Cold and Cholinergic Urticaria: Predictors of Anaphylaxis and Therapeutic Approaches—What We Know and What We Do Not Know?
Abstract
1. Introduction
2. Cold and Cholinergic Urticaria
2.1. The Relevance of Investigating Inducible Urticaria
2.1.1. Epidemiology
2.1.2. Debut and Duration
2.1.3. Frequent Comorbidities
2.2. Pathogenesis
2.3. Pathognomonic Characteristics
- Symptoms occur exclusively after contact with specific physical or non-physical triggers—such as cold or factors leading to increased sweating [68].
- Urticarial elements persist for a brief duration, typically regressing within one hour.
- Both ColdU and CholU are characterized by the occurrence of angioedema; however, this phenomenon is observed less frequently than in CSU [69].
- Repeated and intense exposure to the etiological trigger exacerbates the course of ColdU and CholU [55].
- A low threshold level, indicated by the rapid onset of clinical symptoms following exposure to the causative factor, reflects high disease activity in ColdU and CholU [22].
2.4. Anaphylaxis
- Skin and mucous membranes: wheals, angioedema affecting the lips, tongue, oropharynx, larynx, phalanges of fingers, and ear lobes.
- Respiratory system: dyspnea, difficulty breathing, dizziness, hoarseness, and nasal congestion.
- Cardiovascular system: hypotension (potentially leading to collapse), tachycardia, and chest pain.
- Gastrointestinal tract: abdominal pain, nausea, vomiting, diarrhea, and spastic abdominal pain.
- Central nervous system: headache, vertigo, weakness, disorientation, and syncope.
2.4.1. Common Triggers
- physical exertion;
- heightened emotional intensity or stress;
- consumption of hot and/or spicy foods and beverages;
- elevated ambient temperatures;
- utilization of baths or saunas;
- taking a hot shower or bath;
- medical procedures (such as hemodialysis and physiotherapy; administration of medications from the class of M-cholinomimetics);
- active sun exposure or visits to a tanning salon;
- exposure to low air temperatures;
- bathing in open water, utilizing pools, or showering with cold water;
- ingestion of cold beverages and/or foods;
- contact with cold surfaces or objects within the home and during occupational activities;
- participation in water sports;
- undergoing cryoprocedures;
- prolonged surgical interventions;
2.4.2. Occurrence of Systemic Reactions
- Group I—Local reactions (urticaria and angioedema) confined to the area of contact with cold stimuli.
- Group II—Urticarial elements and/or angioedema with involvement of another organ system, excluding the cardiovascular system.
- Group III—Generalized urticarial elements and/or angioedema accompanied by hypotension, dizziness, syncope, and disorientation.
2.4.3. Risk Factors for Anaphylaxis
- A previous systemic reaction to stings from webworms;
- The presence of angioedema, particularly involving oropharyngeal and laryngeal symptoms;
- Coexisting bronchial asthma;
- Itching of the earlobes.
2.4.4. Kounis Syndrome
2.4.5. Exercise-Induced Anaphylaxis
- Etiological factors: AnIPhE occurs exclusively as a result of physical activity (e.g., walking, participating in various sports, swimming), whereas systemic reactions in ColdU may arise from an elevation in body temperature due to diverse factors, including physical exertion, exposure to hot environments (such as rooms, climates, baths, or saunas), fever, or the consumption of hot food and beverages.
- Clinical features: AnIPhE is characterized by urticarial elements averaging 10 to 15 mm in size, whereas ColdU typically presents with smaller pinpoint lesions measuring 1 to 3 mm in diameter.
- In some instances, the onset of AnIPhE symptoms may necessitate prior exposure to additional triggers, which may include specific foods, medications, hot weather conditions, or particular phases of the menstrual cycle [106].
2.4.6. Other Differential Pathologies
2.5. Provocation Testing
2.6. Therapy
2.6.1. Antihistamines
2.6.2. Monoclonal Antibodies
- Dupilumab is a recombinant human monoclonal antibody that specifically inhibits interleukin-4 and interleukin-13 signaling by binding to the IL-4Rα subunit;
- Ligelizumab is a humanized anti-IgE monoclonal antibody;
2.6.3. Cyclosporine and Glucocorticoids
2.6.4. Alternative Therapies
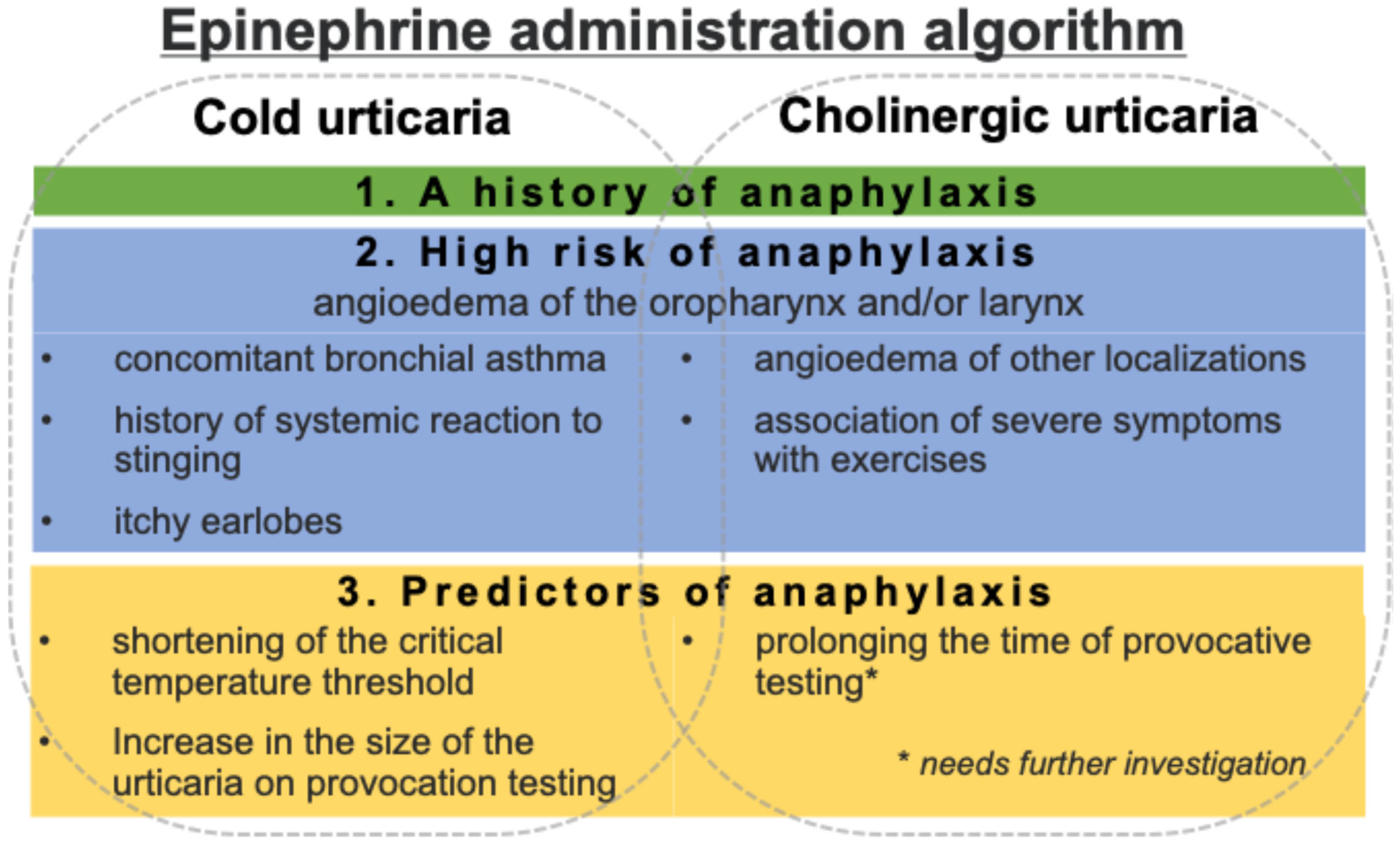
2.6.5. Epinephrine
- Oropharyngeal or laryngeal angioedema accompanied by pruritus of the earlobes;
- Concomitant bronchial asthma;
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AnIPhE | exercise-induced anaphylaxis |
| CINDU | chronic inducible urticaria |
| CholU | cholinergic urticaria |
| ColdU | cold urticaria |
| CSU | chronic spontaneous urticaria |
| AIGA | acquired idiopathic generalized anhidrosis |
| CURE | Chronic Urticaria Registry |
| IgE | immunoglobulin E |
| TRP | transient receptor potential |
References
- Peck, G.; Hashim, M.; Shaughnessy, C.; Muddasani, S.; Elsayed, N.; Fleischer, A.B., Jr. Global Epidemiology of Urticaria: Increasing Burden Among Children, Females and Low-Income Regions. Acta Derm.-Venereol. 2021, 101, adv00433. [Google Scholar] [CrossRef]
- Kubanov, A.A.; Baranov, A.A.; Namazova-Baranova, L.S.; Araviiskaia, E.R.; Astafieva, N.G.; Bazaev, V.T.; Borzova, E.Y.; Vishneva, E.A.; Gallyamova, Y.A.; Danilycheva, I.V.; et al. Urticaria. Russ. J. Allergy 2024, 21, 112–166. [Google Scholar] [CrossRef]
- Abajian, M.; Schoepke, N.; Altrichter, S.; Zuberbier, T.; Maurer, M. Physical Urticarias and Cholinergic Urticaria. Immunol. Allergy Clin. N. Am. 2014, 34, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Latiff, A.H.A.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef] [PubMed]
- Weller, K.; Giménez-Arnau, A.; Grattan, C.; Asero, R.; Mathelier-Fusade, P.; Bizjak, M.; Hanna, M.; Maurer, M.; The CURE Investigators. The Chronic Urticaria Registry: Rationale, methods and initial implementation. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 721–729. [Google Scholar] [CrossRef]
- Shen, M.; Xiao, Y.; Su, J.; Zhao, S.; Li, J.; Tao, J.; Kang, X.; Wu, B.; Shan, S.; Wang, X.; et al. Prevalence and patient-reported outcomes of noncommunicable skin diseases among college students in China. JAAD Int. 2020, 1, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Weller, K.; Maurer, M.; Bauer, A.; Wedi, B.; Wagner, N.; Schliemann, S.; Kramps, T.; Baeumer, D.; Multmeier, J.; Hillmann, E.; et al. Epidemiology, comorbidities, and healthcare utilization of patients with chronic urticaria in Germany. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 91–99. [Google Scholar] [CrossRef]
