Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of Covid-19
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Metrics for Airborne Microorganisms Inactivation
3. Results
3.1. Ozone Virucidal Efficacy
3.2. Total Ozone Dose
3.3. Ozone Production
- -
- Electrolysis of water (water);
- -
- Photochemical method (air, oxygen);
- -
- Dielectric barrier discharge (air, oxygen).
3.4. Disposable Masks and Personal Protective Equipment Disinfection for Reuse
- -
- That disinfection for reuse should not be applied to PPE that are exhausted for another specific use (for example protection from dusts or fibers);
- -
- That an individual PPE would be reused only by the same individual;
- -
- That the acceptable number of reuses has been determined and has not been reached.
3.5. Ozone Negative Impact on Consumer Goods and Other Materials
3.6. Occupational Exposure Limits
3.7. Safety Issues
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ISS Working Group on Biocides. Interim recommendations on cleaning and disinfection of non-healthcare settings during COVID-19 health emergency: Surfaces, indoor environments and clothing. In Rapporto ISS COVID-19 n. 25/2020; ISS: Rome, Italy, 2020. [Google Scholar]
- Knobler, S.; Mahmoud, A.; Lemon, S.; Mack, A.; Sivitz, L.; Oberholtzer, K. Learning from SARS: Preparing for the Next Disease Outbreak; National Academies Press: Washington, DC, USA, 2004. [Google Scholar] [CrossRef]
- Wolf, C.; Von Gunten, U.; Kohn, T. Kinetics of Inactivation of Waterborne Enteric Viruses by Ozone. Environ. Sci. Technol. 2018, 52, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Wong, P.K.Y.; Engelbrecht, R.S.; Chian, E.S.K. Mechanism of enteroviral inactivation by ozone. Appl. Environ. Microbiol. 1981, 41, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.B.; Sharma, M.; Vimalanathan, S. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone Sci. Eng. 2009, 31, 216–223. [Google Scholar] [CrossRef]
- Tanaka, H.; Sakurai, M.; Ishii, K.; Matsuzawa, Y. Inactivation of Influenza virus by ozone gas. IHI Eng. Rev. 2009, 42, 108–111. [Google Scholar]
- Petry, G.; Rossato, L.G.; Nespolo, J.; Kreutz, L.C.; Bertol, C.D. In Vitro Inactivation of Herpes Virus by Ozone. Ozone Sci. Eng. 2014, 36, 249–252. [Google Scholar] [CrossRef]
- Tseng, C.C.; Li, C.S. Ozone for inactivation of aerosolized bacteriophages. Aerosol Sci. Technol. 2006, 40, 683–689. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). U.S. EPA Integrated Science Assessment (ISA) for Ozone and Related Photochemical Oxidants (Final Report); U.S. Environmental Protection Agency: Washington, DC, USA, 2020. [Google Scholar]
