation products: Current state and potential. Greenh. Gases Sci. Technol. 2019, 9, 1096–1113. [Google Scholar] [CrossRef]
- Li, N.; Mo, L.; Unluer, C. Emerging CO2 utilization technologies for construction materials: A review. J. CO2 Util. 2022, 65, 102237. [Google Scholar] [CrossRef]
- Bonfim-Rocha, L.; Silva, A.B.; de Faria, S.H.B.; Vieira, M.F.; de Souza, M. Production of Sodium Bicarbonate from CO2 Reuse Processes: A Brief Review. Int. J. Chem. React. Eng. 2019. [Google Scholar] [CrossRef]
- Rezk, M.G.; Foroozesh, J. Effect of CO2 mass transfer on rate of oil properties changes: Application to CO2-EOR projects. J. Pet. Sci. Eng. 2019, 180, 298–309. [Google Scholar] [CrossRef]
- Alfarge, D.; Wei, M.; Bai, B. Data analysis for CO2-EOR in shale-oil reservoirs based on a laboratory database. J. Pet. Sci. Eng. 2018, 162, 697–711. [Google Scholar] [CrossRef]
- Bayat, A.E.; Junin, R.; Hejri, S.; Fazeli, A.; Afsari, K. Application of CO2-based vapor extraction process for high pressure and temperature heavy oil reservoirs. J. Pet. Sci. Eng. 2015, 135, 280–290. [Google Scholar] [CrossRef]
- Mansour, M.; Al-Sabagh, E.; Desouky, A.; Zawawy, S.; Ramzi, F. Experimental approach of minimum miscibility pressure for CO2 miscible flooding: Application to Egyptian oil fields. Int. J. New Technol. Res. 2016, 2, 105–112. [Google Scholar]
- Pérez-Fortes, M.; Bocin-Dumitriu, A.; Tzimas, E. CO2 Utilization Pathways: Techno-Economic Assessment and Market Opportunities. Energy Procedia 2014, 63, 7968–7975. [Google Scholar] [CrossRef]
- Cui, X.; Kær, S.K.; Nielsen, M.P. Energy analysis and surrogate modeling for the green methanol production under dynamic operating conditions. Fuel 2022, 307, 121924. [Google Scholar] [CrossRef]
- Muslim, A.; Ardy, S.; Syaubari, S. Response Surface Methodology-Based Model and Optimization of CO2 Absorption Using Methyldiethanolamine Activated by Piperazine. Int. Rev. Model. Simul. 2017, 10, 296. [Google Scholar] [CrossRef]
- AlDhaheri, Z.; Ajish, S.A.; Rahman, S.; Owda, L.; AlNowahi, S.; Chaalal, O. A Novel Global Warming Solution: Use of Flue Gas to Produce Urea. MATEC Web Conf. 2018, 187, 03003. [Google Scholar] [CrossRef][Green Version]
- Di Marcoberardino, G.; Invernizzi, C.M.; Iora, P.; Ayub, A.; Di Bona, D.; Chiesa, P.; Binotti, M.; Manzolini, G. Experimental and analytical procedure for the characterization of innovative working fluids for power plants applications. Appl. Therm. Eng. 2020, 178, 115513. [Google Scholar] [CrossRef]
- AspenOne, Version 12.1; AspenTech: Bedford, MA, USA, 2023.
- Pro/II, Version 2024; AVEVA PRO/II Simulation: Cambridge, UK, 2024.
- ProSimPlus, Version 3.7.8; Fives ProSim: Labège, France, 2023.