- Fomina, D.S.; Maltseva, N.P.; Serdotetskova, S.A.; Danilycheva, I.V.; Lebedkina, M.S.; Mikhaylova, V.I.; Kovalkova, E.V.; Chikunov, N.S.; Karaulov, A.V.; Lysenko, M.A.; et al. Epidemiology of chronic inducible urticaria in Moscow. Russ. J. Allergy 2022, 19, 317–327. [Google Scholar] [CrossRef]
- Li, J.; Mao, D.; Liu, S.; Liu, P.; Tian, J.; Xue, C.; Liu, X.; Qi, R.; Bai, B.; Nie, J.; et al. Epidemiology of urticaria in China: A population-based study. Chin. Med. J. 2022, 135, 1369–1375. [Google Scholar] [CrossRef]
- Möller, A.; Henz, B.M. Cold urticaria. In Urticaria; Henz, B.M., Zuberbier, T., Grabbe, J., Monroe, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 69–78. [Google Scholar] [CrossRef]
- Katsarou-Katsari, A.; Makris, M.; Lagogianni, E.; Gregoriou, S.; Theoharides, T.; Kalogeromitros, D. Clinical features and natural history of acquired cold urticaria in a tertiary referral hospital: A 10-year prospective study. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1405–1411. [Google Scholar] [CrossRef]
- Godse, K.; Farooqui, S.; Nadkarni, N.; Patil, S. Prevalence of cholinergic urticaria in Indian adults. Indian Dermatol. Online J. 2013, 4, 62–63. [Google Scholar] [CrossRef]
- Seo, J.-H.; Kwon, J.-W. Epidemiology of urticaria including physical urticaria and angioedema in Korea. Korean J. Intern. Med. 2019, 34, 418–425. [Google Scholar] [CrossRef]
- Rujitharanawong, C.; Tuchinda, P.; Chularojanamontri, L.; Chanchaemsri, N.; Kulthanan, K.; Heffler, E. Cholinergic urticaria: Clinical presentation and natural history in a tropical country. BioMed Res. Int. 2020, 2020, 7301652. [Google Scholar] [CrossRef] [PubMed]
- Kontou-Fili, K.; Borici-Mazi, R.; Kapp, A.; Matjevic, L.J.; Mitchel, F.B. Physical urticaria: Classification and diagnostic guidelines. Allergy 1997, 52, 504–513. [Google Scholar] [CrossRef]
- Silpa-Archa, N.; Kulthanan, K.; Pinkaew, S. Physical urticaria: Prevalence, type and natural course in a tropical country. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.F.; Motta, A.A.; Kalil, J.; Agondi, R.C. Chronic inducible urticaria: Confirmation through challenge tests and response to treatment. Einstein 2020, 18, eAO5175. [Google Scholar] [CrossRef] [PubMed]
- Van Der Valk, P.; Moret, G.; Kiemeney, L. The natural history of chronic urticaria and angioedema in patients visiting a tertiary referral centre. Br. J. Dermatol. 2002, 146, 110–113. [Google Scholar] [CrossRef]
- Stepaniuk, P.; Vostretsova, K.; Kanani, A. Review of cold-induced urticaria characteristics, diagnosis and management in a Western Canadian allergy practice. Allergy Asthma Clin. Immunol. 2018, 14, 85. [Google Scholar] [CrossRef]
- Deza, G.; Brasileiro, A.; Bertolín-Colilla, M.; Curto-Barredo, L.; Pujol, R.M.; Giménez-Arnau, A.M. Acquired cold urticaria: Clinical features, particular phenotypes, and disease course in a tertiary care center cohort. J. Am. Acad. Dermatol. 2016, 75, 918–924.e2. [Google Scholar] [CrossRef]
- Neittaanmäki, H. Cold urticaria. Clinical findings in 220 patients. J. Am. Acad. Dermatol. 1985, 13, 636–644. [Google Scholar] [CrossRef]
- Wanderer, A.A.; Grandel, K.E.; Wasserman, S.I.; Farr, R.S. Clinical characteristics of cold-induced systemic reactions in acquired cold urticaria syndromes: Recommendations for prevention of this complication and a proposal for a diagnostic classification of cold urticaria. J. Allergy Clin. Immunol. 1986, 78, 417–423. [Google Scholar] [CrossRef]
- Mathelier-Fusade, P.; Aïssaoui, M.; Bakhos, D.; Chabane, M.H.; Leynadier, F. Clinical predictive factors of severity in cold urticaria. Arch. Dermatol. 1998, 134, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Althaus, C.; Chantraine-Hess, S.; Czarnetzki, B.M. Prevalence of cholinergic urticaria in young adults. J. Am. Acad. Dermatol. 1994, 31, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Curto-Barredo, L.; Pujol, R.M.; Roura-Vives, G.; Gimenez-Arnau, A.M. Chronic urticaria phenotypes: Clinical differences regarding triggers, activity, prognosis and therapeutic response. Eur. J. Dermatol. 2019, 29, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Bal, F.; Kahveci, M.; Soyer, O.; Sekerel, B.E.; Sahiner, U.M.; Genuneit, J. Chronic inducible urticaria subtypes in children: Clinical features and prognosis. Pediatr. Allergy Immunol. 2021, 32, 146–152. [Google Scholar] [CrossRef]
- Asady, A.; Ruft, J.; Ellrich, A.; Hawro, T.; Maurer, M.; Altrichter, S. Cholinergic urticaria patients of different age groups have distinct features. Clin. Exp. Allergy 2017, 47, 1609–1614. [Google Scholar] [CrossRef]
- Kozel, M.M.; Mekkes, J.R.; Bossuyt, P.M.; Bos, J.D. Natural course of physical and chronic urticaria and angioedema in 220 patients. J. Am. Acad. Dermatol. 2001, 45, 387–391. [Google Scholar] [CrossRef]
- Krause, K.; Spohr, A.; Zuberbier, T.; Church, M.K.; Maurer, M. Up-dosing with bilastine results in improved effectiveness in cold contact urticaria. Allergy 2013, 68, 921–928. [Google Scholar] [CrossRef]
- Magerl, M.; Altrichter, S.; Borzova, E.; Giménez-Arnau, A.; Grattan, C.E.H.; Lawlor, F.; Mathelier-Fusade, P.; Meshkova, R.Y.; Zuberbier, T.; Metz, M.; et al. The definition, diagnostic testing, and management of chronic inducible urticarias—The EAACI/GA2LEN/EDF/UNEV consensus recommendations 2016 update and revision. Allergy 2016, 71, 780–802. [Google Scholar] [CrossRef]
- Sibbald, R.G. Physical urticaria. Dermatol. Clin. 1985, 3, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, J.V.; Lawlor, F.; English, J.S.; Louback, J.B.; Winkelmann, R.K.; Greaves, M.W. Cholinergic urticaria: A clinical and histologic study. Arch. Dermatol. 1987, 123, 462–467. [Google Scholar] [CrossRef]
- Kolkhir, P.; Altrichter, S.; Asero, R.; Daschner, A.; Ferrer, M.; Giménez-Arnau, A.; Hawro, T.; Jakob, T.; Kinaciyan, T.; Kromminga, A.; et al. Autoimmune Diseases Are Linked to Type IIb Autoimmune Chronic Spontaneous Urticaria. Allergy Asthma Immunol. Res. 2021, 13, 545–559. [Google Scholar] [CrossRef]
- Möller, A.; Henning, M.; Zuberbier, T.; Czarnetzki-Henz, B.M. Epidemiologie und Klinik der Kälteurtikaria. Epidemiology and clinical aspects of cold urticaria. Hautarzt 1996, 47, 510–514. (In German) [Google Scholar] [CrossRef]