- Dubuis, M.E.; Dumont-Leblond, N.; Laliberté, C.; Veillette, M.; Turgeon, N.; Jean, J.; Duchaine, C. Ozone efficacy for the control of airborne viruses: Bacteriophage and norovirus models. PLoS ONE 2020, 15, e0231164. [Google Scholar] [CrossRef]
- Dennis, R.; Cashion, A.; Emanuel, S.; Hubbard, D. Ozone Gas: Scientific Justification and Practical Guidelines for Improvised Disinfection using Consumer-Grade Ozone Generators and Plastic Storage Boxes. J. Sci. Med. 2020, 2. [Google Scholar] [CrossRef]
- Tseng, C.; Li, C. Inactivation of surface viruses by gaseous ozone. J. Environ. Health 2008, 70, 56–63. [Google Scholar]
- Zhang, J.; Zheng, C.; Xiao, G.; Zhou, Y.; Gao, R. Examination of the Efficacy of Ozone Solution Disinfectant in in Activating Sars Virus. Chin. J. Disinfect. 2004, 1, 32–33. [Google Scholar]
- Li, C.S.; Wang, Y.C. Surface germicidal effects of ozone for microorganisms. Am. Ind. Hyg. Assoc. J. 2003, 64, 533–537. [Google Scholar] [CrossRef]
- Sharma, M.; Hudson, J.B. Ozone gas is an effective and practical antibacterial agent. Am. J. Infect. Control 2008, 36, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Foarde, K.K.; Vanosdell, D.W.; Steiber, R.S. Investigation of gas-phase ozone as a potential biocide. Appl. Occup. Environ. Hyg. 1997, 12, 535–542. [Google Scholar] [CrossRef]
- Blanchard, E.L.; Lawrence, J.D.; Noble, J.A.; Xu, M.; Joo, T.; Ng, N.L.; Schmidt, B.E.B.; Santangelo, P.J.; Finn, M.G. Enveloped Virus Inactivation on Personal Protective Equipment by Exposure to Ozone. MedRxiv 2020. [Google Scholar] [CrossRef]
- Yano, H.; Nakanoa, R.; Suzukia, Y.; Nakanoa, A.; Kasaharab, K.; Hoso, H. Inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by gaseous ozone treatment. J. Hosp. Infect. 2020, 106, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamma’a, A.I.; Pandithas, I.; Lucas, J. Low-pressure microwave plasma ultraviolet lamp for water purification and ozone applications. J. Phys. D. Appl. Phys. 2001, 34, 2775. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, B.J.; Im, H.G.; Cha, M.S. Ozone Production with Dielectric Barrier Discharge: Effects of Power Source and Humidity. IEEE Trans. Plasma Sci. 2016, 44, 2288–2296. [Google Scholar] [CrossRef]
- McClurkin, J.D.; Maier, D.E.; Ileleji, K.E. Half-life time of ozone as a function of air movement and conditions in a sealed container. J. Stored Prod. Res. 2013, 55, 41–47. [Google Scholar] [CrossRef]