- Lin, H.; Lu, J.; Abed, A.M.; Nag, K.; Fayed, M.; Deifalla, A.; Bin Mahfuz, A.S.; Galal, A.M. Simulation of CO2 capture from natural gas by cyclic pressure swing adsorption process using activated carbon. Chemosphere 2023, 329, 138583. [Google Scholar] [CrossRef]
- Asadi, J.; Kazempoor, P. Techno-economic analysis of membrane-based processes for flexible CO2 capturing from power plants. Energy Convers. Manag. 2021, 246, 114633. [Google Scholar] [CrossRef]
- Ahmad, F.; Lau, K.K.; Shariff, A.M.; Murshid, G. Process simulation and optimal design of membrane separation system for CO2 capture from natural gas. Comput. Chem. Eng. 2012, 36, 119–128. [Google Scholar] [CrossRef]
- Miandoab, E.S.; Scholes, C.A. A Rigorous Membrane Gas-Solvent Contactor Model for Flowsheet Simulation of the Carbon Capture Process. Ind. Eng. Chem. Res. 2022, 61, 9381–9393. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, H.; Lee, K.S.; Won, W. A new modeling approach for a CO2 capture process based on a blended amine solvent. J. Nat. Gas Sci. Eng. 2019, 61, 206–214. [Google Scholar] [CrossRef]
- Xue, B.; Yu, Y.; Chen, J.; Luo, X.; Wang, M. A comparative study of MEA and DEA for post-combustion CO2 capture with different process configurations. Int. J. Coal Sci. Technol. 2017, 4, 15–24. [Google Scholar] [CrossRef]
- Kvamsdal, H.M.; Jordal, K.; Bolland, O. A quantitative comparison of gas turbine cycles with CO2 capture. Energy 2007, 32, 10–24. [Google Scholar] [CrossRef]
- Li, K.; Cousins, A.; Yu, H.; Feron, P.; Tade, M.; Luo, W.; Chen, J. Systematic study of aqueous monoethanolamine-based CO2 capture process: Model development and process improvement. Energy Sci. Eng. 2016, 4, 23–39. [Google Scholar] [CrossRef]
- Deschamps, T.; Kanniche, M.; Grandjean, L.; Authier, O. Modeling of Vacuum Temperature Swing Adsorption for Direct Air Capture Using Aspen Adsorption. Clean Technol. 2022, 4, 258–275. [Google Scholar] [CrossRef]
- Marchese, M.; Buffo, G.; Santarelli, M.; Lanzini, A. CO2 from direct air capture as carbon feedstock for Fischer-Tropsch chemicals and fuels: Energy and economic analysis. J. CO2 Util. 2021, 46, 101487. [Google Scholar] [CrossRef]
- Anselmi, H.; Mirgaux, O.; Bounaceur, R.; Patisson, F. Simulation of Post-Combustion CO2 Capture, a Comparison among Absorption, Adsorption and Membranes. Chem. Eng. Technol. 2019, 42, 797–804. [Google Scholar] [CrossRef]
- Sherman, S.R.; Gray, J.R.; Brinkman, K.S.; Huang, K. Combustion-assisted CO2 capture using MECC membranes. J. Membr. Sci. 2012, 401–402, 323–332. [Google Scholar] [CrossRef]
- Lim, Y.; Kim, J.; Jung, J.; Lee, C.S.; Han, C. Modeling and Simulation of CO2 Capture Process for Coal- based Power Plant Using Amine Solvent in South Korea. Energy Procedia 2013, 37, 1855–1862. [Google Scholar] [CrossRef]
- Øi, L.E.; Bråthen, T.; Berg, C.; Brekne, S.K.; Flatin, M.; Johnsen, R.; Moen, I.G.; Thomassen, E. Optimization of Configurations for Amine based CO2 Absorption Using Aspen HYSYS. Energy Procedia 2014, 51, 224–233. [Google Scholar] [CrossRef]
- Jensen, M.J.; Russell, C.S.; Bergeson, D.; Hoeger, C.D.; Frankman, D.J.; Bence, C.S.; Baxter, L.L. Prediction and validation of external cooling loop cryogenic carbon capture (CCC-ECL) for full-scale coal-fired power plant retrofit. Int. J. Greenh. Gas Control 2015, 42, 200–212. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A.; Gamba, S. Simulation of CO2 Capture by MEA Scrubbing with a Rate-Based Model. Procedia Eng. 2012, 42, 1651–1661. [Google Scholar] [CrossRef]
