1. Introduction
Severe inflammatory pulmonary diseases represent an escalating global health burden, tragically claiming infant lives due to underdeveloped respiratory systems and silent progression, while in adults, they precipitate devastating complications such as cytokine storms (as witnessed in COVID-19 [
1]), irreversible fibrosis in conditions like chronic obstructive pulmonary disease (COPD) [
2], and profound reductions in quality of life. Current diagnostic paradigms, reliant on clinical presentation and systemic blood analyses, are demonstrably inadequate in precision and, crucially, fundamentally fail to provide prognostic insights into the risk of life-threatening complications. While bronchoalveolar lavage (BAL) is a widely established method for respiratory tract examination [
3], particularly when critical states are anticipated, its full diagnostic potential remains untapped. It is within this urgent context that our central thesis emerges: to comprehensively investigate the pivotal role of CD68+ cells—including macrophages, where CD68 is prominently expressed (CD68, a transmembrane protein weighing 101 kDa), and their functionally intertwined eosinophils (given the established co-occurrence and influence of macrophage CD68 activity on eosinophil-driven pathology)—as dynamic biomarkers for both inflammation development and exquisitely precise diagnostic and prognostic stratification of these complex respiratory disorders. Analysis of the BAL fluid cellular composition directly from the alveolar space of the lungs is crucial for detecting pathological processes in the lungs [
4,
5,
6,
7].
Macrophages regulate inflammatory responses, with the delicate balance of their subpopulations critically determining disease progression and resolution. Our recent, in-depth investigation into macrophage receptors and their specific ligands has unveiled a groundbreaking insight: we discovered not merely discrete M1/M2 subsets, but a continuous spectrum of macrophage subpopulations, profoundly differing in receptor distribution and, consequently, in their capacity to bind diverse carbohydrate-containing ligands. To precisely map this previously unrecognized depth of functional heterogeneity, we developed a unique suite of fluorescently labeled specific ligands—acting as sophisticated antibody surrogates—which, when applied to patient BALF, consistently revealed that each distinct pulmonary disease manifests a characteristic ‘fingerprint’ of ligand binding to immune cells [
8,
9,
10].
Recent research in respiratory disease monitoring has largely focused on metabolomic profiling, such as gas chromatography–mass spectrometry (GC-MS), to assess metabolic alterations in conditions like acute pulmonary toxicity, inflammation, asthma, bronchitis, and fibrosis [
11]. Nuclear magnetic resonance (NMR) spectroscopy has similarly been applied to analyze bronchoalveolar lavage fluid (BALF), offering valuable biochemical insights [
7,
12,
13]. Meanwhile, conventional techniques such as flow cytometry [
14,
15,
16] and immunohistochemistry [
17,
18] predominantly rely on a limited set of antibody-based biomarkers to characterize immune cell populations.
Typically, antibodies (e.g., against CD68) indicate the presence or absence of these cells; however, such methods face challenges in discerning subtle, patient-specific phenotypic variations among macrophage subsets. For example, a study analyzing 36 confirmed lung cancer cases [
19] employed flow cytometry to evaluate macrophage markers such as CD206, CD163, CD80, CD86, CD40, CD45, and CD68. Despite comprehensive marker panels, only minor variations were detected, limiting the approach’s predictive accuracy at the individual level. Similarly, flow cytometric techniques developed for enumerating white blood cells in BALF [
20] provide quantitative data but fall short in correlating findings with clinical presentations or disease heterogeneity. In line with these observations, our recent studies [
21,
22] applied flow cytometry to characterize CD206+ macrophage populations in BALF samples. This allowed us to directly measure ligand binding affinities to specific macrophage subsets, offering more granular insight. Nevertheless, the flow cytometry data primarily reflect single-marker-based identification, which limits the ability to capture the full spectrum of macrophage phenotypes.
In contrast, our multiparametric approach to BAL characterization integrates a broader range of biomarkers and analytical parameters, enabling a more nuanced dissection of cellular phenotypes. This high-dimensional profiling surpasses the constraints of traditional antibody panels by capturing complex cellular states and interactions unique to each patient. Consequently, it offers enhanced sensitivity and specificity for differentiating immune cell subpopulations and linking their profiles to clinical outcomes. Compared to standard methods, our strategy provides deeper insight into the lung microenvironment’s immunological landscape, facilitating improved disease monitoring and personalized therapeutic decision-making.
By analyzing CD68, a key receptor found on all macrophages, we gain insights into macrophage functioning. Crucially, this also helps us understand the broader inflammatory state and the functional role of other immune cells, particularly eosinophils, as macrophage CD68 activity is frequently linked to eosinophil involvement in many respiratory diseases. Both CD68+ macrophages and eosinophils play an important role in the pathogenesis of a wide range of respiratory diseases, including asthma, COPD, interstitial lung disease (ILD), allergic rhinitis, and other conditions [
23,
24,
25,
26] (
Table 1). Elevated levels of eosinophils in the BAL fluid are a characteristic feature of asthma in both children and adults [
27]. In acute eosinophilic pneumonitis (AEP), a rare and severe condition characterized by rapid accumulation of eosinophils in the alveoli, BAL fluid analysis reveals a significant increase in their number [
28,
29,
30].
Functional diversity of eosinophils is manifested both in their proinflammatory activity and in their involvement in tissue remodeling and other processes [
31]. Determining the level of eosinophils in BALF at more than 5% of the differential count of leukocytes indicates the presence of a pathological process in the lungs. In eosinophilic lung diseases, the proportion of eosinophils in the BAL fluid can be >10% or more (
Table 1). Thus, the quantitative determination of eosinophils in the BAL fluid is an important element in the diagnosis of many respiratory diseases, especially those characterized by eosinophilic inflammation.
Given the close relationship (in terms of coexistence and complementary action) of CD68+ macrophages with eosinophils [
19,
49,
50,
51,
52], especially in eosinophilic asthma, and the functional uniqueness of M1/M2 macrophage phenotypes, a comprehensive diagnostic approach is needed. Simultaneous typing of CD68+ macrophages and assessment of the contribution of eosinophils, supplemented by CD206+ cell analysis to determine the specific roles of macrophages, provide accurate data for the diagnosis of diseases. It has been shown that CD68+ macrophages can engage in interactions with eosinophils, affecting their function through, for instance, the release of chemokine CCL24 [
49], which elicit the migration of eosinophils towards the respiratory tract.
This research aims to explore the role and analytical significance of CD68+ cells in the differential diagnosis of respiratory disorders and the creation of a liposome-based assay using a phosphatidylserine (PS)-based marker for CD68+ cells. We developed a novel approach leveraging DOPS (dioleoylphosphatidylserine) as a specific ligand for CD68 receptor [
50,
53]. Our methodology includes precisely engineered DOPS/lecithin liposomes of different compositions, fluorescently labeled with Rhodamine 6G (R6G), for the sensitive and specific typing of CD68+ cells in BALF. The precise identification and quantification of both CD68+ macrophages and their co-occurring eosinophils in BALF, facilitated by this innovative system, promise to yield invaluable insights into the intricate mechanisms of specific respiratory inflammatory processes.
2. Materials and Methods
2.1. Reagents
Dioleoylphosphatidylserine (DOPS) was purchased from Chimmed (Moscow, Russia). Lecithin from soybeans was purchased from Reachim (Moscow, Russia). 4′,6-diamidino-2-phenylindole (DAPI) and rhodamine 6G (R6G) were purchased from Sigma Aldrich (St. Louis, MO, USA).
2.2. Ethical Approval and Sample Collection
BALF samples were obtained from the Morozovskaya Children’s City Clinical Hospital following patient consent and strict adherence to ethical guidelines (approved by the Local Ethics Committee, Protocol #2024-15A). Cellular components from BALF were enriched by combining conventional histological methods with a lipid-based capture strategy. Lipid films of DOPS/lecithin or lecithin were fabricated on capture surfaces. The BALF cell suspension was incubated for 60 min at 37 °C to facilitate selective cell adhesion. Cell suspensions were prepared by cytocentrifugation using an Eppendorf 5810 R centrifuge (Hamburg, Germany) and stained with a Romanowsky stain. Differential cell counts were performed under a light microscope. To process and analyze the images, we utilized the Paint (version 11.2402.32.0, Microsoft, Redmond, WA, USA) and ImageJ software (version 1.54d, developed by the National Institutes of Health, Bethesda, MD, USA).
2.3. Liposome Preparation and Characterization
The development of a liposomal formulations was made with Liposome Extruder LiposoFast LF-50, 5-50 ml, 50-1000 nm, (Avestin China, distributer DIA-M, Russia, Moscow). A range of liposomal formulations were prepared to investigate the cellular interactions of different lipid compositions. These included:
Lecithin-based liposomes: Pure lecithin liposomes served as a control formulation.
Dioleoyl phosphatidylserine (DOPS)/Lecithin liposomes: Liposomes were prepared with varying ratios of DOPS to lecithin (25/75, 50/50, and 75/25 w/w) to explore the influence of anionic lipid content on cellular uptake.
PEGylated Stealth Liposomes: To impart stealth properties and potentially prolong circulation time, DOPS/lecithin liposomes (specifically the 75/25 w/w ratio was used for these, though you can specify if others were PEGylated) were further functionalized with DSPE-PEG2000 at varying concentrations (1–33 mass%).
For all liposome preparations, the respective lipids (lecithin, DOPS, and DSPE-PEG2000 where applicable) were initially dissolved in ethanol at a final lipid concentration of 10 mg/mL (for binary formulations, e.g., lecithin or DOPS/lecithin 50/50, the specified concentrations were used). The solvent was then removed by rotary evaporation, followed by drying under a gentle stream of air to ensure complete residual solvent removal, yielding a thin lipid film.
These dried lipid films were subsequently hydrated with either PBS or PBS containing the encapsulated fluorescent dye R6G. Samples were further treated in a water bath sonicator to ensure complete dispersion and to potentially reduce vesicle aggregation. Then, rehydrated lipid dispersions were then subjected to extrusion through polycarbonate membranes with a pore size of 200 nm. This process was performed to achieve a defined and uniform liposome size distribution. Unencapsulated R6G was separated from the liposomal formulations via dialysis using a dialysis membrane with a 3 kDa molecular weight cut-off (MWCO). The dialyzed liposomes were then collected.