- Jain, S.; Mullins, R. Cold urticaria: A 20-year follow-up study. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, M.; Košnik, M.; Dinevski, D.; Thomsen, S.F.; Fomina, D.; Borzova, E.; Kulthanan, K.; Meshkova, R.; Ahsan, D.M.; Al-Ahmad, M.; et al. Risk factors for systemic reactions in typical cold urticaria: Results from the COLD-CE study. Allergy 2022, 77, 2185–2199. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Weller, K.; Mlynek, A.; Magerl, M.; Altrichter, S.; dos Santos, R.V.; Maurer, M.; Zuberbier, T. Acquired cold urticaria: Clinical picture and update on diagnosis and treatment. Clin. Exp. Dermatol. 2007, 32, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Petalas, K.; Kontou-Fili, K.; Gratziou, C. Bronchial hyperresponsiveness in patients with cholinergic urticaria. Ann. Allergy Asthma Immunol. 2009, 102, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Altrichter, S.; Koch, K.; Church, M.; Maurer, M. Atopic predisposition in cholinergic urticaria patients and its implications. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2060–2065. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Wang, W.; Wang, B.; Li, L. Symptomatic dermographism in Chinese population: An epidemiological study of hospital-based multicenter questionnaire survey. Curr. Med. Res. Opin. 2022, 38, 131–137. [Google Scholar] [CrossRef]
- Maltseva, N.P.; Fomina, D.S.; Serdotetskova, S.A.; Kovalkova, E.V.; Lebedkina, M.S.; Skvortsova, A.Y.; Chernov, A.A.; Karaulov, A.V.; Lysenko, M.A. Cholinergic urticaria: Search for predictors of course severity and response to therapy. Russ. J. Allergy 2023, 20, 19–28. [Google Scholar] [CrossRef]
- Zimmer, S.; Peveling-Oberhag, A.; Weber, A.; Gilfert, T.T.; Rady-Pizarro, U.; Staubach, P. Unique coexistence of cold and solar urticaria and its efficient treatment. Br. J. Dermatol. 2016, 174, 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- Mathelier-Fusade, P.; Aissaou, M.; Chabane, M.H.; Mounedji, N.; Leynadier, F. Association of cold urticaria and aquagenic urticaria. Allergy 1997, 52, 678–679. [Google Scholar] [CrossRef]
- Ohashi, T.; Kan, Y.; Takahashi, H.; Yoneta, D.; Kase, K.; Sumikawa, Y.; Uhara, H. Cold urticaria comorbid with heat urticaria: A case report. J. Dermatol. 2020, 47, E325–E326. [Google Scholar] [CrossRef]
- Sánchez, J.; Amaya, E.; Acevedo, A.; Celis, A.; Caraballo, D.; Cardona, R. Prevalence of inducible urticaria in patients with chronic spontaneous urticaria: Associated risk factors. J. Allergy Clin. Immunol. Pract. 2017, 5, 464–470. [Google Scholar] [CrossRef]
- Pitsios, C.; Vithoulka, A.; Roumana, A.; Kompoti, E.; Kontou-Fili, K. Multiple physical urticarias: Report of three cases and review of the literature. Allergy Asthma Proc. 2003, 24, 313–317. [Google Scholar] [PubMed]
- Cheon, H.-W.; Han, S.-J.; Yeo, S.-J.; Lee, S.-H.; Kim, M.-J.; Kim, S.-H.; Jang, A.-S. A case of combined cholinergic and cold urticaria. Korean J. Intern. Med. 2012, 27, 478–479. [Google Scholar] [CrossRef]
- Farnam, J.; Grant, J.; Lettbrown, M.; Lord, R.; Russell, W.; Henry, D. Combined cold- and heat-induced cholinergic urticaria+. J. Allergy Clin. Immunol. 1986, 78, 353–356. [Google Scholar] [CrossRef]
- Benelli, E.; Longo, G.; Barbi, E.; Berti, I. Anaphylaxis in atypical cold urticaria: Case report and review of literature. Ital. J. Pediatr. 2018, 44, 135. [Google Scholar] [CrossRef]
- Oda, Y.; Fukunaga, A.; Tsujimoto, M.; Hatakeyama, M.; Washio, K.; Nishigori, C. Combined cholinergic urticaria and cold-induced cholinergic urticaria with acquired idiopathic generalized anhidrosis. Allergol. Int. 2015, 64, 214–215. [Google Scholar] [CrossRef]
- Curto-Barredo, L.; Archilla, L.; Vives, G.; Pujol, R.; Giménez-Arnau, A. Clinical Features of Chronic Spontaneous Urticaria that Predict Disease Prognosis and Refractoriness to Standard Treatment. Acta Derm.-Venereol. 2018, 98, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Maurer, M. Urticaria and Angioedema; Zuberbier, T., Grattan, C., Maurer, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 77–107. [Google Scholar]
- Ormerod, A.; Kobza-Black, A.; Milford-Ward, A.; Greaves, M. Combined cold urticaria and cholinergic urticaria clinical characterization and laboratory findings. Br. J. Dermatol. 1988, 118, 621–627. [Google Scholar] [CrossRef]
- Newcomb, R.W.; Nelson, H. Dermographia mediated by immunoglobulin E. Am. J. Med. 1973, 54, 174–180. [Google Scholar] [CrossRef]
- Gruber, B.L.; Baeza, M.L.; Marchese, M.J.; Agnello, V.; Kaplan, A.P. Prevalence and functional role of anti-IgE autoantibodies in urticarial syndromes. J. Investig. Dermatol. 1988, 90, 213–217. [Google Scholar] [CrossRef]
- Cho, Y.; Jang, Y.; Yang, Y.D.; Lee, C.-H.; Lee, Y.; Oh, U. TRPM8 mediates cold and menthol allergies associated with mast cell activation. Cell Calcium 2010, 48, 202–208. [Google Scholar] [CrossRef]
- Medic, N.; Desai, A.; Komarow, H.; Burch, L.H.; Bandara, G.; Beaven, M.A.; Metcalfe, D.D.; Gilfillan, A.M. Examination of the role of TRPM8 in human mast cell activation and its relevance to the etiology of cold-induced urticaria. Cell Calcium 2011, 50, 473–480. [Google Scholar] [CrossRef]
- Bizjak, M.; Maurer, M.; Košnik, M.; Terhorst-Molawi, D.; Zver, S.; Burmeister, T.; Siebenhaar, F. Severe cold urticaria can point to an underlying clonal mast cell disorder. Allergy 2021, 76, 2609–2613. [Google Scholar] [CrossRef]
- Bito, T.; Sawada, Y.; Tokura, Y. Pathogenesis of cholinergic urticaria in relation to sweating. Allergol. Int. 2012, 61, 539–544. [Google Scholar] [CrossRef]
- Horikawa, T.; Fukunaga, A.; Nishigori, C. New concepts of hive formation in cholinergic urticaria. Curr. Allergy Asthma Rep. 2009, 9, 273–279. [Google Scholar] [CrossRef]
- Dice, J.P. Physical urticaria. Immunol. Allergy Clin. N. Am. 2004, 24, 225–246. [Google Scholar] [CrossRef]
- Kobayashi, H.; Aiba, S.; Yamagishi, T.; Tanita, M.; Hara, M.; Saito, H.; Tagami, H. Cholinergic urticaria, a new pathogenic concept: Hypohidrosis due to interference with the delivery of sweat to the skin surface. Dermatology 2002, 204, 173–178. [Google Scholar] [CrossRef]
- Fukunaga, A.; Washio, K.; Hatakeyama, M.; Oda, Y.; Ogura, K.; Horikawa, T.; Nishigori, C. Cholinergic urticaria: Epidemiology, physiopathology, new categorization, and management. Clin. Auton. Res. 2018, 28, 103–113. [Google Scholar] [CrossRef]
- Sawada, Y.; Nakamura, M.; Bito, T.; Sakabe, J.-I.; Kabashima-Kubo, R.; Hino, R.; Kobayashi, M.; Tokura, Y. Decreased expression of acetylcholine esterase in cholinergic urticaria with hypohidrosis or anhidrosis. J. Investig. Dermatol. 2014, 134, 276–279. [Google Scholar] [CrossRef]
- Munetsugu, T.; Fujimoto, T.; Oshima, Y.; Sano, K.; Murota, H.; Satoh, T.; Iwase, S.; Asahina, M.; Nakazato, Y.; Yokozeki, H. Revised guideline for the diagnosis and treatment of acquired idiopathic generalized anhidrosis in Japan. J. Dermatol. 2016, 44, 394–400. [Google Scholar] [CrossRef]
- Fukunaga, A.; Horikawa, T.; Sato, M.; Nishigori, C. Acquired idiopathic generalized anhidrosis: Possible pathogenic role of mast cells. Br. J. Dermatol. 2009, 160, 1337–1340. [Google Scholar] [CrossRef]