- Plaza, J.M.; Van Wagener, D.; Rochelle, G.T. Modeling CO2 capture with aqueous monoethanolamine. Energy Procedia 2009, 1, 1171–1178. [Google Scholar] [CrossRef]
- Garcia, M.; Knuutila, H.K.; Gu, S. ASPEN PLUS simulation model for CO2 removal with MEA: Validation of desorption model with experimental data. J. Environ. Chem. Eng. 2017, 5, 4693–4701. [Google Scholar] [CrossRef]
- Escudero, A.I.; Espatolero, S.; Romeo, L.M.; Lara, Y.; Paufique, C.; Lesort, A.L.; Liszka, M. Minimization of CO2 capture energy penalty in second generation oxy-fuel power plants. Appl. Therm. Eng. 2016, 103, 274–281. [Google Scholar] [CrossRef]
- Cabral, R.P.; Heldebrant, D.J.; Dowell, N.M. A Techno-Economic Analysis of a Novel Solvent-Based Oxycombustion CO2 Capture Process. Ind. Eng. Chem. Res. 2019, 58, 6604–6612. [Google Scholar] [CrossRef]
- Lozano, E.M.; Petersen, S.B.; Paulsen, M.M.; Rosendahl, L.A.; Pedersen, T.H. Techno-economic evaluation of carbon capture via physical absorption from HTL gas phase derived from woody biomass and sewage sludge. Energy Convers. Manag. X 2021, 11, 100089. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Moya, C.; Gazzani, M.; Palomar, J. Direct air capture based on ionic liquids: From molecular design to process assessment. Chem. Eng. J. 2023, 468, 143630. [Google Scholar] [CrossRef]
- Asgharian, H.; Marques, D.L.; Iov, F.; Liso, V.; Nielsen, M.P.; Thellufsen, J.Z.; Lund, H. The role of cryogenic carbon capture in future carbon-neutral societies. Int. J. Greenh. Gas Control 2024, 135, 104161. [Google Scholar] [CrossRef]
- Asgharian, H.; Baxter, L.; Iov, F.; Cui, X.; Araya, S.S.; Nielsen, M.P.; Liso, V. Techno-economic analysis of blue ammonia synthesis using cryogenic CO2 capture Process-A Danish case investigation. Int. J. Hydrogen Energy 2024, 69, 608–618. [Google Scholar] [CrossRef]
- Asgharian, H.; Iov, F.; Nielsen, M.P.; Liso, V.; Burt, S.; Baxter, L. Analysis of cryogenic CO2 capture technology integrated with Water-Ammonia Absorption refrigeration cycle for CO2 capture and separation in cement plants. Sep. Purif. Technol. 2024, 353, 128419. [Google Scholar] [CrossRef]














| Process | Solvent | Operation Conditions | Advantages | Disadvantages |
|---|---|---|---|---|
| Selexol | Dimethyl ether of polyethylene glycol | 3.6 MPa, 35 °C | High H2S selectivity; Capable of the simultaneous removal of H2S/CO2 and targeted H2S removal; Possessing high chemical and thermal stability with minimal loss of solvent; Low initial investment and operational costs | High solvent viscosity at low temperatures, lowering the mass transfer rate; Exclusively applicable for CO2 removal if the CO2 concentration is higher than H2S |
| Rectisol | Methanol | 3.6 MPa, −25 °C | Simultaneous removal of H2S/CO2, and good removal efficiency (<0.1 ppm); Reasonable solvent viscosity; Chemically and thermally stable, with minimal solvent loss or degradation; More energy-efficient than amine-based processes | Expensive cryogenic process for low-temperature operation; Possible amalgam formation due to mercury absorption at low temperatures |