- Criegee, R. Mechanism of Ozonolysis. Angew. Chemie Int. Ed. English 1975. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Rubber, Vulcanized or Thermoplastic—Resistance to Ozone Cracking Part 1: Static Strain Test (ISO 1431-1); ISO: Geneva, Switzerland, 1989. [Google Scholar]
- International Organization for Standardization (ISO). Rubber, Vulcanized or Thermoplastic—Resistance to Ozone Cracking—Part 2: Dynamic Strain Test (ISO 1431-2); ISO: Geneva, Switzerland, 1994. [Google Scholar]
- International Organization for Standardization (ISO). Rubber, Vulcanized or Thermoplastic—Resistance to Ozone Cracking—Part 3: Reference and Alternative Methods for Determining the Ozone Concentration in Laboratory Test Chambers (ISO 1431-3); ISO: Geneva, Switzerland, 2000. [Google Scholar]
- International Organization for Standardization (ISO). Rubber—Or Plastics-Coated Fabrics—Determination of Resistance to Ozone Cracking under Static Conditions (ISO 3011); ISO: Geneva, Switzerland, 1997. [Google Scholar]
- Jaffe, L.S. The effects of photochemical oxidants on materials. J. Air Pollut. Control Assoc. 1967, 17, 375–378. [Google Scholar] [CrossRef]
- Van Rossem, A.; Talen, H.W. The Appearance of Atmospheric Cracks in Stretched Rubber. Rubber Chem. Technol. 1931, 4, 490–504. [Google Scholar] [CrossRef]
- Peeling, J.; Clark, D.T. Surface Ozonation And Photooxidation Of Polyethylene Film. J. Polym. Sci. A1 1983, 21, 2047–2055. [Google Scholar] [CrossRef]
- Walzak, M.J.; Flynn, S.; Foerch, R.; Hill, J.M.; Karbashewski, E.; Lin, A.; Strobel, M. UV and ozone treatment of polypropylene and poly(Ethylene terephthalate). J. Adhes. Sci. Technol. 1995, 9, 1229–1248. [Google Scholar] [CrossRef]
- James, R.D. Ozone: The Intractable Problem; Western Association for Art Conservation (WAAC) Newsletter: Alaska, HI, USA, 1985. [Google Scholar]
- Crabtree, J.; Malm, F. Deterioration of Rubber from Use and with Age. In Engineering Uses of Rubber; Reinhold Publishing Corp: New York, NY, USA, 1956. [Google Scholar]
- Manning, E.P.; Stephens, M.D.; Patel, S.; Dufresne, S.; Silver, B.; Gerbarg, P.; Gerbarg, Z.; Cruz, C.D.; Sharma, L. Disinfection of N95 Respirators with Ozone. MedRxiv 2020. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Rational Use of Personal Protective Equipment for Coronavirus Disease (COVID-19) and Considerations during Severe Shortages Interim Guidance; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Bogaty, H.; Campbell, K.S.; Appel, W.D. The Oxidation of Cellulose by Ozone in Small Concentrations. Text. Res. J. 1952, 22, 81–83. [Google Scholar] [CrossRef]
- Prabaharan, M.; Rao, J.V. Study on ozone bleaching of cotton fabric-process optimisation, dyeing and finishing properties. Color. Technol. 2001, 117, 92–103. [Google Scholar] [CrossRef]