- Itakura, E.; Urabe, K.; Yasumoto, S.; Nakayama, J.; Furue, M. Cholinergic urticaria associated with acquired generalized hypohidrosis: Report of a case and review of the literature. Br. J. Dermatol. 2000, 143, 1064–1066. [Google Scholar] [CrossRef]
- Trevisonno, J.; Balram, B.; Netchiporouk, E.; Ben-Shoshan, M. Physical urticaria: Review on classification, triggers and management with special focus on prevalence including a meta-analysis. Postgrad. Med. 2015, 127, 565–570. [Google Scholar] [CrossRef]
- Maurer, M.; Giménez-Arnau, A.; Ensina, L.F.; Chu, C.-Y.; Jaumont, X.; Tassinari, P. Chronic urticaria treatment patterns and changes in quality of life: AWARE study 2-year results. World Allergy Organ. J. 2020, 13, 100460. [Google Scholar] [CrossRef] [PubMed]
- Doeglas, H.; Rijnten, W.; Schroder, F.; Schirm, J. Cold urticaria and virus infections: A clinical and serological study in 39 patients. Br. J. Dermatol. 1986, 114, 311–318. [Google Scholar] [CrossRef]
- Montgomery, S.L. Cholinergic urticaria and exercise-induced anaphylaxis. Curr. Sports Med. Rep. 2015, 14, 61–63. [Google Scholar] [CrossRef]
- Prosty, C.; Gabrielli, S.; Le, M.; Ensina, L.F.; Zhang, X.; Netchiporouk, E.; Ben-Shoshan, M. Prevalence, Management, and Anaphylaxis Risk of Cold Urticaria: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2022, 10, 586–596.e4. [Google Scholar] [CrossRef]
- Gernez, Y.; Sicherer, S.H.; Wang, J. Variability in diagnosis and management of acquired cold-induced urticaria. J. Allergy Clin. Immunol. Pract. 2018, 6, 1396–1399. [Google Scholar] [CrossRef]
- Wanderer, A.A.; Hoffman, H.M. The spectrum of acquired and familial cold-induced urticaria/urticaria-like syndromes. Immunol. Allergy Clin. N. Am. 2004, 24, 259–286. [Google Scholar] [CrossRef]
- Vadas, P.; Sinilaite, A.; Chaim, M. Cholinergic urticarial with anaphylaxis: An under recognized clinical entity. J. Allergy Clin. Immunol. Pract. 2016, 4, 284–291. [Google Scholar] [CrossRef]
- Fukunaga, A.; Oda, Y.; Imamura, S.; Mizuno, M.; Fukumoto, T.; Washio, K. Cholinergic Urticaria: Subtype Classification and Clinical Approach. Am. J. Clin. Dermatol. 2023, 24, 41–54. [Google Scholar] [CrossRef]
- Washio, K.; Fukunaga, A.; Onodera, M.; Hatakeyama, M.; Taguchi, K.; Ogura, K.; Horikawa, T.; Nishigori, C. Clinical characteristics in cholinergic urticaria with palpebral angioedema: Report of 15 cases. J. Dermatol. Sci. 2017, 85, 135–137. [Google Scholar] [CrossRef]
- Brevik, C.; Zuckerman, M. Cold Anaphylaxis: A Case Report. J. Emerg. Med. 2021, 60, 226–228. [Google Scholar] [CrossRef]
- Confino-Cohen, R.; Goldberg, A.; Magen, E.; Mekori, Y.A. Hemodialysis-induced rash: A unique case of cholinergic urticaria. J. Allergy Clin. Immunol. 1995, 96, 1002–1004. [Google Scholar] [CrossRef]
- Borzova, E.Y.; Popova, K.Y.; Kurowski, M.; Rukhadze, M.T.; Darlenski, R.; Zaborova, V.A.; Kurshev, V.V. Cholinergic urticaria: Novel aspects of pathogenesis, diagnosis and management. Russ. J. Ski. Vener. Dis. 2021, 24, 211–226. [Google Scholar] [CrossRef]
- Claudy, A. Cold urticaria. J. Investig. Dermatol. Symp. Proc. 2001, 6, 141–142. [Google Scholar] [CrossRef]
- Alangari, A.A.; Twarog, F.J.; Shih, M.-C.; Schneider, L.C. Clinical features and anaphylaxis in children with cold urticaria. Pediatrics 2004, 113, e313–e317. [Google Scholar] [CrossRef]
- González-Díaz, S.N.; Fuentes-Lara, E.I.; Quezada, C.E.D.L.; González, R.V.V.; de la Cruz-Cruz, R.A. Anaphylaxis in Cold Induced Urticaria: A Case Report and Review of the Literature. Iran. J. Allergy Asthma Immunol. 2022, 21, 98–100. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Degener, F.; Zuberbier, T.; Martus, P.; Maurer, M. High-dose desloratadine decreases wheal volume and improves cold provocation thresholds compared with standard-dose treatment in patients with acquired cold urticaria: A randomized, placebo-controlled, crossover study. J. Allergy Clin. Immunol. 2009, 123, 672–679. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Harari, Z.; Sheonfeld, Y.; Keren, G. Cholinergic urticaria: A seasonal disease. Arch. Intern. Med. 1981, 141, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Kulthanan, K.; Tuchinda, P.; Chularojanamontri, L.; Kiratiwongwan, R. Cold urticaria: Clinical features and natural course in a tropical country. Allergy Asthma Immunol. Res. 2019, 11, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.S.; El Khoury, K.; Albuhairi, S.; Broyles, A.; Schneider, L.; Rachid, R. Acquired coldinducedurticaria in pediatric patients: A 22-year experience in a tertiary care center (1996–2017). J. Allergy Clin. Immunol. Pract. 2019, 7, 1024–1031.e3. [Google Scholar] [CrossRef]
- Metz, M.; Scholz, E.; Ferrán, M.; Izquierdo, I.; Giménez-Arnau, A.; Maurer, M. Rupatadine and its effects on symptom control, stimulation time, and temperature thresholds in patients with acquired cold urticaria. Ann. Allergy Asthma Immunol. 2010, 104, 86–92. [Google Scholar] [CrossRef]
- Bizjak, M.; Korošec, P.; Košnik, M.; Šelb, J.; Bidovec-Stojkovič, U.; Svetina, M.; Zver, S.; Dinevski, D.; Rijavec, M. Cold-induced anaphylaxis: New insights into clinical and genetic characteristics. Front. Immunol. 2025, 16, 1558284. [Google Scholar] [CrossRef]
- Işk, S.; Arkan-Ayyldz, Z.; Sozmen, S.C.; Karaman, Ö.; Uzuner, N. Idiopathic cold urticaria and anaphylaxis. Pediatr. Emerg. Care 2014, 30, 38–39. [Google Scholar] [CrossRef]
- Maciag, M.C.; Nargozian, C.; Broyles, A.D. Intraoperative anaphylaxis secondary to systemic cooling in a pediatric patient with cold-induced urticaria. J. Allergy Clin. Immunol. Pract. 2018, 6, 1394–1395. [Google Scholar] [CrossRef] [PubMed]
- Magerl, M.; Pisarevskaja, D.; Staubach, P.; Martus, P.; Church, M.; Maurer, M. Critical temperature threshold measurement for cold urticaria: A randomized controlled trial of H (1) -antihistamine dose escalation. Br. J. Dermatol. 2012, 166, 1095–1099. [Google Scholar] [CrossRef]
- Citlak, H.K.; Azkur, D.; Yildiz, Y.K.; Demirel, A.C.; Kot, H.; Vezir, E.; Kilic, M.; Guc, B.U.; Kilic, M.; Yakici, N.; et al. Cold-induced urticaria in children: A multicenter, retrospective cohort study. Allergy Asthma Proc. 2023, 44, e36–e43. [Google Scholar] [CrossRef]
- McManus, N.; Zehrung, R.; Armstrong, T.; Offman, R. Anaphylaxis Caused by Swimming: A Case Report of Cold-induced Urticaria in the Emergency Department. Clin. Pract. Cases Emerg. Med. 2021, 5, 307–311. [Google Scholar] [CrossRef]
- Alrafiaah, A.S.; Netchiporouk, E.; Ben-Shoshan, M. Cold-induced anaphylaxis triggered by drinking cold water. Allergol. Immunopathol. 2024, 52, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Cordes, F.; Ellermann, C.; Ehrchen, J.; Ullerich, H.; Eckardt, L.; Khan, H.; Sommer, P.; Bera, D.; Sayers, M.; Green, P. Diagnosis and management of cold urticaria in cryoablation of atrial fibrillation: A case report. Eur. Heart J.—Case Rep. 2019, 3, 1–5. [Google Scholar] [CrossRef]