The determination of the hydrodynamic diameter and ζ-potential of the prepared liposomes was performed using nanoparticle tracking analysis with the NanoSight LM10-HS device (Salisbury, UK). The samples were diluted with MilliQ-purified water to obtain a concentration of 109–1010 particles per milliliter. The hydrodynamic diameter was calculated based on the Stokes-Einstein equation, taking into account the Brownian motion of particles. Five measurements were performed for each sample, and the average value and standard deviation were calculated. Liposomes had an average diameter of 120–250 nm (depending on sample) with a polydispersity index (PDI) < 0.2 and a zeta potential of +5 to +15 mV (depending on sample), indicating good aggregative stability.
Following the approach described in our recent study [
10], we analyzed characteristic absorption bands corresponding to CH
2 and C=O groups stretching, which remained stable over the storage period, confirming the chemical integrity of the liposomal membranes. Based on our repeated measurements, the liposomal formulations demonstrated high stability over the following time frames under storage at 4 °C:
Approximately 90% of the initial liposome population maintained consistent particle size (±10 nm) and zeta potential (±5 mV) for up to 4 days.
By two weeks of storage, stability declined to about 50%, with noticeable increases in particle size distribution due to initial aggregation.
Beyond two weeks, more pronounced aggregation and changes in physicochemical properties were observed, indicating that liposomes require re-preparation to ensure assay reliability.
2.4. Liposome-Based Sandwich Assay for CD68 Receptor Quantification
A novel, lipid-film based assay was meticulously developed for the selective capture and quantitative analysis of CD68-positive macrophages and eosinophils from heterogeneous cellular suspensions. Briefly, a cell suspension derived from BALF was introduced onto pre-fabricated lipid films in wells of 96-well clear bottom Costar plate. These films, consisting of either dioleoyl phosphatidylserine (DOPS)/lecithin or lecithin-only (serving as a control for non-specific binding), were prepared with in situ encapsulated rhodamine 6G (R6G) to enable fluorescent detection. The cell suspensions were then incubated with the lipid films at a physiological temperature of 37 °C for a standardized period of 60 min. This incubation facilitated the specific interaction between the surface-exposed CD68 receptors on target cells and the complementary lipid components within the DOPS/lecithin films. Following incubation, non-adherent cells were meticulously dislodged and removed through two sequential washing steps with phosphate-buffered saline (PBS), ensuring the isolation of only bound cellular populations. The successfully captured CD68-positive cells, now associated with the DOPS/lecithin films pre-loaded with R6G, were subsequently visualized and quantified using fluorimetry and fluorescence microscopy. The intensity of the fluorescent signal emitted by the R6G served as a direct quantitative measure, linearly correlating with the number of specifically bound liposomes and, by extension, the abundance of CD68-positive cells. Lecithin-only lipid films, devoid of DOPS and R6G, were employed as a crucial control to determine the extent of non-specific cellular adsorption and to enable accurate background fluorescence subtraction. The binding kinetics of R6G-labeled lecithin-based and DOPS/lecithin-based liposomes to eosinophils in BALF samples from patients with various respiratory conditions were assessed. Measurements were performed with 5 × 105 cells per well, a lipid concentration of 0.1 mg/mL, and R6G concentration of 1 µg/mL in PBS (0.01M, pH 7.4) at 37 °C. Normalized kinetic curves of R6G fluorescence changes were recorded over time on SpectraMax M5 Microplate Reader (Molecular Devices, MDS Analytical Technologies, Sunnyvale, CA, USA).
2.5. Confocal Laser Scanning Microscopy (CLSM) Analysis
BALF samples from patients with bronchial asthma and acute bronchitis were incubated with R6G-labeled lecithin-based and DOPS/lecithin-based liposomes. Eosinophils were visualized using CLSM Olympus FluoView FV1000 (Olympus Corporation, Tokyo, Japan), with nuclei stained with DAPI. DAPI fluorescence was detected at λexci,max = 340 nm, λemi = 410–500 nm. R6G fluorescence was detected at λexci,max = 520 nm, λemi = 570–700 nm. Integral values of fluorescence signal intensity, background intensity, signal-to-background ratio, and co-localization of R6G with DAPI were calculated using ImageJ software (v. 1.54d, NIH, Bethesda, MD, USA).
2.6. FTIR Spectroscopy Analysis
To validate binding specificity, FTIR spectroscopy was employed to analyze interactions between liposomes and eosinophils isolated from BAL samples of patients with bronchial asthma using a Bruker TensorFlow 27 device (Bruker, Ettlingen, Germany) and MICRAN-3 FTIR microscope (Simex, Novosibirsk, Russia). Spectra of cells (eosinophils and CD68+ macrophages) were obtained at different time points (step 5 min) during incubation with R6G-loaded lecithin-based or DOPS/lecithin-based liposomes in PBS (0.01M, pH 7.4) at 37 °C. Analysis focused on the amide I region (1600–1700 cm−1) and the ν(CH2) region (2800–3000 cm−1). Kinetic curves of FTIR peak positions for Amide I (maximum position and intensity) and ν(CH2,s) and ν(CH2,as) (maximum positions) were generated as a marker of liposome-cell interaction.
2.7. Evaluation of Assay Performance and Clinical Correlation
To rigorously assess the diagnostic potential and clinical relevance of the developed lipid-film-based capture assay, its performance was evaluated using patient-derived samples. Two key quantitative parameters were determined for both the experimental (DOPS/lecithin) and control (lecithin-only) liposome formulations: the initial binding rate (expressed as %/min), reflecting the kinetics of cellular capture, and the overall effective binding index after a 1-h incubation period (expressed as %), indicating the total cellular capture efficiency—were measured on SpectraMax M5 Microplate Reader (Molecular Devices, MDS Analytical Technologies, Sunnyvale, CA, USA).
Measurements were conducted on BALF samples obtained from a carefully selected cohort of six distinct patients. These patients were chosen to represent a diverse spectrum of clinical diagnoses, thereby enhancing the generalizability of the findings regarding the utility of this assay across varying pathological conditions. For each patient sample, all binding parameters were meticulously assessed in triplicate (n = 3 independent technical replicates) to ensure statistical robustness and reproducibility. Subsequently, these newly derived liposome-binding indices were correlated against established clinical biomarkers, including comprehensive complete blood count (CBC) parameters and C-reactive protein (CRP) levels, to investigate their potential as novel diagnostic indicators reflective of underlying disease states or inflammatory processes.
3. Results and Discussion
3.1. CD68+ Cells Isolation Technique and CD68 Validation
We have developed a novel, specific and effective approach for the direct analysis of CD68+ immune cells, primary macrophages, crucial for understanding respiratory inflammatory processes. We suggest applying a series of fluorescently labeled liposomal ligands based on phosphatidylserine (PS), specifically dioleoylphosphatidylserine (DOPS) as a primary, high-affinity (Kd ≈ 10–7 M) ligand for CD68. This strategy leverages the inherent binding properties of DOPS, enabling its use as a powerful, antibody-independent alternative for routine in vitro diagnostics in cellularly limited samples, such as BALF.
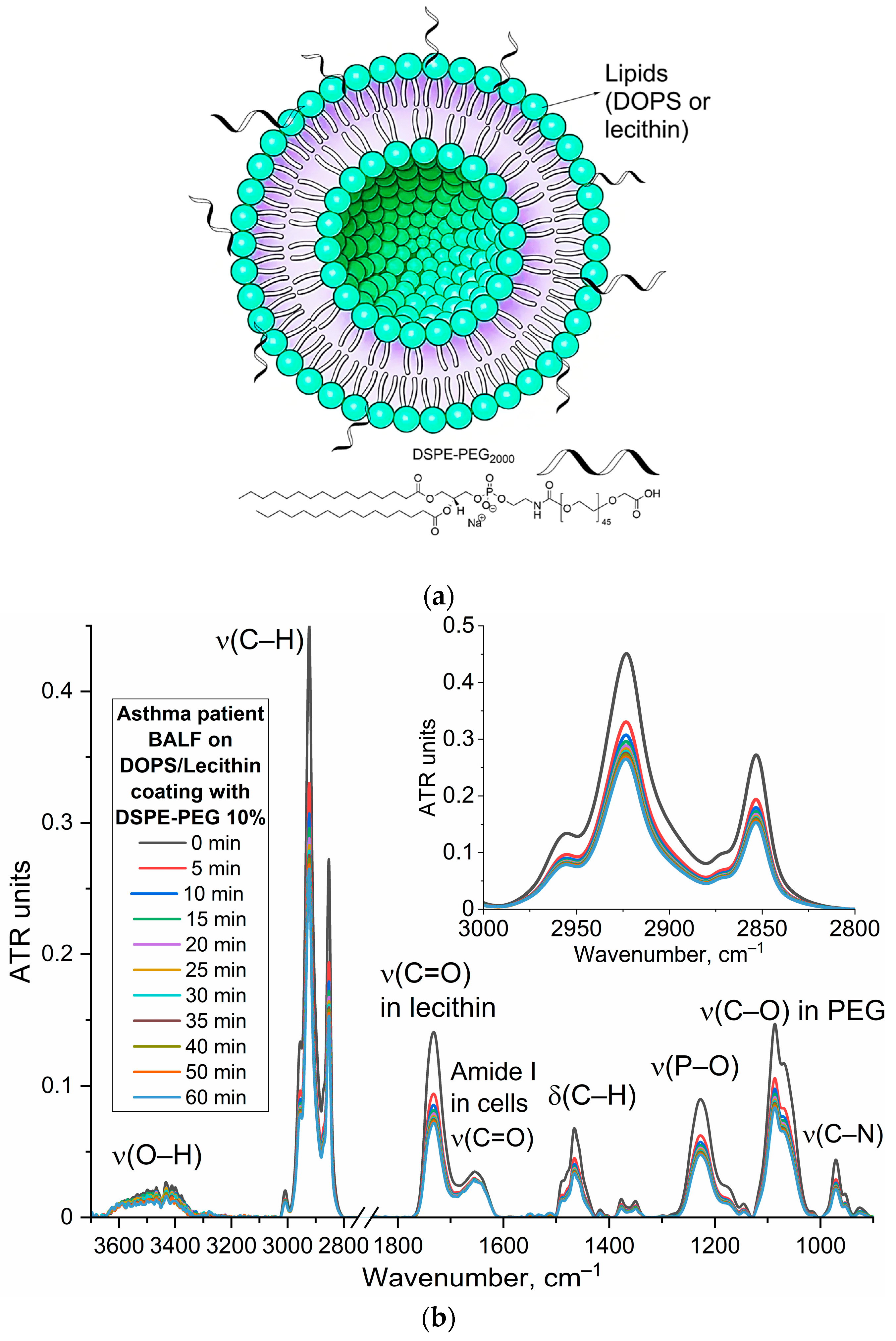
The base of our approach involves DOPS/lecithin liposomes as convenient, self-assembling lipid carriers (
Figure 1a). These liposomes contain fluorescent reporters like Rhodamine 6G, forming a tunable system where specificity can be regulated through the ligand’s content and architecture. The remarkable specificity of DOPS binding is rooted in CD68’s well-established role as a scavenger receptor, whose three-dimensional structure reveals distinct hydrophobic pockets ideally suited for binding anionic phospholipids like DOPS.