| Purisol | N-methyl-2-pyrolidone | 6.8 MPa, −15~0 °C | High H2S selectivity; Compatible with combined removal of H2S/CO2, and specific H2S removal | Volatile solvent, requiring water wash to avoid excessive solvent loss |
| Morphysorb | Morpholine | 6.9 MPa, −48.8 °C | Solvent possesses high loading capacity; Removes H2S and CO2 with exceedingly high selectivity; Environmentally friendly with lower corrosion risk; High economic viability due to low operational costs and initial investment costs; Lower energy requirement, recirculation requirement, and hydrocarbon co-absorption | Comparatively novel process with comparatively low technology readiness level (pilot-testing) |
| Fluor | Propylene carbonate | 2.72~5.78 MPa, 25 °C | Low-viscosity and non-corrosive solvent; Enables selective H2S removal; High CO2 solubility; No need for make-up water | Expensive solvent; Not economically viable to reach high purity; Requirement of cryogenic operation condition and more efficient gas–liquid contactor |
| CC Technology | Purity of the Captured CO2 (%) | Temperature (°C) | or Power Plant Output Reduction (PPOR%) | Technology Readiness Level (TRL) | |||
|---|---|---|---|---|---|---|---|
| Oxy combustion | 99.94 [90] 99.5 [91] 83–99.9 [92] 99.3 [93] 85–99.5 [94] 97–98 [95] 99 [96] | −30 to −50 [97] −54 [93] −54.9 to 13.2 [94] −55 to −25 [95] | 7–11 PPOR% [98] 12 PPOR% [99] 11.59 PPOR% [100] 10.36 PPOR% [96] 9.53 PPOR% output [101] 12.11 PPOR% [102] 10.7 PPOR% t [103] | 36–67 [98] 25.3–34.3 [103] 27.8–53.2 [104] 54 [105] | 8 [106] 7–8 [107] >7 [108] 8 [28] 7 [105] 5–7 [109] | 45–73 [98] 47.4–74.8 [92] 29.18–46 [103] 37.6–66.83 [104] 22.355–22.42 [110] 116–137.64 [111] 63.27 [105] | |
| Pre-/post-combustion | Chemical absorption | 99.9 [112] 90–95 [113] 97 [114] 99 [115] >98 [116] 98 [117] >99 [118] >95 [73] | 108–128 [119] 119.1 [116] 101–120.8 [120] 90 [121] 150 [122] 120 [123] 96 [118] 100–120 [124] | 3.6 * [112] 3.8 * [114] 3.29 * [115] 4 * [125] 3.9 * [119] 3.63 * [116] 3.8 * [117] 3.5 * [126] 1.379 ** [77] | 28–103.5 [127] 58.8–76.6 [115] 35–53 [117] 59.1 [128] 42.2–45.45 [129] 42.06 [130] 44 [105] 49 [131] 80 [132] 73 [133] | 9 [98] 9 [134] 8–9 [135] 9 [105] 9–11 [109] 9 [136] | 50–80 [115] 106 [98] 68.8 [105] 72.2 [133] |
| Solid adsorption | >95 [137] 47.65 [138] 23.33–99.8 [139] | 120–150 [137] 105 [140] 80–90 [141] 150–200 [142] 50–100 [143] 100 [138] 200 [139] | 3.78 * [137] 1.79–2.14 ** [139] | 51–57 [131] 40–49 [137] 19.6–41 [144] | 7 [105] 6 [145] | n/a | |
| Membranes | 90 [113] 98 [126] >80 [146] 80–95 [73] 88.5–96.3 [147] 90 [148], [149] | 25 [146], [150] 40 [149] 29 [147] | 0.89 ** [113] 0.5–1 ** [146] 1.49–2.17 ** [126] | 16.96–158.9 [127] 34.4–46.6 [115] | 7 [134] 2–6 [135] 6–7 [109] | n/a | |
| Cryogenic | 99.99 [73] 99.8 [151] 99.7 [152] 99.17 [153] 99.9 [154] 99.99 [155] 99.9 [156] 99.2 [77] 99.7–99.99+ [130] 99.99 [105] | −55.97 [153] −63 to −30 [154] −122 to −103 [156] −119 [77] −75 [105] | 1.024 ** [156] 0.71–0.92 ** [77] 0.854 ** [130] | 10.28 [154] 12.36 [130] | 6 [134] 3–6 [157] <6 [135] 9 [136] | 6.47 [130] | |
| Direct air capture (DAC) | 99.5–99.96 [158] 99.9 [159] >95 [160] 88.5 [161] | 850–900 [162] <100 [163] 80–120 [164] 120 [165] 25–60 [166] 900 [167] 250 [158] 25 [160] 250 [168] 90 [161] | 12 * [169] 6.7 * [162] 8.81 * [164] 8.3–11.1 * [167] | 138 [162] <300 [170] 77–142 [166] 94–232 [167] 250–690 [159] >1000 [161] 1100–1500 [171] 250–690 [172] | 7 [173] 9 [174] 7 [175] 7 [176] | n/a | |