- Kounis, N.G.; Zavras, G.M. Histamine-induced coronary artery spasm: The concept of allergic angina. Int. J. Clin. Pract. 1991, 45, 121–128. [Google Scholar] [CrossRef]
- Mazarakis, A.; Bardousis, K.; Almpanis, G.; Mazaraki, I.; Markou, S.; Kounis, N.G. Kounis syndrome following cold urticaria: The swimmer’s death. Int. J. Cardiol. 2014, 176, e52–e53. [Google Scholar] [CrossRef]
- Kalatsei, L.V.; Snezhitskiy, V.A.; Hrib, S.N.; Liskovich, T.G.; Filistovich, I.S. Clinical case of allergic acute coronary syndrome (Kounis syndrome) caused by cold urticaria. J. Grodno State Med. Univ. 2021, 19, 229–235. [Google Scholar] [CrossRef]
- Sravan, A.; Tse, R.; Cala, A.D. A decline in 2 consecutive postmortem serum tryptase levels in an anaphylactic death. Am. J. Forensic. Med. Pathol. 2015, 36, 233–235. [Google Scholar] [CrossRef]
- Yaegashi, T.; Nakamura, Y.; Sakagami, S.; Saeki, T.; Omi, W.; Ikeda, K. Acute myocardial infarction following food dependent exercise induced anaphylaxis. Intern. Med. 2011, 50, 451–454. [Google Scholar] [CrossRef]
- Kounis, N.G.; Kounis, G.N.; Soufras, G.D. Exercise-induced urticaria, cholinergic urticaria, and Kounis syndrome. J. Pharmacol. Pharmacother. 2016, 7, 48–50. [Google Scholar] [CrossRef]
- Kounis, N.G.; Almpanis, G.; Tsigkas, G.; Kounis, G.N.; Mazarakis, A. Kounis syndrome following food-dependent exercise-induced anaphylaxis. Intern. Med. 2011, 50, 1451. [Google Scholar] [CrossRef]
- Soter, N.A.; Wasserman, S.I.; Austen, K.F.; McFadden, E.R. Release of mast-cell mediators and alterations in lung function in patients with cholinergic urticaria. N. Engl. J. Med. 1980, 302, 604–608. [Google Scholar] [CrossRef]
- Volcheck, G.W.; Li, J.T. Exercise-induced urticaria and anaphylaxis. Mayo Clin. Proc. 1997, 72, 140–147. [Google Scholar] [CrossRef]
- Geller, M. Clinical Management of Exercise-Induced Anaphylaxis and Cholinergic Urticaria. J. Allergy Clin. Immunol. Pract. 2020, 8, 2209–2214. [Google Scholar] [CrossRef]
- Sheffer, A.; Austen, K. Exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 1980, 66, 106–111. [Google Scholar] [CrossRef]
- Baadsgaard, O.; Lindskov, R. Cholinergic urticaria with anaphylaxis induced by exercise or heating. Acta Derm.-Venereol. 1984, 64, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Natbony, S.; Tawil, A.; Fruchter, L.; Foster, M. Exercise-induced anaphylaxis as a manifestation of cholinergic urticaria. J. Allergy Clin. Immunol. 1981, 68, 319–324. [Google Scholar] [CrossRef]
- Fukunaga, A.; Bito, T.; Tsuru, K.; Oohashi, A.; Yu, X.; Ichihashi, M.; Nishigori, C.; Horikawa, T. Responsiveness to autologous sweat and serum in cholinergic urticaria classifies its clinical subtypes. J. Allergy Clin. Immunol. 2005, 116, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Mellerowicz, E.J.; Asady, A.; Maurer, M.; Altrichter, S. Angioedema frequently occurs in cholinergic urticaria. J. Allergy Clin. Immunol. Pract. 2019, 7, 1355–1357.e1. [Google Scholar] [CrossRef]
- Özkaya, E.; Bozkurt, P.K. An unusual case of triclosan-induced immunological contact urticaria. Contact Dermat. 2013, 68, 121–123. [Google Scholar] [CrossRef]
- Bruusgaard-Mouritsen, M.A.; Mortz, C.; Winther, L.; Garvey, L.H. Repeated idiopathic anaphylaxis caused by povidone. Ann. Allergy Asthma Immunol. 2021, 126, 598–600. [Google Scholar] [CrossRef]
- Pasche-Koo, F.; French, L.; Piletta-Zanin, P.; Hauser, C. Contact urticaria and shock to hair dye. Allergy 1998, 53, 904–905. [Google Scholar] [CrossRef]
- Paiva, M.; Piedade, S.; Gaspar, Â. Toothpaste-induced anaphylaxis caused by mint (Mentha) allergy. Allergy 2010, 65, 1201–1202. [Google Scholar] [CrossRef]
- Maurice, F.; Rivory, J.; Larsson, P.; Johansson, S.; Bousquet, J. Anaphylactic shock caused by formaldehyde in a patient undergoing long-term hemodialysis. J. Allergy Clin. Immunol. 1986, 77, 594–597. [Google Scholar] [CrossRef]
- Johansen, J.D.; Aalto-Korte, K.; Agner, T.; Andersen, K.E.; Bircher, A.; Bruze, M.; Cannavó, A.; Giménez-Arnau, A.; Gonçalo, M.; Goossens, A.; et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—Recommendations on best practice. Contact Dermat. 2015, 73, 195–221. [Google Scholar] [CrossRef]
- Maltseva, N.; Borzova, E.; Fomina, D.; Bizjak, M.; Terhorst-Molawi, D.; Košnik, M.; Kulthanan, K.; Meshkova, R.; Thomsen, S.F.; Maurer, M.; et al. Cold urticaria—What we know and what we do not know. Allergy 2021, 76, 1077–1094. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Staubach, P.; Metz, M.; Magerl, M.; Jung, J.; Maurer, M. Peltier effect–based temperature challenge: An improved method for diagnosing cold urticaria. J. Allergy Clin. Immunol. 2004, 114, 1224–1225. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.G.; Agner, T.; Thomsen, S.F. Diagnostic properties of provocation tests for cold, heat, and delayed-pressure urticaria. Eur. J. Dermatol. 2017, 27, 406–408. [Google Scholar] [CrossRef]
- Magerl, M.; Abajian, M.; Krause, K.; Altrichter, S.; Siebenhaar, F.; Church, M. An improved Peltier effect-based instrument for critical temperature threshold measurement in cold- and heat-induced urticaria. J. Eur. Acad. Dermatol. Venereol. 2014, 29, 2043–2045. [Google Scholar] [CrossRef]
- Inuzuka, Y.; Yamamoto-Hanada, K.; Saito-Abe, M.; Ohya, Y. Pediatric cold-induced anaphylaxis and evaluation using TempTest®. Allergol. Int. 2022, 71, 412–413. [Google Scholar] [CrossRef]
- Krause, K.; Zuberbier, T.; Maurer, M. Modern approaches to the diagnosis and treatment of cold contact urticarial. Curr. Allergy Asthma Rep. 2010, 10, 243–249. [Google Scholar] [CrossRef]
- Młynek, A.; Magerl, M.; Siebenhaar, F.; Weller, K.; dos Santos, R.V.; Zuberbier, T.; Zalewska-Janowska, A.; Maurer, M. Results and relevance of critical temperature threshold testing in patients with acquired cold urticaria. Br. J. Dermatol. 2010, 162, 198–200. [Google Scholar] [CrossRef]
- Serdotetskova, S.A.; Danilycheva, I.V.; Fomina, D.S.; Maltseva, N.P.; Kovalkova, E.V.; Lebedkina, M.S.; Karaulov, A.V.; Namazova-Baranova, L.S.; Vishneva, E.A.; Levina, J.G.; et al. Chronic-induced urticaria; classification, actual aspects of diagnosis and therapy. Russ. J. Allergy 2023, 20, 74–96. [Google Scholar] [CrossRef]
- Zuberbier, T.; Aberer, W.; Asero, R.; Latiff, A.H.A.; Baker, D.; Ballmer-Weber, B.; Bernstein, J.A.; Bindslev-Jensen, C.; Brzoza, Z.; Bedrikow, R.B.; et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef] [PubMed]