The protocol includes the preparation of DOPS/lecithin film at the bottom of a fluorescent plate (1), through solvent evaporation techniques. These films create capture layers (2a). BALF, containing a heterogeneous population of CD68+ cells including macrophages and eosinophils, is then added and incubated at 37 °C for 1 h to facilitate interaction between CD68 receptors on the target cells and the DOPS ligands in films (2b). Washing steps (2c) remove unbound cells, leaving only CD68+ cells interacting with the DOPS/lecithin liposomes (different composition) containing R6G (2d). This interaction is specific, mediated by the phospholipid DOPS. Finally, the captured CD68+ cells are visualized via fluorescence microscopy (3) by detecting the emitted fluorescence of R6G incorporated within the liposomes bound to CD68+ cells. The changes in the intensity of the fluorescent signal (binding index relative to control) are directly correlated with the content of DOPS/lecithin liposomes bound to CD68+ cells. Lecithin-based liposomes without DOPS were used as a control, and serve to assess non-specific binding of liposomes to the cells.
Figure 1b presents the predicted three-dimensional structure of the human CD68 protein (UniProt P34810), offering crucial structural insights into its function. A prominent feature highlighted is the hydrophobic anchor, vital for the protein’s stable integration into the cell membrane, reflecting its role as a surface-expressed receptor. Furthermore, the structure reveals distinct hydrophobic pockets. These pockets are essential for the interaction with a diverse range of ligands, encompassing oxidized low-density lipoproteins (LDL) that are specifically targeted due to their origin as byproducts of cellular degradation. CD68 serves as a receptor-like scavenger, recognizing and binding to these ligands, including cellular debris, and specifically, anionic phospholipids like DOPS (dioleoylphosphatidylserine). This structural depiction supports CD68’s established role as a scavenger receptor involved in the recognition and internalization of diverse extracellular materials, providing a basis for understanding its interaction with specific liposomal formulations and model lipid films containing DOPS.
To further quantify these interactions, computational analysis was performed using the open-access platform (open.playmolecule.org) to predict the binding affinity of CD68 with DOPS and lecithin. DOPS exhibits a more favorable (more negative) binding free energy (ΔG) of –9.7 kcal/mol and a higher predicted pKd of 7.2, compared to lecithin’s ΔG of –8.1 kcal/mol and pKd of 6.0. This computational finding indicates a significantly stronger predicted binding affinity of CD68 for DOPS than for lecithin (selectivity is more than 10 times). This aligns with CD68’s well-documented function as a scavenger receptor that preferentially recognizes and binds to anionic phospholipids like phosphatidylserine (PS), which is widely exposed on the surface of apoptotic cells to facilitate their clearance. Lecithin (phosphatidylcholine, PC), being a zwitterionic lipid, would consequently be expected to interact less strongly.
The remarkable specificity of our DOPS-based liposomes for the CD68 receptor arises from a sophisticated interplay of biophysical forces. We’ve identified three primary factors that contribute to this highly selective binding (
Figure S1):
Electrostatic complementarity (Major factor): At the core of this selectivity lies electrostatic complementarity. Detailed analysis of the CD68 receptor’s surface reveals a distinct region characterized by a concentrated positive charge, primarily due to the presence of arginine and lysine residues. Phosphatidylserine (DOPS), in turn, possesses a net negative charge on its headgroup. This fundamental charge difference drives a powerful electrostatic attraction, facilitating the formation of robust ionic bonds, or ‘salt bridges,’ with CD68’s positively charged pocket. In contrast, lecithin, being zwitterionic (with a net neutral charge), lacks the focused negative charge required for such strong, directed ionic interactions. Furthermore, its positively charged moiety can experience electrostatic repulsion from the CD68 binding site.
Geometric and steric contributions: Beyond charge, geometric fit plays a crucial role. The headgroup of DOPS is notably more compact, with a cross-sectional area of approximately 65.3 Å2. Conversely, the lecithin headgroup is bulkier, measured at around 72.5 Å2. This smaller footprint of the DOPS headgroup allows it to fit more precisely and deeply into the CD68 binding pocket, effectively minimizing steric hindrance and maximizing the number of favorable molecular contacts. This optimal fit enhances the overall binding affinity and specificity.
Hydrophobic interactions and a universal recognition mechanism: While the primary specificity is determined by the polar headgroup interactions, the overall affinity to lipid structures is significantly enhanced by the hydrophobic tails of the phospholipids. These tails engage with a complementary hydrophobic channel within the CD68 receptor, providing a substantial contribution to the binding energy for both DOPS and lecithin.
Importantly, this mechanism of recognizing negatively charged motifs appears to be a universal function for CD68. It explains why CD68 effectively binds not only phosphatidylserine presented on apoptotic cells but also oxidized low-density lipoproteins (oxLDL). During the oxidation process, oxLDL acquires negatively charged groups on its surface, which are recognized by the very same positively charged pocket on CD68. This phenomenon strongly reinforces CD68’s critical role as a versatile pattern recognition receptor for various danger-associated molecular patterns (DAMPs), highlighting its significance in innate immunity and inflammatory responses. These in silico predictions provide a molecular basis for understanding the selective interactions observed in experimental systems, reinforcing the potential for targeted delivery strategies leveraging CD68.
3.2. CD68+ Macrophages, Eosinophils and Other Cells in BALF Characterization
According to the available literature, cells that express the CD68 receptors exhibit characteristics mainly associated with the pro-inflammatory M1 macrophage phenotype, whereas cells bearing the CD206 antigen mainly display an anti-inflammatory profile [
54]. BALF comprises a heterogeneous population of cells reflecting the underlying pulmonary condition.
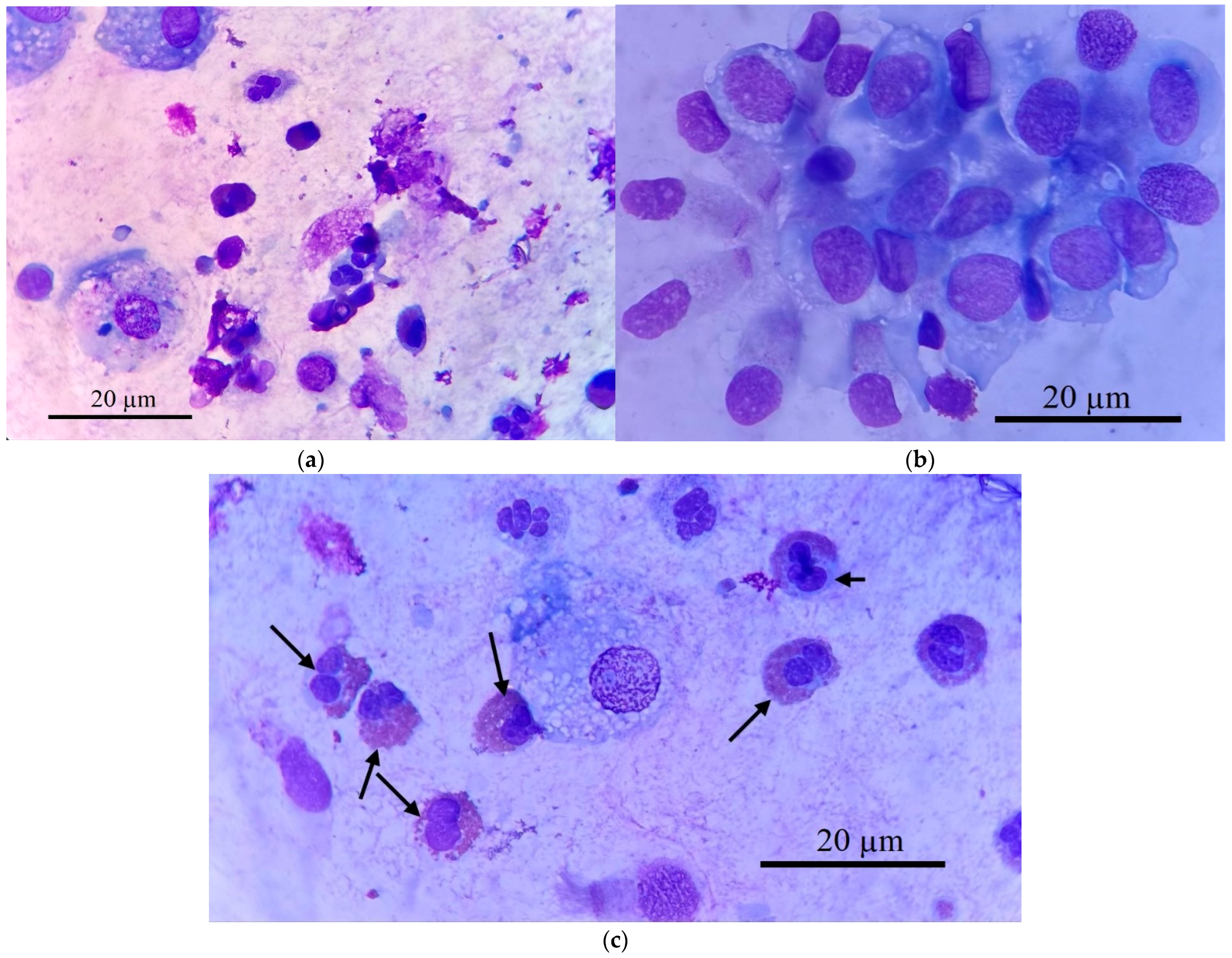
Figure 2 demonstrates this cellular heterogeneity, showing macrophages, eosinophils, neutrophils, and epithelial cells (
Figure 2a,b). Our analysis focused on isolating specific target cells (
Figure 2c), primarily macrophages, for subsequent CD68 receptor analysis.
Figure 2 provides representative micrographs illustrating the heterogeneous cellular composition of BALF samples from a patient with bronchial asthma. Histological characterization, employing Romanowsky staining, revealed distinct cell populations critical to understanding respiratory inflammation.