| Industries | CO2 Emissions | Flue Gas Temperature (°C) | CO2 vol% in the Flue Gas |
|---|---|---|---|
| Cement | [132] [195] [196] [197] | 100–150 [136] 380 [198] 100 [199] 232 [200] | 25 [132] 20–30 [136] 15–30 [195] 14–33 [196] 12–16 [201] 20 [202] 17 [203] |
| Iron and steel | [195,204] [204] | 100 [136] <200 [204] | 7–30 [201] 20–30 [202] 30 [204] 21–44 [205] 20–27 [206] |
| Pulp and paper | [207] | 184–250 [208] | 23–26 [136] 13–20 [202] 13–20.4 [208] 20 [201,209] 10–20 [210] 13–14 [211] |
| Coal-fired power plants | [212] | 120 [213] | 13.3 [133] 12–15 [136] 13 [205] 13.7 [212] 13.1 [213] 10–15 [206,214] |
| Petrochemical | [215] | 160–215 [206] 189 [216] | 12 [195] 7–12 [206] 10.74 [216] |
| Application | CO2 Purity (vol%) | Temperature (°C) | Pressure (MPa) |
|---|---|---|---|
| EOR | >95 [245,258] 99.9 [296] | 95.5 [296] 110 [297] 82.2 [298] 89 [299] | 6.34–13.73 [296] 34.47 [297] 20.685 [298] 23 [299] |
| Methanol production | 98 [288] | 210 [300] 250 [288] 220 [301] | 7.8 [288] 8 [288] 3.5 [301] |
| Urea production | 99.46 [302] | 180 [300] 180–185 [303] | 14.1 [300] 14 [303] |
| Working fluid in power plants | 99.99 [304] | 50–400 [304] | 15–35 [304] |
| Feature/Aspect | AspenOne | Pro/II | ProSimPlus |
|---|---|---|---|
| User Interface | Comprehensive, customisable | Simple, user-friendly | Intuitive, user-friendly |
| Additional Features | Advanced economic optimisation | Robust simulation for complex chemical processes | Thermodynamic property estimation, heat transfer calculations |
| Software Integration | Best with other AspenTech products | Can interface with software like Excel | Can interface with other software, but not as strongly integrated with other products |
| Academic Usage | Widely used in academia, extensive resources | Widely used in academia, some resources | Used in academic research, fewer resources |
| Market Presence | Market leader, largest user base | Second most popular, significant user base | Growing popularity, smaller user base |
| Flexibility | Broad range of applications | Focused on chemical processes | Focused on specific industries |
| Technical Support | Comprehensive support and training | Comprehensive support and training | Standard support and training |
| Simulation Method/Software | Maximum Error | Application | Thermodynamic Model | Reference |
|---|---|---|---|---|
| Aspen Plus | 0.95% | Techno-economic analysis of CO2 capture process using membranes | Peng–Robinson | [309] |
| Aspen HYSYS | 6.89% | Establishing a process model for capturing CO2 from natural gas through membrane technology | Ideal | [310] |
| Aspen Plus | 3.56% | Modelling membranes for CO2 capture | Redlich−Kwong | [311] |
| Pro/II | 5% | Modelling CO2 capture using amine-based solvent | NRTL | [312] |
| Pro/II (version 9.0) | 5% | Modelling MEA and DEA for CO2 capture | Amine package and electrolyte algorithm | [313] |
| Pro/II | - | Modelling post-combustion technologies for gas turbine cycles | GPSA | [314] |
| Aspen Plus | 5.8% | Post-combustion using MEA-based CC | Electrolyte NRTL with Redlich–Kwon | [315] |
| Aspen Adsorption | - | Temperature-vacuum-swing-adsorption for DAC | Ideal | [316] |