- Agbenyefia, P.; Shilliam, L.A.; Stoicea, N.; Roth, A.; Moran, K.R. Perioperative management of a patient with cold urticaria. Front. Med. 2017, 4, 222. [Google Scholar] [CrossRef] [PubMed]
- Starsmore, L.; Durbridge, J. Anaesthetic implications of a patient with cold-induced anaphylaxis presenting to the labour ward. Int. J. Obstet. Anesth. 2019, 37, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Kotera, A. General anesthetic management in two patients with an anaphylaxis history cholinergic urticaria. JA Clin. Rep. 2023, 9, 47. [Google Scholar] [CrossRef]
- Maltseva, N.P.; Ryabova, K.A.; Zhernov, Y.V.; Sebekina, O.V. Aspects of pathogenesis, differential and modifying approaches in cholinergic urticaria. Russ. J. Allergy 2024, 21, 479–491. [Google Scholar] [CrossRef]
- Magerl, M.; Schmolke, J.; Siebenhaar, F.; Zuberbier, T.; Metz, M.; Maurer, M. Acquired cold urticaria symptoms can be safely prevented by ebastine. Allergy 2007, 62, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Wanderer, A.; St Pierre, J.; Ellis, E.F. Primary acquired cold urticaria: Double-blind comparative study of treatment with cyproheptadine, chlorpheniramine, and placebo. Arch. Dermatol. 1977, 113, 1375–1377. [Google Scholar] [CrossRef]
- Martínez, F.V.; Contreras, F.J.; Cazaña, J.M.L.; Serrano, M.C.L.; Alzamora, F.M. A comparison of new nonsedating and classical antihistamines in the treatment of primary acquired cold urticaria (ACU). J. Investig. Allergol. Clin. Immunol. 1992, 2, 258–262. [Google Scholar] [PubMed]
- Neittaanmäki, H.; Myöhänen, T.; Fräki, J.E. Comparison of cinnarizine, cyproheptadine, doxepin, and hydroxyzine in treatment of idiopathic cold urticaria: Usefulness of doxepin. J. Am. Acad. Dermatol. 1984, 11, 483–489. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Kobric, M.; Rackham, A. Effect of ketotifen treatment on cold-induced urticaria. Ann. Allergy 1985, 55, 840–843. [Google Scholar] [PubMed]
- Kulthanan, K.; Hunnangkul, S.; Tuchinda, P.; Chularojanamontri, L.; Weerasubpong, P.; Subchookul, C.; Maurer, M. Treatments of cold urticaria: A systematic review. J. Allergy Clin. Immunol. 2019, 143, 1311–1331. [Google Scholar] [CrossRef]
- Magerl, M.; Pisarevskaja, D.; Staubach, P.; Martus, P.; Church, M.K.; Maurer, M. Critical temperature threshold reduction in acquired cold urticaria Patients treated with rupatadine: A randomised, doubleblind, cross-over, placebo-controlled study. In Proceedings of the XXVIII EAACI Congress of the European Academy of Allergy and Clinical Immunology, Warszawa, Poland, 6–10 June 2009. [Google Scholar] [CrossRef]
- Abajian, M.; Curto-Barredo, L.; Krause, K.; Santamaria, E.; Izquierdo, I.; Church, M.; Maurer, M.; Giménez-Arnau, A. Rupatadine 20 mg and 40 mg are effective in reducing the symptoms of chronic cold urticaria. Acta Derm.-Venereol. 2016, 96, 56–59. [Google Scholar] [CrossRef]
- Levnadier, F.; Dubertret, L.; Murrieta-Aguttes, M. Effect of mizolastine vs placebo in acquired cold induced urticaria (ACIU): The cold stimulation time test. In Proceedings of the Congrès Annuel de Recherche Dermatologique 97, Tour, France, 10–11 April 1997; p. 823. [Google Scholar] [CrossRef]
- Dressler, C.; Werner, R.N.; Eisert, L.; Zuberbier, T.; Nast, A.; Maurer, M. Chronic inducible urticaria: A systematic review of treatment options. J. Allergy Clin. Immunol. 2018, 141, 1726–1734. [Google Scholar] [CrossRef]
- Zuberbier, T.; Münzberger, C.; Haustein, U.; Trippas, E.; Burtin, B.; Mariz, S.; Henz, B. Double-blind crossover study of high-dose cetirizine in cholinergic urticarial. Dermatology 1996, 193, 324–327. [Google Scholar] [CrossRef]
- Koch, K.; Weller, K.; Werner, A.; Maurer, M.; Altrichter, S. Antihistamine updosing reduces disease activity in patients with difficult-to-treat cholinergic urticaria. J. Allergy Clin. Immunol. 2016, 138, 1483–1485.e9. [Google Scholar] [CrossRef]
- Nakamizo, S.; Egawa, G.; Miyachi, Y.; Kabashima, K. Cholinergic urticaria: Pathogenesis-based categorization and its treatment options. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 114–116. [Google Scholar] [CrossRef]
- Zuberbier, T. The personalized treatment for urticaria. In Personalized Treatment Options in Dermatology; Bieber, T., Nestle, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 111–120. [Google Scholar]
- Metz, M.; Schütz, A.; Weller, K.; Gorczyza, M.; Zimmer, S.; Staubach, P.; Merk, H.F.; Maurer, M. Omalizumab is effective in cold urticaria—Results of a randomized placebo-controlled trial. J. Allergy Clin. Immunol. 2017, 140, 864–867.e5. [Google Scholar] [CrossRef]
- Maurer, M.; Metz, M.; Brehler, R.; Hillen, U.; Jakob, T.; Mahler, V.; Pföhler, C.; Staubach, P.; Treudler, R.; Wedi, B.; et al. Omalizumab treatment in patients with chronic inducible urticaria: A systematic review of published evidence. J. Allergy Clin. Immunol. 2018, 141, 638–649. [Google Scholar] [CrossRef]
- Gastaminza, G.; Azofra, J.; Nunez-Cordoba, J.M.; Baeza, M.L.; Echechipía, S.; Gaig, P.; García, B.E.; Labrador-Horrillo, M.; Sala-Cunill, A.; Brescó, M.S.; et al. Efficacy and safety of omalizumab (xolair) for cholinergic urticaria in patients unresponsive to a double dose of antihistamines: A randomized mixed double-blind and open-label placebo-controlled clinical trial. J. Allergy Clin. Immunol. Pract. 2019, 7, 1599–1609.e1. [Google Scholar] [CrossRef]
- Metz, M.; Ohanyan, T.; Church, M.K.; Maurer, M. Omalizumab is an effective and rapidly acting therapy in difficult-to-treat chronic urticaria: A retrospective clinical analysis. J. Dermatol. Sci. 2014, 73, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Altrichter, S.; Ardelean, E.; Keβler, B.; Krause, K.; Magerl, M.; Siebenhaar, F.; Weller, K.; Zuberbier, T.; Maurer, M. Anti-immunoglobulin E treatment of patients with recalcitrant physical urticaria. Int. Arch. Allergy Immunol. 2011, 154, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Schütz, A.; Weller, K.; Schoepke, N.; Peveling-Oberhag, A.; Staubach, P.; Müller, S.; Jakob, T.; Metz, M. Omalizumab is effective in symptomatic dermographism—Results of a randomized placebo-controlled trial. J. Allergy Clin. Immunol. 2017, 140, 870–873.e5. [Google Scholar] [CrossRef]
- Grabbe, J. Pathomechanisms in physical urticaria. J. Investig. Dermatol. Symp. Proc. 2001, 6, 135–136. [Google Scholar] [CrossRef]
- Pesqué, D.; Ciudad, A.; Andrades, E.; Soto, D.; Gimeno, R.; Pujol, R.M.; Giménez-Arnau, A.M. Solar Urticaria: An Ambispective Study in a Long-Term Follow-Up Cohort with Emphasis on Therapeutic Predictors and Outcomes. Acta Derm.-Venereol. 2024, 104, adv25576. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.V.; Bidese, B.L.; de Souza, J.R.; Maurer, M. Effects of omalizumab in a patient with three types of chronic urticaria. Br. J. Dermatol. 2014, 170, 469–471. [Google Scholar] [CrossRef]