Macrophages (
Figure 2a,c) are typically identified by their large size, abundant cytoplasm, and often a lobed nucleus. Within this population, a significant subtype are
epithelioid cells (
Figure 2b), as one of the types of differentiated macrophages with pronounced secretory activity characteristic of M1-phenotype reflecting an active inflammatory response [
55]. These cells often lack phagosomes and possess a well-defined lobed nucleus with a prominent nucleolus. A hallmark morphological feature of epithelioid cells is their characteristic “lacy” irregular plasma membrane edge, resulting from continuous vesicle fusion, and they can reach sizes up to 100 µm, frequently containing 2–3 lobed nuclei. Their presence, alongside elevated pro-inflammatory cytokines such as IL-8 (and to a lesser extent IL-2 and IL-1β) and decreased IL-4 and IL-5, strongly indicates a predominance of M1-phenotype macrophages, reflecting an active inflammatory response.
Eosinophils (indicated by arrows in
Figure 2c, also visible in
Figure 2a) are readily distinguishable by their bilobed or trilobed nucleus and, most characteristically, their cytoplasm filled with numerous distinct, bright reddish-orange granules when stained with Romanowsky dyes.
Neutrophils (
Figure 2a) exhibit a multi-lobed nucleus and pale, fine granules. This detailed cellular analysis allows for a comprehensive assessment of inflammatory profiles in respiratory diseases.
Table 2 summarizes the differential cell counts obtained from BALF samples of several patients representing various respiratory diseases. These data highlight the distinct cellular profiles characteristic of each condition.
CD68 serves as one of the markers of macrophages [
54,
55,
56,
57]. While eosinophils exhibit minimal CD68, their frequent co-occurrence with macrophages in inflammatory conditions means their combined presence can help predict the overall CD68-related inflammatory profile. Consequently, in bronchiectasis—a chronic respiratory disease characterized by permanently widened airways—the typically high macrophage percentage (in this case around 19%) strongly indicates significant CD68 staining, reflecting substantial inflammatory activity. The substantial macrophage population in pneumonia (14%) also suggests potential for CD68 positivity, although further investigation is needed to correlate staining intensity with inflammatory state. In eosinophilic asthma, despite a low macrophage percentage (1.3%), the remarkably high eosinophil count (65.4%) suggests sufficient CD68 expression might still be present, warranting further analysis to determine its contribution. Conversely, bronchial asthma, primary ciliary dyskinesia (PCD), and bronchitis, all exhibiting low macrophage percentages (1.3%, 2%, and 1%, respectively), and minimal eosinophils, are anticipated to show minimal CD68 staining. Immunohistochemical analysis using CD68 staining would validate these predictions, offering a quantitative assessment of macrophage and eosinophil contributions to CD68 expression.
3.3. Confocal Microscopy Analysis of Liposome Binding to BALF Cells
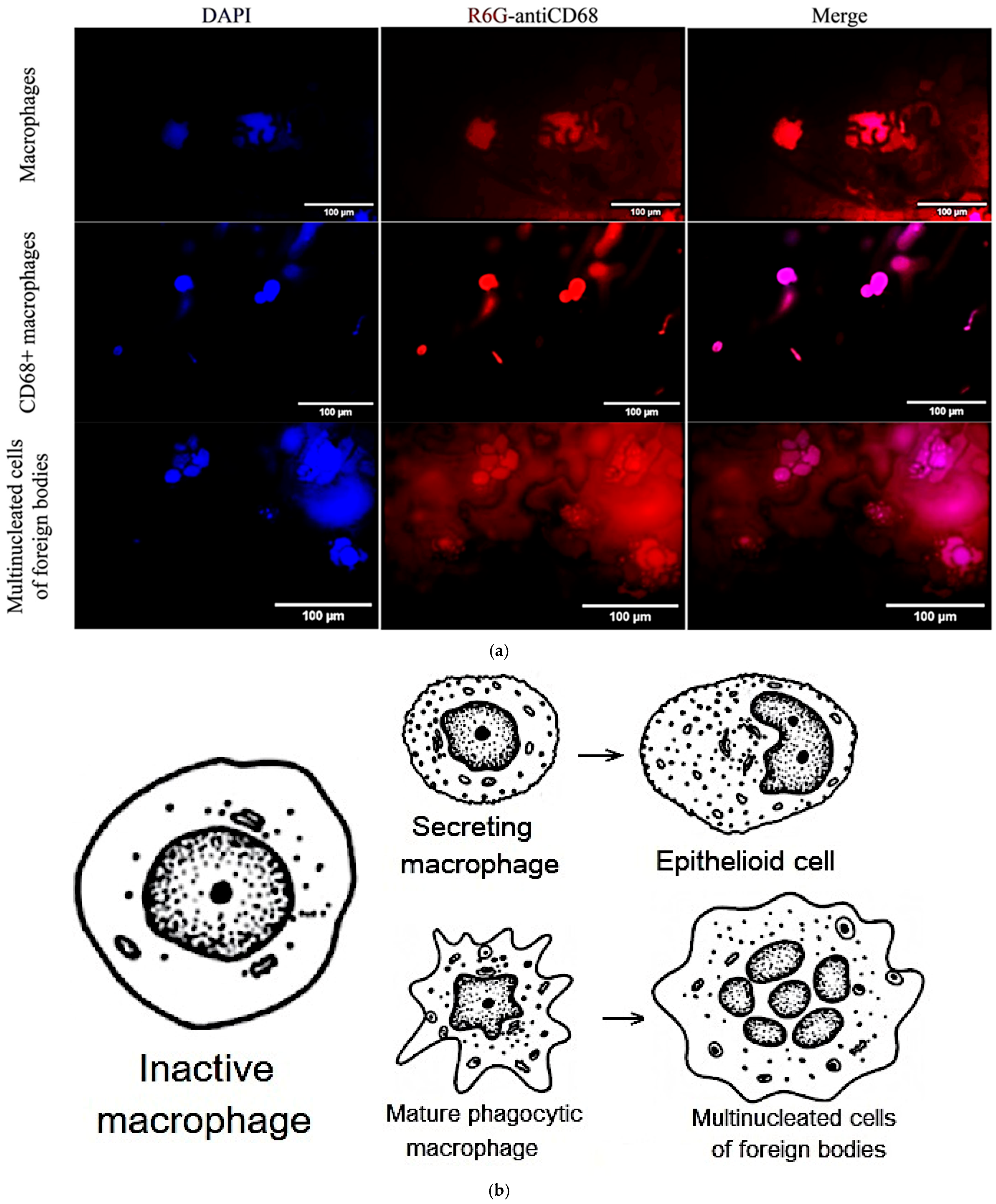
In order to further explore the interaction between our R6G-labelled DOPS/lecithin liposomes and BALF cells in the context of various respiratory disorders, we employed confocal laser scanning microscopy (CLSM) as a tool. BALF samples obtained from individuals with bronchitis (illustrated in the first two rows of
Figure 3a) and bronchial asthma (third row) were carefully incubated with liposomes based on DOPS and lecithin. This approach provided invaluable insights into the morphological features of these critical immune and inflammatory cells, as well as the specific patterns of liposome attachment.
In the first row of
Figure 3a, we have BALF cells (at bronchitis) incubated with lecithin-based liposomes. Within this field, we can observe two distinct types of cells. On the left side, there is a typical phagocytic macrophage. These cells display a characteristic polymorphic morphology, characterized by an irregular shape and a single, distinct nucleus. On the right side, we observe a multinucleated macrophage, which has formed through the fusion of several cells. Its cytoplasm is densely filled with various vacuoles and phagosomes, indicating its active involvement in phagocytosis and the clearance of debris from the respiratory tract. This morphological feature aligns with the general representation of macrophages depicted in
Figure 3b.
In row 2 of
Figure 3a, which involved BALF cells (at bronchitis) incubated with DOPS/lecithin liposomes, we observed a preponderance of M1-phenotype CD68+ macrophages. These macrophages typically exhibit a more rounded morphology with a more prominent cytoplasm compared to phagocytic macrophages. The strong expression of CD68, visualized through the binding of DOPS ligands, indicates an activated state, reflecting their increased secretory activity characteristic of the M1 phenotype. This is conceptually represented as “secretory type” macrophages in
Figure 3b.
The third row in the experiment involving Bronchial asthma BALF cells incubated with DOPS/lecithin liposomes demonstrates the formation of foreign body giant cells (FBGCs), which are large, multinuclear cells that are a hallmark of chronic inflammation. These cells arise from the fusion of multiple macrophages in response to substances that are too large or insoluble for individual cells to clear, a process known as frustrated phagocytosis. Morphologically, FBGCs are characterized by their substantial size, multiple nuclei frequently clustered centrally, and expansive cytoplasm containing numerous phagosomes or entrapped material. Conceptually, as illustrated in
Figure 3b, they represent the final stage of differentiation of mononuclear phagocytic cells and play a crucial role in the intricate inflammatory processes occurring within BALF. It is worth noting that minimal cell capture was observed when using plain lecithin, hence its exclusion from this study.
Figure 3b serves as a schematic representation that further aids in the classification of distinct cellular populations within BALF. It specifically depicts the characteristic morphologies of mature phagocytic and secretory macrophages, as well as epithelioid cells. This schematic serves as a complementary visual reference for CLSM images.
To quantitatively assess the liposome binding, we analyzed the integral values of cell-associated fluorescence signals obtained from processing these CLSM images, as summarized in
Table 3.
As is evident from
Table 3, the CD68+ secreting type macrophages exhibited the highest signal intensity (157 a.u.) and, crucially, the most impressive Signal/Background ratio of 79. This significantly higher ratio, compared to other type of macrophages (11) and multinucleated cells (21), strongly indicates that our DOPS/lecithin liposomes preferentially bind to cells with elevated CD68 expression, confirming the specificity of our DOPS-based marker for mature, activated macrophages. While all three cell types showed detectable binding, the enhanced signal in CD68+ mature macrophages underscores the utility of our system in identifying and quantifying this specific, diagnostically relevant subpopulation.
3.4. FTIR Spectroscopy Analysis of CD68 Status of BALF Immune Cells
3.4.1. Methodology and Confirmation of Liposome- BALF CD68+ Cells Binding Specificity
To investigate the molecular-scale interactions between our liposomes and immune cells, particularly CD68+ cells (macrophages) isolated from BALF, and gain insights into their binding dynamics, we utilized Fourier Transform Infrared (FTIR) spectroscopy. This powerful analytical technique provides detailed information about chemical structure, secondary structure changes, and conformational dynamics of biomolecules, allowing for real-time monitoring of subtle spectral alterations in liposomes upon interaction with target cells.