- Perincek, S.D.; Duran, K.; Korlu, A.E.; Bahtiyari, I.M. An investigation in the use of ozone gas in the bleaching of cotton fabrics. Ozone Sci. Eng. 2007, 29, 325–333. [Google Scholar] [CrossRef]
- Rai, A.C.; Guo, B.; Lin, C.H.; Zhang, J.; Pei, J.; Chen, Q. Ozone reaction with clothing and its initiated VOC emissions in an environmental chamber. Indoor Air 2014, 24, 49–58. [Google Scholar] [CrossRef]
- California Environmental Protection Agency. Available online: https://ww2.arb.ca.gov/sites/default/files/2017-10/ozone-fs.pdf (accessed on 20 November 2020).
- Lee, D.S.; Holland, M.R.; Falla, N. The potential impact of ozone on materials in the U.K. Atmos. Environ. 1996, 30, 1053–1065. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA) Helsinki, Finland. Ozone—Substance Infocard. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.158.222 (accessd on 22 November 2020).
- Institut fur Arbeitsschutz der Deutschen Gesetzlichen Unfallversicherung (IFA). Database GESTIS. Available online: https://limitvalue.ifa.dguv.de/ (accessd on 22 November 2020).
- ACGIH. 2020 TLVs and BEIs; ACGIH: Cincinnati, OH, Canada, 2020. [Google Scholar]
- Working Group ISS-INAIL. Focus on professional use of ozone also in reference to COVID-19. In Rapporto ISS COVID-19 n. 25/2020; ISS: Rome, Italy, 2020; p. 42. [Google Scholar]
- Moongilan, D. Corona and arcing in power and RF devices. In Proceedings of the 2009 IEEE Symposium on Product Compliance Engineering, Toronto, ON, Canada, 26–28 October 2009; pp. 1–7. [Google Scholar]
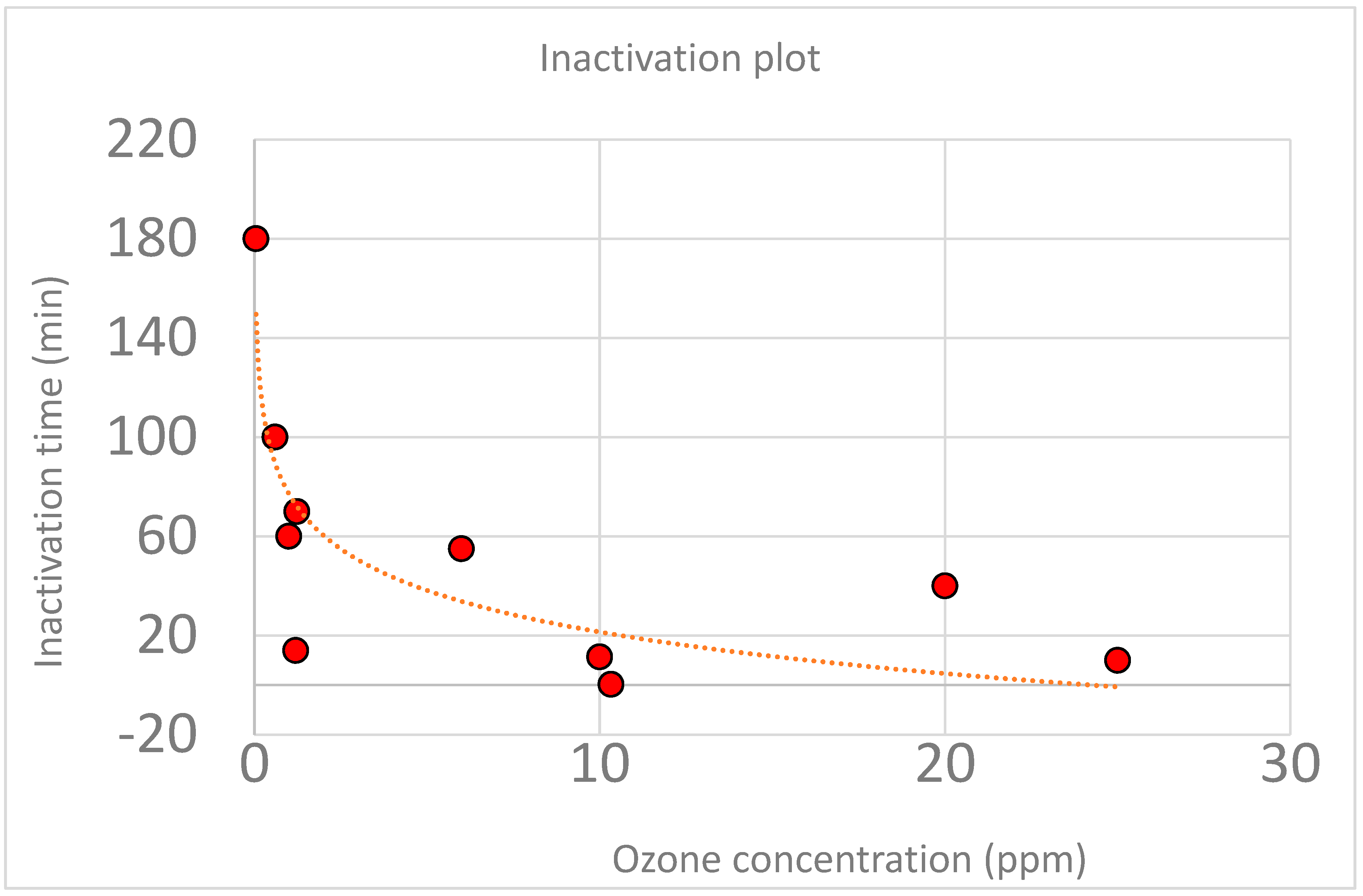
| Ozone Concentration (ppm) | 90% Inactivation Time (min) | Relative Humidity | Viruses | Reference |
|---|---|---|---|---|
| 25 | 15 | >95% after cycle | 12 different viruses | [5] |
| 0.05 | 180 | 35% | Herpes | [7] |
| 10.33 | 0.3 | 55% | 4 kind: ssDNA, ssRNA, dsDNA, Enveloped dsRNA | [8] |
| 1.23 | 70 | 55% | 4 phages | [10] |
| 10 | 11.36 | 55% | Different viruses | [11] |
| 0.6 | 100 | 55% | 4 kind: ssDNA, ssRNA, dsDNA, Enveloped dsRNA | [12] |
| 1.2 | 14 | 55% | 4 kind: ssDNA, ssRNA, dsDNA, Enveloped dsRNA | [12] |
| 20 | 40 | >70% | Influenza A and respiratory syncytial virus | [17] |
| 1 | 60 | 60–80% | SARS-CoV-2 | [18] |
| 6 | 55 |
| Country or Agency | Limit Value—Eight Hours | Limit Value—Short Term | ||
|---|---|---|---|---|
| ppm | mg/m3 | ppm | mg/m3 | |
| Austria | 0.1 | 0.2 | 0.2 | 0.4 |
| Belgium | 0.1 | 0.2 | ||
| Denmark | 0.1 | 0.2 | 0.1 | 0.2 |
| Finland | 0.05 | 0.1 | 0.2 | 0.4 |
| France | 0.1 | 0.2 | 0.2 | 0.4 |