- Boyce, J. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J. Allergy Clin. Immunol. 2006, 117, 1415–1418. [Google Scholar] [CrossRef]
- Terhorst-Molawi, D.; Hawro, T.; Grekowitz, E.; Kiefer, L.; Metz, M.; Alvarado, D.; Hawthorne, T.; Merchant, K.; Crew, L.; Crowley, E.; et al. The anti-KIT antibody, CDX-0159, reduces mast cell numbers and circulating tryptase and improves disease control in patients with chronic inducible urticaria (CIndU). J. Allergy Clin. Immunol. 2022, 149, AB178. [Google Scholar] [CrossRef]
- Maurer, M.; Giménez-Arnau, A.M.; Sussman, G.; Metz, M.; Baker, D.R.; Bauer, A.; Bernstein, J.A.; Brehler, R.; Chu, C.-Y.; Chung, W.-H.; et al. Ligelizumab for chronic spontaneous urticaria. N. Engl. J. Med. 2019, 381, 1321–1332. [Google Scholar] [CrossRef]
- Maurer, M.; Casale, T.B.; Saini, S.S.; Ben-Shoshan, M.; Giménez-Arnau, A.M.; Bernstein, J.A.; Yagami, A.; Stjepanovic, A.; Radin, A.; Staudinger, H.W.; et al. Dupilumab in patients with chronic spontaneous urticaria (LIBERTY-CSU CUPID): Two randomized, double-blind, placebo-controlled, phase 3 trials. J. Allergy Clin. Immunol. 2024, 154, 184–194. [Google Scholar] [CrossRef]
- Alvarado, D.; Maurer, M.; Gedrich, R.; Seibel, S.B.; Murphy, M.B.; Crew, L.; Goldstein, J.; Crocker, A.; Vitale, L.A.; Morani, P.A.; et al. Anti-KIT monoclonal antibody CDX-0159 induces profound and durable mast cell suppression in a healthy volunteer study. Allergy 2022, 77, 2393–2403. [Google Scholar] [CrossRef]
- Sabag, D.A.; Matanes, L.; Bejar, J.; Sheffer, H.; Barzilai, A.; Church, M.K.; Toubi, E.; Maurer, M.; Vadasz, Z. Interleukin-17 is a potential player and treatment target in severe chronic spontaneous urticaria. Clin. Exp. Allergy 2020, 50, 799–804. [Google Scholar] [CrossRef]
- Kolkhir, P.; Giménez-Arnau, A.M.; Kulthanan, K.; Peter, J.; Metz, M.; Maurer, M. Urticaria. Nat. Rev. Dis. Prim. 2022, 8, 61. [Google Scholar] [CrossRef]
- Bonnekoh, H.; Butze, M.; Spittler, S.; Staubach, P.; Weller, K.; Scheffel, J.; Maurer, M.; Krause, K. Inhibition of interleukin-1 with rilonacept is not effective in cold urticaria—Results of a randomized, placebo-controlled study. Clin. Transl. Allergy 2023, 13, e12226. [Google Scholar] [CrossRef]
- Bodar, E.J.; Simon, A.; De Visser, M.; Van Der Meer, J.W.M. Complete remission of severe idiopathic cold urticaria on interleukin-1 receptor antagonist (anakinra). Neth. J. Med. 2009, 67, 302–305. [Google Scholar] [PubMed]
- Gualdi, G.; Monari, P.; Rossi, M.; Crotti, S.; Calzavara-Pinton, P. Successful treatment of systemic cold contact urticaria with etanercept in a patient with psoriasis. Br. J. Dermatol. 2012, 166, 1373–1374. [Google Scholar] [CrossRef] [PubMed]
- Altrichter, S.; Staubach, P.; Pasha, M.; Singh, B.; Chang, A.T.; Bernstein, J.A.; Rasmussen, H.S.; Siebenhaar, F.; Maurer, M. An open-label, proof-of-concept study of lirentelimab for antihistamine-resistant chronic spontaneous and inducible urticaria. J. Allergy Clin. Immunol. 2022, 149, 1683–1690.e7. [Google Scholar] [CrossRef] [PubMed]
- Grekowitz, E.; Metz, M.; Altrichter, S.; Bauer, A.; Brockow, K.; Heine, G.; Lionnet, L.; Saday, K.K.; Hultsch, T.; Søerensen, E.O.; et al. Targeting histamine receptor 4 in cholinergic urticaria with izuforant (LEO 152020): Results from a phase IIa randomized double-blind placebo-controlled multicentre crossover trial. Br. J. Dermatol. 2024, 190, 825–835, https://doi.org/10.1093/bjd/ljae038. Erratum in Br. J. Dermatol. 2024, 191, e1. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.C.; Walker, A.; Grattan, C.; Perry, H.; Williams, N.; Ratia, N.; Dewit, O.; Gisbert, S.; Metz, M.; Maurer, M. Effects of a topical treatment with spleen tyrosine kinase inhibitor in healthy subjects and patients with cold urticaria or chronic spontaneous urticaria: Results of a phase 1a/b randomised double-blind placebo-controlled study. Br. J. Clin. Pharmacol. 2021, 87, 4797–4808. [Google Scholar] [CrossRef]
- Sabroe, R.A. Failure of omalizumab in cholinergic urticaria. Clin. Exp. Dermatol. 2010, 35, e127–e129. [Google Scholar] [CrossRef]
- Ghazanfar, M.; Sand, C.; Thomsen, S. Effectiveness and safety of omalizumab in chronic spontaneous or inducible urticaria: Evaluation of 154 patients. Br. J. Dermatol. 2016, 175, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Marsland, A.; Beck, M. Cold urticaria responding to systemic ciclosporin. Br. J. Dermatol. 2003, 149, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.; Arora, V.; Gupta, L.; Khare, A.K.; Vyas, K.; Mittal, A. Cyclosporine in Cholinergic Itch. Indian Dermatol. Online J. 2022, 13, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, A.; Hatakeyama, M.; Tsujimoto, M.; Oda, Y.; Washio, K.; Nishigori, C. Steroid treatment can improve the impaired quality of life of patients with acquired idiopathic generalized anhidrosis. Br. J. Dermatol. 2015, 172, 537–538. [Google Scholar] [CrossRef]
- Ohshima, Y.; Yanagishita, T.; Ito, K.; Tamada, Y.; Nishimura, N.; Inukai, Y.; Iwase, S.; Sugenoya, J.; Watanabe, D. Treatment of patients with acquired idiopathic generalized anhidrosis. Br. J. Dermatol. 2013, 168, 430–432. [Google Scholar] [CrossRef]
- Tosoni, C.; Lodi-Rizzini, F.; Bettoni, L.; Toniati, P.; Zane, C.; Capezzera, R.; Venturini, M.; Calzavara-Pinton, P.; Bettoni, L. Cinnarizine is a useful and well-tolerated drug in the treatment of acquired cold urticaria (ACU). Eur. J. Derm. 2003, 13, 54–56. [Google Scholar] [PubMed]
- Gorczyza, M.; Schoepke, N.; Krause, K.; Hawro, T.; Maurer, M. Patients with chronic cold urticaria may benefit from doxycycline therapy. Br. J. Dermatol. 2017, 176, 259–261. [Google Scholar] [CrossRef]
- Hani, N.; Hartmann, K.; Casper, C.; Peters, T.; Schneider, L.A.; Hunzelmann, N.; Scharffetter-Kochanek, K. Improvement of cold urticaria by treatment with the leukotriene receptor antagonist montelukast. Acta Derm. Venereol. 2000, 80, 229. [Google Scholar] [CrossRef]
- Altrichter, S.; Wosny, K.; Maurer, M. Successful treatment of cholinergic urticaria with methantheliniumbromide. J. Dermatol. 2015, 42, 422–424. [Google Scholar] [CrossRef]
- Ujiie, H.; Shimizu, T.; Natsuga, K.; Arita, K.; Tomizawa, K. Severe cholinergic urticaria successfully treated with scopolamine butylbromide in addition to antihistamines. Clin. Exp. Dermatol. 2006, 31, 588–589. [Google Scholar] [CrossRef]
- Sheraz, A.; Halpern, S. Cholinergic urticaria responding to botulinum toxin injection for axillary hyperhidrosis. Br. J. Dermatol. 2013, 168, 1369–1370. [Google Scholar] [CrossRef]