Figure 4a presents the time-resolved FTIR spectra of DOPS/lecithin-based liposomes (10/90
w/
w) loaded with R6G, obtained upon incubation with CD68+ cells isolated from BALF samples of patients with bronchial asthma. Control FTIR spectra for pure lecithin-based and DOPS-based liposomes are provided in
Figure S2. Our analysis primarily focuses on the Amide I region (1600–1700 cm
−1), sensitive to protein secondary structures, and the ν(CH2) region (2800–3000 cm
−1), which reflects the lipid acyl chain organization.
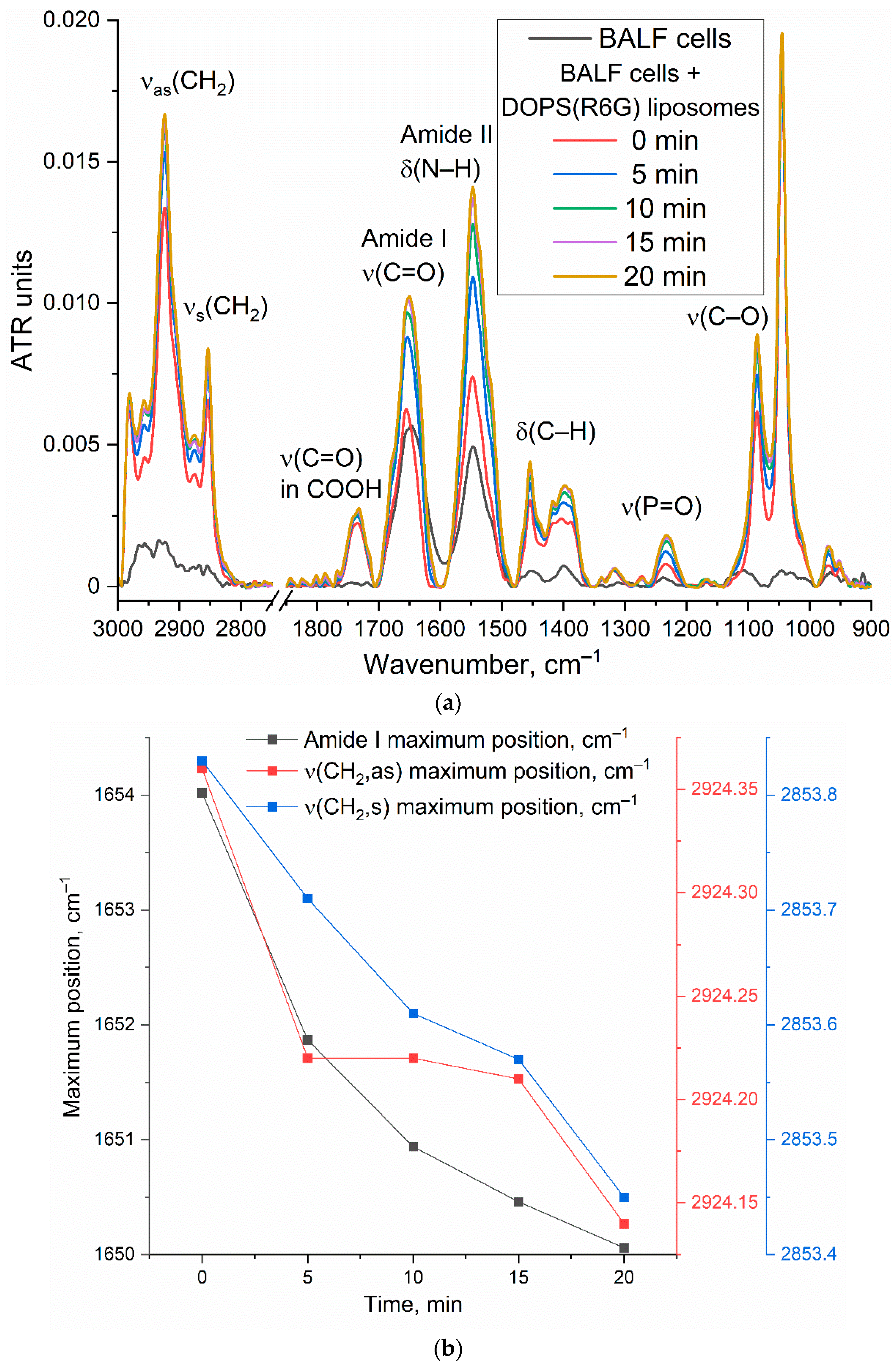
Figure 4b further illustrates the kinetic changes in key FTIR spectral features (Amide I maximum position and intensity, ν(CH2,s) and ν(CH2,as) maximum positions), reflecting dynamic alterations upon liposome interaction with the cell surface.
The observed time-dependent changes in the FTIR spectra provide strong evidence for the interaction of DOPS-containing liposomes with CD68+ cells. The shift in the Amide I peak position, coupled with an increase in intensity, suggests conformational changes or an altered microenvironment of cellular proteins upon liposome binding. The shifts observed in the ν(CH2,s) and ν(CH2,as) positions indicate reorganization of lipid acyl chains, likely involving both liposomal and cellular membrane lipids. These changes are consistent with mechanisms such as liposome adsorption, insertion, or fusion into the cell membrane. While further experiments are needed to definitively quantify selectivity, the observed spectral changes and their specific kinetic profiles are consistent with a significant interaction between DOPS-containing liposomes and BALF CD68+ cells, supporting the potential for targeted cellular interaction by our liposomal system.
3.4.2. FTIR-Based Optimization of Liposome Composition for Differential Diagnosis of Respiratory Diseases
This section details the strategic use of FTIR spectroscopy to optimize liposome composition, specifically DOPS content, for enhanced differential diagnostic capability based on distinct cellular interactions in BALF from different patient disease states (e.g., Bronchiectasis vs. Bronchial Asthma). FTIR’s ability to reveal subtle, disease-specific characteristics through quantitative differences in binding indices offers a novel method for identifying molecular signatures not always discernible via standard analyses.
To determine the optimal lipid film composition, particularly the DOPS mass content, for improved diagnostic differentiation across patient groups, we employed FTIR spectroscopy to monitor subtle alterations in cell-lipid interactions dependent on the disease state (
Figure 5). Kinetic analyses focused on two analytically significant frequencies: the 1732 cm
−1 (ν(C=O)) band, reflecting lipid headgroup hydration and conformation, and the 2923 cm
−1 (ν(CH
2)) band, sensitive to hydrophobic core packing and fluidity. While both frequencies provided complementary information, the 2923 cm
−1 peak was identified as particularly critical for optimizing DOPS concentration due to its direct correlation with membrane fluidity and its utility in characterizing robust binding dynamics (as exemplified in
Figure 5d,f).
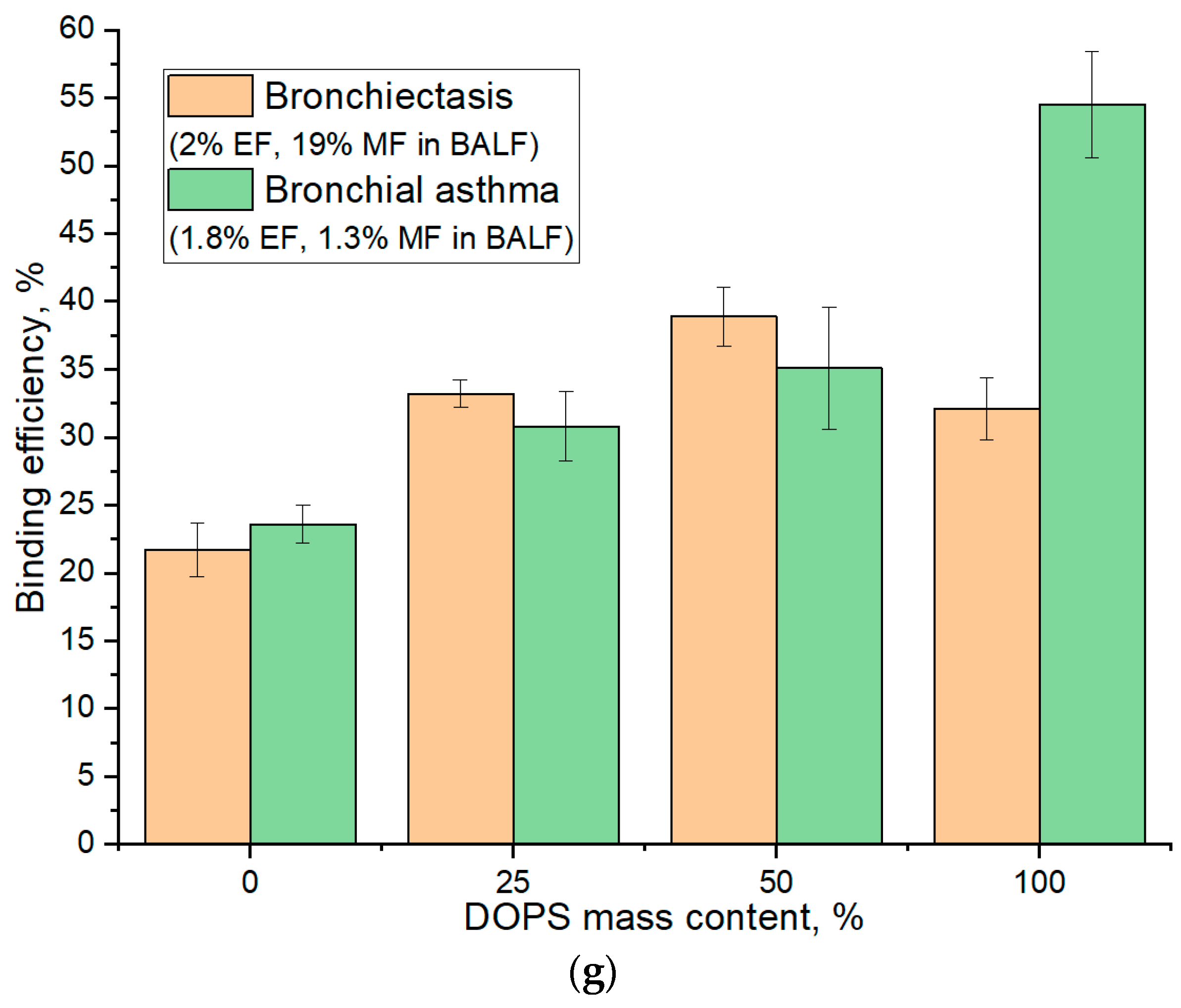
Figure 5g illustrates the binding efficiency as a function of DOPS mass content, revealing distinct optimal compositions tailored to specific patient conditions. For BALF samples from patients with Bronchiectasis (2% eosinophils and 19% macrophages), the maximal binding efficiency (39%) was observed at a 50% DOPS mass content. In contrast, for Bronchial Asthma samples (1.8% eosinophils and 1.3% macrophages), the binding efficiency is lower (35%) at 50% DOPS and continually increased with the concentration of DOPS phospholipid, reaching its maximum (55%) at 100% DOPS. The largest absolute difference in binding efficiency between these two patient groups was also observed at 100% DOPS mass content (32% for Bronchiectasis vs. 55% for Bronchial Asthma).