| Aspen Plus | - | High-temperature DAC integrated with Fischer–Tropsch synthesis | Unsymmetric electrolyte NRTL with Redlich–Kwon | [317] |
| Aspen Plus and Aspen Adsorption | - | Comparison of post-combustion CC including membranes, MEA-based absorption and solid adsorption | Membrane: Peng–RobinsonMEA: Electrolyte NRTLAdsorption: Ideal | [318] |
| ProSimPlus (version 3.1.2.1.2) | - | Modelling membranes for CO2 capture from a power plant | - | [319] |
| Aspen Plus | - | Modelling the CO2 process using amine-based solvent | Electrolyte NRTL | [320] |
| Aspen HYSYS (version 7.2) | - | Modelling amine-based CO2 capture from a power plant | Li–Mather model and Redlich–Kwong equation of state | [321] |
| Aspen Plus | - | Modelling the cryogenic CO2 capture from a coal-fired power plant | Peng–Robinson equation of state for SVE calculations | [322] |
| Aspen Plus | 7% | Modelling CO2 capture from power plants Using MEA | Electrolyte NRTL | [323] |
| Aspen Plus | 3.8% | Simulation of MEA-based CO2 capture process for a coal-fired power | Electrolyte NRTL | [324] |
| Aspen Plus (version 8.6) | 6.9% | Modelling CO2 capture by chemical absorption | ENRTL-RK | [325] |
| Aspen Plus | - | Modelling and optimisation of oxyfuel combustion | - | [326] |
| Aspen HYSYS | - | Techno-economic analysis of oxyfuel combustion when solvent is used to for CO2 separation | NRTL | [327] |
| Aspen Plus (version 10) | Techno-economic analysis of CO2 capture and utilisation | Techno-economic analysis of CO2 capture and utilisation | Electrolyte NRTL for modelling the CO2 capture process andLangmuir–Hinshelwood–Hougen–Watson model to simulate the methanol production reactor | [288] |
| Aspen HYSYS | - | Techno-economic analysis of CO2 capture by physical absorption | SRK | [328] |
| Aspen Plus (V.12) | - | Modelling DAC using ionic liquids | COSMOSAC property method | [329] |
| EnergyPLAN | - | Modelling and comparing the cryogenic and amine-based technologies in the future of Denmark | - | [330] |
| Aspen Plus | - | A techno-economic analysis of blue ammonia production using a cryogenic process | Peng–Robinson | [331] |
| Aspen Plus | - | A techno-economic analysis of a cryogenic process combined with an absorption refrigeration cycle for capturing CO2 from a cement factory | Peng–Robinson | [332] |
| Aspen Plus Dynamics (version 9) | - | Transient model for green methanol synthesis | Predictive Soave–Redlich–Kwong | [301] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asgharian, H.; Yahyaee, A.; Yin, C.; Liso, V.; Nielsen, M.P.; Iov, F. A Guideline for Cross-Sector Coupling of Carbon Capture Technologies. Gases 2024, 4, 371-420. https://doi.org/10.3390/gases4040021
Asgharian H, Yahyaee A, Yin C, Liso V, Nielsen MP, Iov F. A Guideline for Cross-Sector Coupling of Carbon Capture Technologies. Gases. 2024; 4(4):371-420. https://doi.org/10.3390/gases4040021
Chicago/Turabian StyleAsgharian, Hossein, Ali Yahyaee, Chungen Yin, Vincenzo Liso, Mads Pagh Nielsen, and Florin Iov. 2024. "A Guideline for Cross-Sector Coupling of Carbon Capture Technologies" Gases 4, no. 4: 371-420. https://doi.org/10.3390/gases4040021
APA StyleAsgharian, H., Yahyaee, A., Yin, C., Liso, V., Nielsen, M. P., & Iov, F. (2024). A Guideline for Cross-Sector Coupling of Carbon Capture Technologies. Gases, 4(4), 371-420. https://doi.org/10.3390/gases4040021