| Hungary | 0.1 | 0.2 | 0.1 | 0.2 |
| Ireland | heavy work 0.05 | heavy work 0.1 | heavy, moderate and light works < 2 h 0.2 | heavy, moderate and light works < 2 h 0.4 |
| moderate work 0.08 | moderate work 0.16 | |||
| light work 0.1 | light work 0.2 | |||
| Latvia | 0.05 | 0.1 | ||
| Poland | 0.075 | 0.15 | ||
| Romania | 0.05 | 0.1 | 0.1 | 0.2 |
| Spain | heavy work 0.05 | heavy work 0.1 | heavy, moderate and light works < 2 h 0.2 | heavy, moderate and light works < 2 h 0.4 |
| moderate work 0.08 | moderate work 0.16 | |||
| light work 0.1 | light work 0.2 | |||
| Sweden | 0.1 | 0.2 | 0.3 | 0.6 |
| Switzerland | 0.1 | 0.2 | 0.1 | 0.2 |
| The Netherlands | 0.06 | 0.12 | ||
| ACGIH (American Conference of Governmental Industrial Hygienists) | heavy work 0.05 | heavy work 0.1 | heavy, moderate and light works < 2 h 0.2 | heavy, moderate and light works < 2 h 0.4 |
| moderate work 0.08 | moderate work 0.16 | |||
| light work 0.1 | light work 0.2 | |||
| USA—NIOSH (National Institute for Occupational Safety and Health) | 0.1 | 0.2 | ||
| USA—OSHA (Occupational Safety and Health Administration) | 0.1 | 0.2 | ||
| United Kingdom | 0.2 | 0.4 | ||
| Canada—Ontario | 0.1 | 0.2 | 0.3 | 0.6 |
| Canada—Québec | 0.1 | 0.2 | ||
| Japan JSOH (Japan Society for Occupational Health) | 0.1 | 0.2 | ||
| New Zealand | 0.1 | 0.2 | ||
| Republic of China | 0.15 | 0.3 | ||
| Singapore | 0.1 | 0.2 | ||
| South Korea | 0.08 | 0.16 | 0.2 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grignani, E.; Mansi, A.; Cabella, R.; Castellano, P.; Tirabasso, A.; Sisto, R.; Spagnoli, M.; Fabrizi, G.; Frigerio, F.; Tranfo, G. Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of Covid-19. Gases 2021, 1, 19-32. https://doi.org/10.3390/gases1010002
Grignani E, Mansi A, Cabella R, Castellano P, Tirabasso A, Sisto R, Spagnoli M, Fabrizi G, Frigerio F, Tranfo G. Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of Covid-19. Gases. 2021; 1(1):19-32. https://doi.org/10.3390/gases1010002
Chicago/Turabian StyleGrignani, Elena, Antonella Mansi, Renato Cabella, Paola Castellano, Angelo Tirabasso, Renata Sisto, Mariangela Spagnoli, Giovanni Fabrizi, Francesco Frigerio, and Giovanna Tranfo. 2021. "Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of Covid-19" Gases 1, no. 1: 19-32. https://doi.org/10.3390/gases1010002
APA StyleGrignani, E., Mansi, A., Cabella, R., Castellano, P., Tirabasso, A., Sisto, R., Spagnoli, M., Fabrizi, G., Frigerio, F., & Tranfo, G. (2021). Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of Covid-19. Gases, 1(1), 19-32. https://doi.org/10.3390/gases1010002