Crucially, for optimal diagnostic discrimination between these disease states, particularly when elucidating macrophage interaction profiles, our analysis suggests that a DOPS mass content in the 25% range offers superior analytical value. While 100% DOPS demonstrated the highest overall binding percentages, the lower concentration range provides for more stable liposomal particles and would enhance the selectivity of interaction with CD68+ macrophages, thus providing a more potent diagnostic signal for distinguishing between diseases.
Kinetic curves (
Figure 5c–f) indicated that the most significant spectral changes occurred within the initial 5–10 min of interaction, subsequently reaching a plateau. This rapid stabilization suggests an optimal analysis time within 15–20 min for practical diagnostic applications.
In summary, the optimized FTIR spectroscopic parameters—focusing on 25% DOPS films, kinetic analysis up to 15–20 min, and primary monitoring of the 2923 cm−1 band—provide a refined methodology for sensitive and differential analysis of cellular interactions in BALF, paving the way for improved diagnostic tools.
3.4.3. PEGylated Stealth Liposomes for Defining CD68 Subtypes
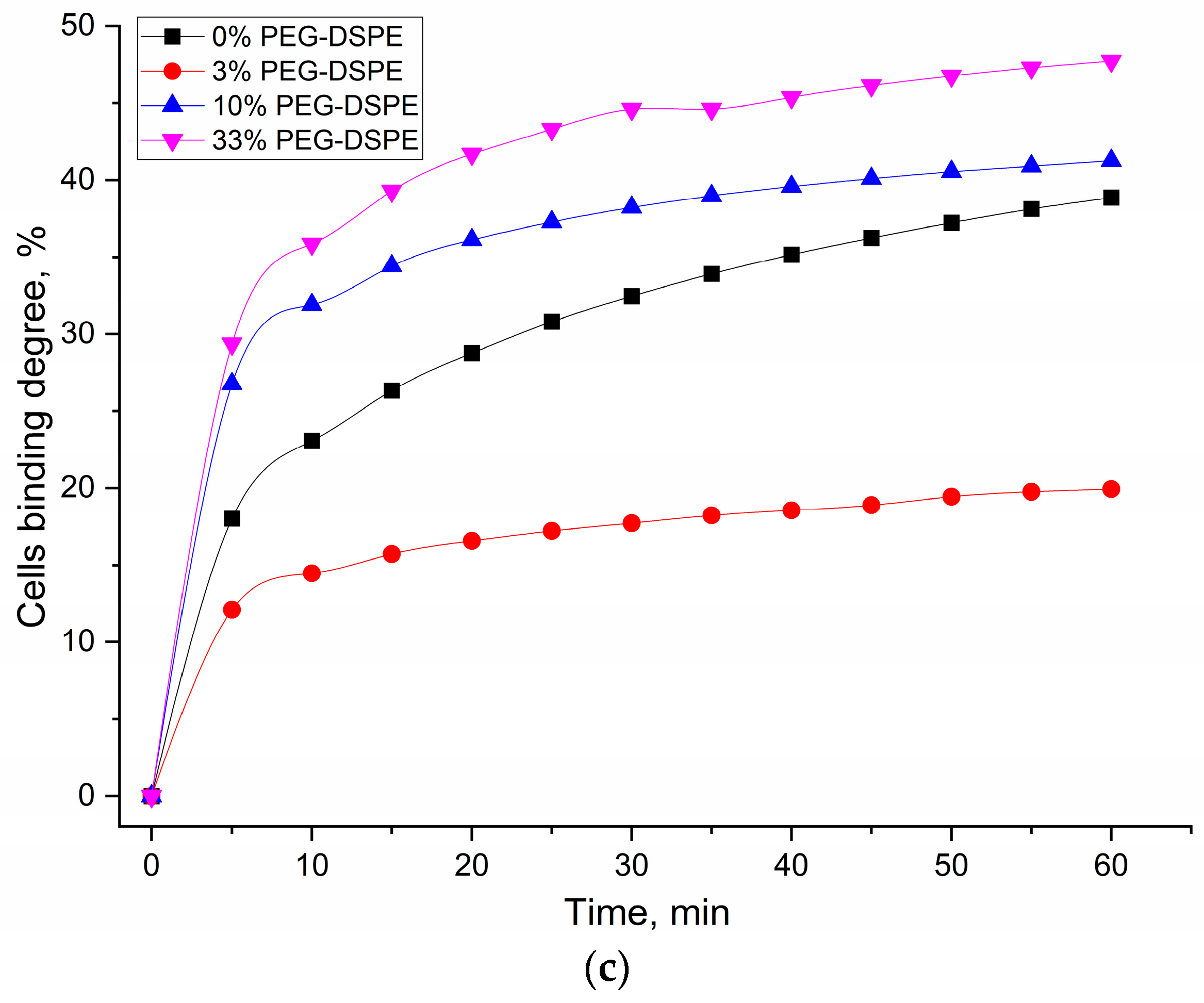
A significant challenge in achieving high binding specificity in biosensing applications is the contribution of non-specific interactions, notably from common membrane components like lecithin, which can lead to considerable baseline binding and confound specific signal detection. To overcome this non-specific binding and enhance the diagnostic specificity of our assay for CD68+ populations, we employed PEGylated stealth liposomes incorporating a PEG-lipid (1–10 mass. %). This modification aims to create a steric barrier on the liposome surface, thereby reducing non-specific protein adsorption and cellular adhesion, ultimately improving the signal-to-noise ratio and thus the diagnostic utility of binding indices DOPS vs. lecithin (e.g., aiming for robust differentiation beyond typical baseline values of 1.3–1.5 for bronchiectasis and ~2.5 for asthma).
Specifically, 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine polyethylene glycol (DSPE-PEG
2000) was incorporated into the lipid traps.
Figure 6b presents FTIR spectra of a DOPS/lecithin (75/25
w/
w) film interacting with BALF cells from bronchial asthma patients, with varying concentrations of incorporated DSPE-PEG
2000. The corresponding kinetic curves (
Figure 6c) illustrate the effect of increasing DSPE-PEG
2000 concentration on the binding kinetics.
In Bronchial Asthma samples, the binding index of liposomes with BALF cells initially decreased with the addition of 3% DSPE-PEG2000, indicating an effective reduction in non-specific interactions. However, a subsequent increase in binding was observed at higher DSPE-PEG2000 concentrations (10–33%). This intriguing phenomenon suggests that excessively high PEGylation might lead to altered surface properties of liposomes or induce alternative non-specific interactions, potentially due to changes in the conformation of PEG chains or effects on membrane mechanics. In contrast, eosinophilic asthma exhibited a distinct and more straightforward trend: the binding index decreased monotonically with increasing DSPE-PEG2000 concentration. This consistent reduction demonstrates effective competition for binding sites and a lack of significant increase in non-specific binding even at higher PEG concentrations. This implies that in eosinophilic asthma, the interaction with the DOPS/lecithin film is predominantly specific to the target (CD68 receptors of immune cells), and PEGylation robustly masks non-specific interactions, leaving primarily the desired DOPS-mediated interaction. These results strongly suggest that the PEGylated liposome approach provides a valuable tool to differentiate the specific binding characteristics of cells in eosinophilic versus non-eosinophilic asthma, potentially reflecting underlying differences in membrane receptor expression or interaction mechanisms.
Here, we investigate the impact of varying DSPE-PEG content on the relative binding index of liposomes and, crucially, on the selectivity of their interaction with CD68, using the relative binding index data presented in
Figure 7. The relative binding index demonstrates distinct trends across different liposome compositions.
The incorporation of DSPE-PEG significantly enhanced the specific binding performance of DOPS-containing liposomes. Both DOPS-only and lecithin/DOPS-based systems exhibited improvements in their specific binding indices. While DOPS-only showed peak binding at 10% DSPE-PEG (index 1.6), the most pronounced enhancement for lecithin/DOPS-based films was observed at 5% DSPE-PEG, achieving a specific binding index of 1.8. Crucially, at this optimal 5% DSPE-PEG concentration for the lecithin/DOPS formulation, the specific binding index (1.8) was substantially higher than that observed for DOPS-only system at the same concentration (index 1.3). This significant difference highlights the superior selectivity achieved with the lecithin/DOPS formulation at 5% PEG, indicating its enhanced ability to differentiate specific interactions. This improvement underscores that PEGylation, by creating a steric barrier, effectively mitigates non-specific interactions, thereby allowing the specific CD68-mediated binding to DOPS to become more prominent and efficient.
Mechanistically, PEGylation forms a hydrophilic “brush” on the liposome surface, which primarily functions by:
Reducing non-specific adhesion: Steric hindrance from PEG chains minimizes non-specific electrostatic or hydrophobic interactions, particularly those involving neutral lecithin components.
Optimizing specific ligand presentation: By suppressing non-specific binding, the relative contribution and effective presentation of the specific CD68-DOPS interaction are significantly amplified (potentially up to 3-fold), thus greatly increasing the signal-to-noise ratio for CD68-mediated recognition of anionic phospholipids.
The observed optimal selectivity at 5% DSPE-PEG suggests a critical balance: the PEG corona is sufficiently dense to mask non-specific binding sites while preserving optimal accessibility of DOPS for specific CD68 recognition. Higher PEG concentrations (e.g., 10–33%) may introduce some liposomes destabilization, excessive steric hindrance, potentially slightly impeding the specific ligand-receptor interaction itself, leading to a minor reduction in selectivity.