- Feinberg, J.H.; Toner, C.B. Successful treatment of disabling cholinergic urticarial. Mil. Med. 2008, 173, 217–220. [Google Scholar] [CrossRef] [PubMed]
- La Shell, M.S.; England, R.W. Severe refractory cholinergic urticaria treated with danazol. J. Drugs Dermatol. 2006, 5, 664–667. [Google Scholar]
- Antolín-Amérigo, D.; Vlaicu, P.C.; De La Hoz Caballer, B.; Cano, M.S. Anaphylax is like cholinergic urticaria. Can. Fam. Physician 2013, 59, 745–746. [Google Scholar] [PubMed]
- Kozaru, T.; Fukunaga, A.; Taguchi, K.; Ogura, K.; Nagano, T.; Oka, M.; Horikawa, T.; Nishigori, C. Rapid desensitization with autologous sweat in cholinergic urticarial. Allergol. Int. 2011, 60, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Black, A.K.; Sibbald, R.; Greaves, M. Cold urticaria treated by induction of tolerance. Lancet 1979, 314, 964. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Lang, D.M.; Khan, D.A.; Craig, T.; Dreyfus, D.; Hsieh, F.; Sheikh, J.; Weldon, D.; Zuraw, B.; Bernstein, D.I.; et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J. Allergy Clin. Immunol. 2014, 133, 1270–1277.e66. [Google Scholar] [CrossRef]
- Tomei, L.; Saretta, F.; Arasi, S.; Sarti, L.; Licari, A.; Giovannini, M.; Barni, S.; Liccioli, G.; Tallarico, V.; Piccorossi, A.; et al. Cold Anaphylaxis in Children: Italian Case Series and Review of the Literature. Diseases 2023, 11, 143. [Google Scholar] [CrossRef]
- Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Brockow, K.; Fernández Rivas, M.; Santos, A.F.; Zolkipli, Z.Q.; Bellou, A.; Beyer, K.; et al. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014, 69, 1026–1045. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, M.; Košnik, M.; Dinevski, D.; Thomsen, S.F.; Fomina, D.; Borzova, E.; Kulthanan, K.; Meshkova, R.; Aarestrup, F.M.; Ahsan, D.M.; et al. Adrenaline autoinjector is underprescribed in typical cold urticaria patients. Allergy 2022, 77, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Sabroe, R.; Lawlor, F.; Grattan, C.; Ardern-Jones, M.; Bewley, A.; Campbell, L.; Flohr, C.; Leslie, T.; Marsland, A.; Ogg, G.; et al. British Association of Dermatologists guidelines for the management of people with chronic urticaria 2021. Br. J. Dermatol. 2022, 186, 398–413. [Google Scholar] [CrossRef] [PubMed]
- ASCIA (Australian Society of Clinical Immunology and Allergy). Available online: https://www.allergy.org.au (accessed on 22 September 2025).
- JSA (Japanese Society of Allergology). Available online: https://www.jsaweb.jp/themes/en_top.html (accessed on 22 September 2025).
- ASBAI (Associação Brasileira de Alergia e Imunologia). Available online: https://anafilaxiabrasil.com.br/ (accessed on 22 September 2025).
- ALLSA (The Allergy Society of South Africa). Available online: https://www.allergysa.org (accessed on 22 September 2025).


| Year of the Study | Country | Author | Number of Patients | Number of Anaphylaxis Cases | References |
|---|---|---|---|---|---|
| 2019 | Thailand | Kulthanan | 27 | 4% | [86] |
| 2004 | USA | Alangari | 30 | 37% | [82] |
| 1986 | USA | Wanderer | 50 | 38% | [22] |
| 2016 | Spain | Deza | 74 | 19% | [20] |
| 1985 | Finland | Neittaanmäki | 220 | 40% | [21] |
| 1986 | Netherlands | Doeglas | 39 | 51% | [70] |
| 2008 | Greece | Katsarou-Katsari | 62 | 29% | [63] |
| 2016 | Australia | Jain | 99 | 28% | [35] |
| 2019 | USA | Yee | 415 | 19% | [87] |
| 2010 | Germany | Metz | 21 | 19% | [88] |
| 2009 | Germany | Siebenhaar | 30 | 46% | [84] |
| 2021 | 17 countries | Bizjak | 551 | 37% | [36] |
| 2025 | Slovenia | Bizjak | 92 | 36% | [89] |
| Medication | Target | Urticaria Type | ClinicalTrials.Gov Identifier | Status |
|---|---|---|---|---|
| Rilonacept | IL-1β, IL-1α | ColdU | NCT02171416 | Completed |
| Dupilumab | IL-4Rα | ColdU CholU | NCT04681729 NCT03749148 | Completed Completed |
| Ligelizumab | FcεRI | ColdU, CholU | NCT04513548 | Terminated |
| Lirentelimab | Siglec 8 | CholU | NCT03436797 | Completed |
| Barzolvolimab | KIT | ColdU, CholU | NCT04548869 | Completed |
| Omalizumab | FcεRI | ColdU, CholU | NCT01580592 NCT05960708 NCT02012387 | Completed Completed Completed |
| Remibrutinib | BTK | ColdU, CholU ColdU, CholU | NCT05976243 NCT06865651 | Recruiting Not yet recruiting |
| BLU-808 | Wild-type KIT | ColdU | NCT06931405 | Not yet recruiting |
| Izuforant | H4R | CholU | NCT04853992 | Completed |
| GSK2646264 | SYK | ColdU | NCT02424799 | Completed |
| Therapy | Notes/Proposed Mechanism of Action | Ref. |
|---|---|---|
| COLD URTICARIA | ||
| Doxepin | Tricyclic antidepressant with potent H1 and H2 antihistamine activity. | [30] |
| Azathioprine | Immunosuppressant. Used for severe, refractory cases. | [1] |
| Mycophenolate mofetil | Immunosuppressant. Considered an alternative to azathioprine. | [1] |
| Cinnarizine | Calcium channel blocker; may exhibit mast-cell-stabilizing properties. | [173] |
| Doxycycline | Tetracycline antibiotic; used for its anti-inflammatory properties. | [174] |
| Montelukast | Leukotriene receptor antagonist. | [175] |
| CHOLINERGIC URTICARIA | ||
| Methantheline bromide | Anticholinergic agent, suppresses acetylcholine-mediated mast cell degranulation. | [176] |
| Butylscopolamine + Antihistamine | Combination of an anticholinergic and an antihistamine agent. | [177] |
| Botulinum toxin | Local injections block the release of acetylcholine from nerve endings. | [178] |
| Antihistamine + Propranolol + Montelukast | Combination therapy using a beta-blocker (use may be controversial), antihistamine, and antileukotriene. | [179] |
| Danazol | Synthetic androgen; reserved for extreme cases due to its side effect profile. | [180] |
| Montelukast | Leukotriene receptor antagonist. | [181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltseva, N.P.; Riabova, K.A.; Zhernov, Y.V. Cold and Cholinergic Urticaria: Predictors of Anaphylaxis and Therapeutic Approaches—What We Know and What We Do Not Know? Immuno 2025, 5, 44. https://doi.org/10.3390/immuno5040044
Maltseva NP, Riabova KA, Zhernov YV. Cold and Cholinergic Urticaria: Predictors of Anaphylaxis and Therapeutic Approaches—What We Know and What We Do Not Know? Immuno. 2025; 5(4):44. https://doi.org/10.3390/immuno5040044
Chicago/Turabian StyleMaltseva, Natalia P., Ksenja A. Riabova, and Yury V. Zhernov. 2025. "Cold and Cholinergic Urticaria: Predictors of Anaphylaxis and Therapeutic Approaches—What We Know and What We Do Not Know?" Immuno 5, no. 4: 44. https://doi.org/10.3390/immuno5040044
APA StyleMaltseva, N. P., Riabova, K. A., & Zhernov, Y. V. (2025). Cold and Cholinergic Urticaria: Predictors of Anaphylaxis and Therapeutic Approaches—What We Know and What We Do Not Know? Immuno, 5(4), 44. https://doi.org/10.3390/immuno5040044