These findings conclusively demonstrate that optimized PEGylation (5% DSPE-PEG) dramatically enhances the selectivity of CD68-mediated binding to DOPS-containing liposomes. This not only confirms PEG’s role in improving liposomal stability but, more critically, refines the specificity of receptor-mediated targeting, offering a powerful tool for developing precise diagnostic agents or therapeutic carriers for CD68-expressing cells like macrophages.
3.5. Fluorimetric Detection of CD68 Receptors on Macrophages and Eosinophils in Order to Differentiate Diagnoses
To complement and further validate our findings from FTIR and CLSM analyses, and to confirm the enhanced selectivity observed with PEG-DOPS liposomes, we developed a fluorimetric method for different diagnosis based on CD68 receptor profiling. This assay specifically quantifies the interaction of BALF-derived CD68-positive cells with DOPS/lecithin-based liposomes. The liposomes were designed to encapsulate rhodamine 6G (R6G) as a fluorescent reporter.
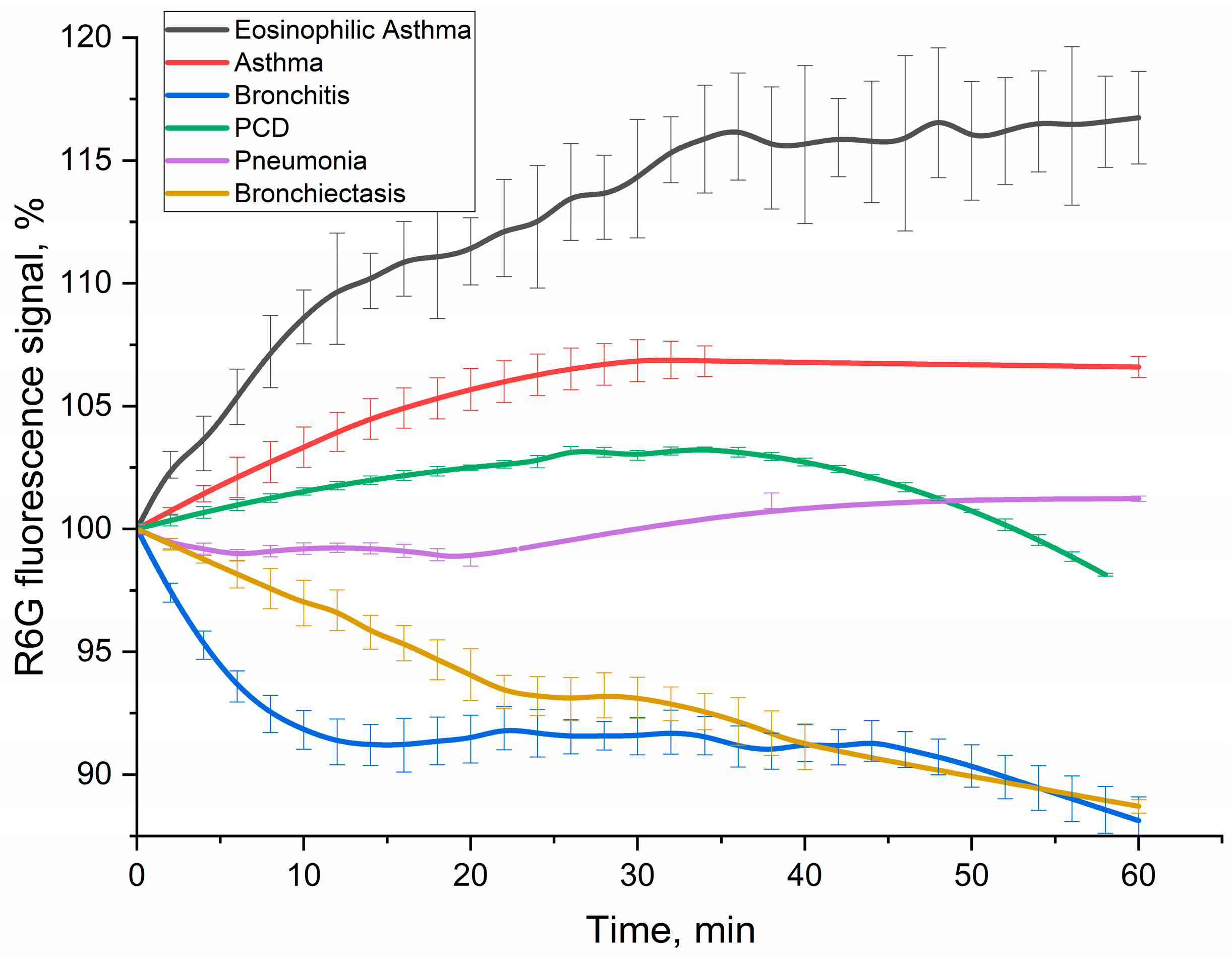
Figure 8 displays the kinetic curves of R6G fluorescence signal over 60 min following incubation with BALF cells from patients across various pulmonary diagnoses. Crucially, the contribution of non-specific binding from lecithin-based liposomes was rigorously accounted for and subtracted, ensuring that the measured fluorescence signal exclusively reflected specific CD68-mediated interactions with DOPS.
It is well-established that R6G fluorescence can be significantly quenched upon cellular uptake or interaction with intracellular environments. Indeed, our previous control experiments involving direct incubation of rhodamine 6G with cell lines such as A549 and K562 consistently demonstrated such quenching, evident as a reduction in fluorescence intensity [
58]. This phenomenon provides a plausible explanation for the observed decrease in rhodamine fluorescence in our current system upon interaction with BALF cells.
The kinetic profiles of R6G fluorescence signal change varied significantly across different diagnoses (
Figure 8), providing distinct patterns as potential diagnostic signatures. In order to interpret the kinetic profiles, it is necessary to delineate the stages of the process involving rhodamine. Liposomal R6G initially binds to cells, and either fusion or receptor-mediated endocytosis takes place, or phagocytosis, which is a response to large particles such as liposomes. These processes ultimately determine the variation in the fluorescent signal. Binding to the receptor within the hydrophobic pocket appears to amplify the signal emitted by R6G fluorescence. Conversely, fusion with the cellular membrane and the subsequent formation of endosomes lead to a reduction in fluorescence. Subsequently, once rhodamine enters lysosomes after approximately 20 min, it undergoes a process of digestion in an acidic environment. This indicates that regions with more intense fluorescence correspond to areas with a higher concentration of CD68+ receptors. Conversely, regions with a negative slope suggest primarily involve non-specific liposome fusion without receptor involvement.
Positive signal response. In case of eosinophilic asthma BALF samples (with M2 macrophage phenotype) showed the most pronounced and sustained R6G fluorescence increase (over 16% within 1h). This increased fluorescence signal correlates with their exceptionally high BALF eosinophil counts (65.4%), accompanied with intensive CD68 receptor activity and specific liposome binding and internalization by the cells. Other type of BALF (Bronchial Asthma: 1.8% eosinophils, 1.3% macrophages; PCD: 6.0% eosinophils, 2.0% macrophages) also exhibited a fluorescence increase, consistent with active interaction of DOPS/lecithin-based liposomes with CD68+ cells populations.
Negative signal response. In contrast, bronchitis (1.0% macrophages, 96% neutrophils) and bronchiectasis (19% macrophages, 33% neutrophils) patients’ BALFs showed a marked and persistent R6G fluorescence decline (approx. 11% reduction within 1 h). This remarkable quenching phenomenon, observed in the presence of macrophages, suggests the existence of an alternative interaction mechanism. It is possible that this mechanism involves the active phagocytosis of R6G-liposomes or their fusion with the biomembrane, resulting in the loss of fluorescence. Alternatively, it may be associated with a distinct phenotype of macrophages, compared to pro-inflammatory M1-type.
Minimal signal response. The BALF sample from a patient with pneumonia, which contained 14% macrophages and 81% neutrophils, exhibited minor alterations in R6G fluorescence. These findings suggest a delicate equilibrium between CD68-mediated interactions and non-specific interactions, such as phagocytosis of large particles and fusion, which leads to the quenching of rhodamine. This suggests a more intricate or transient cellular response, rather than a clear net uptake or quenching, indicating a mixed M0/M1/M2 phenotype.
The divergent kinetic profiles of R6G fluorescence observed across different disease states underscore the potential of this fluorimetric assay as a tool for differential diagnosis. The unique “kinetic pattern” reflecting the interaction of BALF cells with DOPS-containing liposomes provides valuable insights into the functional state and receptor activity of macrophages in various pulmonary pathologies. The clear distinction between positive (asthma, PCD) and negative (bronchitis, bronchiectasis) fluorescence changes, combined with the minimal response in pneumonia, suggests that this methodology could significantly aid in the sub-classification of respiratory diseases.
3.6. Diagnostic Performance Evaluation: Liposome Binding Kinetics and Correlation with Clinical Parameters
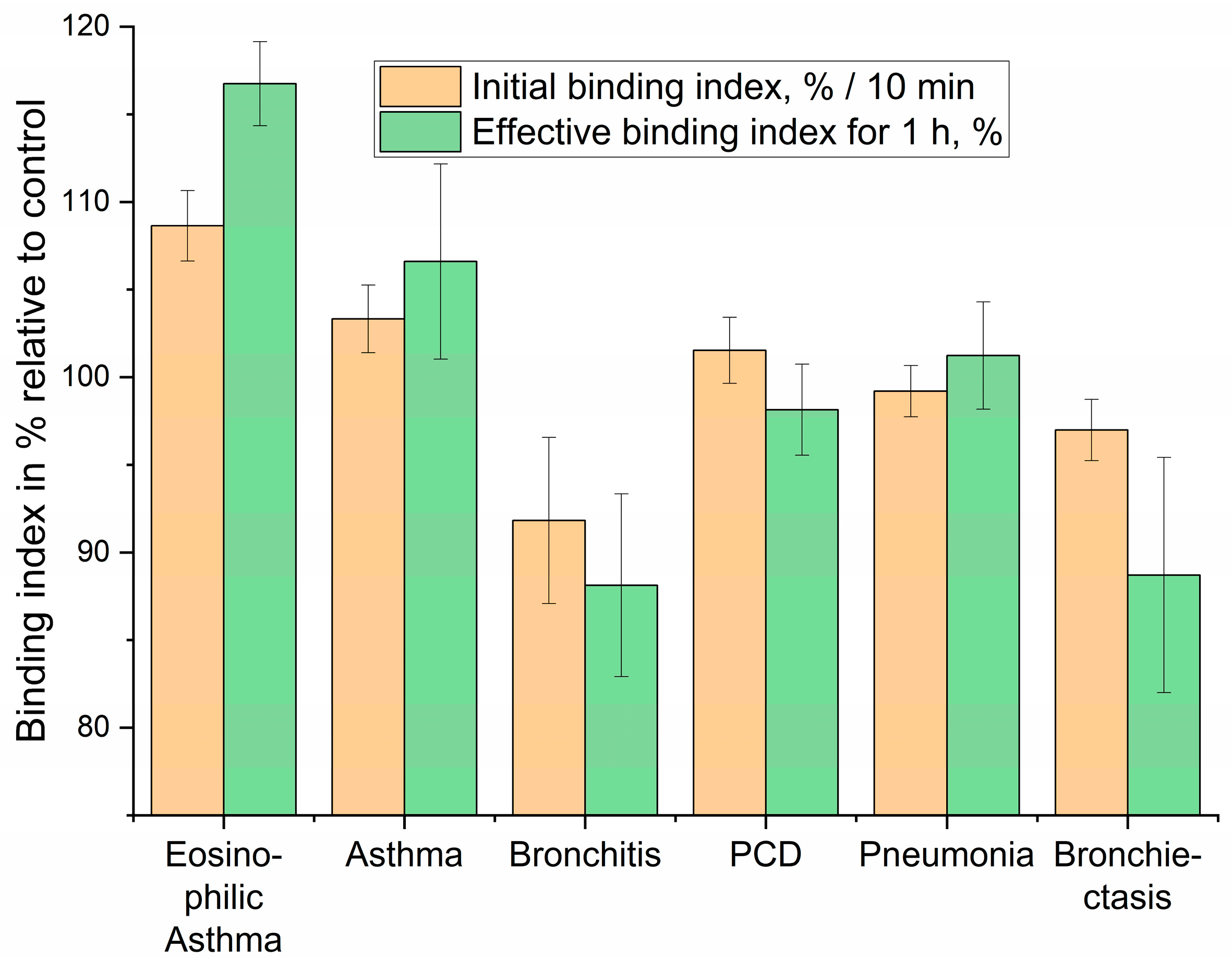
Based on the kinetic profiles of liposome binding to eosinophils in BALF, we aimed to evaluate the diagnostic performance of our approach. We focused on quantifying the initial binding rate (Initial binding index, %/min), reflecting the early interaction kinetics, and the effective binding index over a 1-h period (Effective binding index for 1 h, %), representing the overall interaction outcome (
Figure 9). These indices represent a simplified yet powerful “fingerprint” for patient diagnosis based on the distinct cellular interactions with DOPS/lecithin-based liposomes loaded with R6G. As before, background fluorescence from lecithin-based liposomes was subtracted to isolate specific CD68-mediated interactions.
The “Initial binding index” captures the early phase of the cellular interaction, specifically reflecting the change in R6G fluorescence at the 10-min mark. This metric is indicative of the rapid cellular recognition and initial binding kinetics. The “Effective binding index for 1 h” represents the cumulative fluorescence change, providing insight into the sustained cellular processing of the liposomes and the eventual fate of the encapsulated R6G. This two-parameter approach enables the differentiation of conditions that might otherwise present similar clinical symptoms, highlighting the potential for this assay to refine diagnostic precision in respiratory medicine.
3.7. Statistical Analysis of Lecithin and DOPS/Lecithin Binding to BALF Cells
To demonstrate the selectivity of the fluorescent ligand, we compared the binding affinities of lecithin liposomes and DOPS/lecithin (75/25 w/w) liposomes to cells derived from bronchoalveolar lavage fluid (BALF). Binding indices were derived from fluorescence data obtained from multiple samples (n = 50). The binding index quantitatively reflects the rate of liposome interaction with BALF cells and serves as a measure of binding affinity.
Figure 10 depicts individual binding indices (%/min) measured for lecithin (blue) and DOPS/lecithin (orange) liposomes across the BALF samples. Statistical analysis using two-tailed Student’s
t-tests revealed a significant difference between the lecithin and DOPS/lecithin groups (
p = 0.01). Moreover, the DOPS/lecithin group exhibited a bimodal distribution, indicating two distinct subpopulations with significantly different binding levels (
p < 0.001). This heterogeneity likely reflects variations in macrophage phenotypes or activation states within the BALF and underscores the potential of DOPS-containing liposomes for refined immunophenotyping in respiratory disease research.
Overall, these findings demonstrate that the incorporation of DOPS modifies liposome binding characteristics, enabling the discrimination of distinct immune cell subsets within BALF samples.
For statistical analysis, a two-tailed Student’s
t-test was employed to compare the mean binding indices between the lecithin and DOPS/lecithin groups (
Figure 10). This analysis demonstrated a statistically significant increase in binding affinity for the DOPS/lecithin liposomes relative to lecithin alone, with a
p-value of 0.01.
Moreover, the binding data for the DOPS/lecithin group revealed a bimodal distribution, indicative of two distinct subpopulations of CD68+ cells—one exhibiting normal CD68 expression and the other showing CD68 overexpression (
Figure 10, red curve). Additional two-tailed Student’s
t-tests comparing these subgroups confirmed a highly significant difference (
p < 0.001). These results suggest notable heterogeneity within the CD68+ cell population in BALF, potentially reflecting differences in cellular phenotype or activation states influencing ligand binding behavior.
3.8. Comparative Analysis of Macrophage Receptor Activity in Pulmonary Diseases
To quantitatively compare liposome-cell interactions across different analytical platforms, binding indices were derived from both fluorescence kinetic data (
Figure 8 and
Figure 9) and endpoint FTIR spectroscopy (
Figure 5 and
Figure 6). Fluorescence data yielded an Initial Rate Index (calculated from the first 10 min, %/min) and an Effective Binding Index (% change at 60 min). FTIR data provided a DOPS-Specific Binding Index (calculated as the percentage point increase in binding efficiency between 0% and 100% DOPS liposomes).
Both methodologies demonstrate concordant trends, highlighting a higher Effective Binding Index and thus, significantly stronger ligand-receptor interaction in asthma phenotypes. FTIR analysis reveals a nearly 3-fold greater DOPS-specific binding for bronchial asthma compared to bronchiectasis (+30.5% vs. +10.5%). This aligns with fluorescence data, where eosinophilic and bronchial asthma show positive indices, reflecting high surface binding or accessibility. In contrast, bronchitis and bronchiectasis exhibit negative indices, indicating significant fluorescence quenching likely due to rapid internalization and lysosomal processing.
These insights into the activity of the pan-macrophage marker CD68 complement previous findings on the M2-phenotype marker CD206 [
9] and phagocytic activity [
10], allowing for a more nuanced characterization of the macrophage landscape (
Table 4):
Bronchiectasis: This condition is characterized by chronic infection and a resulting pro-inflammatory state dominated by M1 macrophages. The assay accurately reflects this pathology, yielding a signature of weak binding to the M2-specific CD206 marker coupled with a strong interaction with the CD68-DOPS probe. Critically, this strong interaction manifests as significant fluorescence quenching. M1 macrophages are “classically activated” phagocytes, primed to engulf and destroy pathogens and cellular debris. The observed quenching strongly suggests that the fluorescent DOPS -liposomes, which mimic apoptotic bodies, are not only binding to the M1 cells but are being rapidly internalized and trafficked into the acidic, degradative environment of the phagolysosome. This process denatures or degrades the fluorophore, leading to a measurable decrease in signal. Therefore, the quenching serves as a direct proxy for robust, M1-driven phagocytic activity.
Asthma: In asthma, our assay revealed a high CD206 marker signal, consistent with the literature’s description of a dominant M2 anti-inflammatory/remodeling macrophage phenotype. This was coupled with a high CD68-DOPS probe signal, suggesting a substantial M2 macrophage population or high surface receptor accessibility contributing to the observed immune profile.
Bronchitis: For bronchitis, our data indicated a high CD206 marker signal with fluorescence quenching, aligning with a literature-supported M1-phenotype and mixed macrophage presence. The observed high CD68-DOPS probe signal, particularly with quenching, infers an active pro-inflammatory state, potentially with significant phagocytic activity characteristic of M1 macrophages or a mixed inflammatory response.
Pneumonia: Acute bacterial pneumonia presents a highly dynamic and heterogeneous immune landscape, reflecting the complex and rapidly evolving phases of infection, acute inflammation, and subsequent resolution. The macrophage population is a mixture of resident cells, newly recruited monocytes (M0), pro-inflammatory M1 cells fighting the infection, and emerging M2 cells mediating repair. The assay successfully captures this complexity, generating a signature of intermediate CD206 binding and a minimal, fluctuating signal from the CD68 probe. This fingerprint is consistent with a mixed, transitional population of macrophages in various states of activation, phagocytosis, and functional polarization.
In conclusion, this multi-marker comparison, leveraging quantitative indices from both CD68 and CD206 interactions, provides a more comprehensive understanding of the macrophage activation spectrum in BALF. The functional activity of CD68 (probing general engagement and uptake) combined with the phenotypic specificity of CD206 (profiling M2 polarization) offers enhanced diagnostic precision by characterizing not just the presence but also the functional state of key immune cell populations.
4. Conclusions
In this study, we present a novel approach for the detection and analysis of CD68+ cells using specific non-antibody ligands as an alternative to traditional antibody-based methods. We synthesized a fluorescently labeled liposomal marker incorporating dioleoylphosphatidylserine (DOPS), which serves as a targeted ligand for selective binding to CD68+ cells. Building on this, we developed an innovative liposome-based sandwich fluorometric assay that facilitates both the isolation and quantitative assessment of CD68 receptor expression in bronchoalveolar lavage fluid (BALF). This method offers a promising tool for the differential characterization of respiratory diseases. Our methodology utilizes DOPS-enriched, PEGylated liposomes that generate distinct fluorimetric “binding fingerprints” upon interaction with macrophage subpopulations. These fingerprints reflect the complex phenotypic and functional heterogeneity of macrophages, allowing for robust differentiation between conditions such as asthma, bronchitis, bronchiectasis, and pneumonia. By integrating this CD68-targeted assay with M2-specific markers like CD206, we can construct detailed immunophenotypes. This allows for the stratification of patients based on their macrophage profiles—for instance, distinguishing between a pro-inflammatory M1-dominant signature, often associated with a more severe disease course, and an anti-inflammatory M2-dominant profile, which may indicate a different disease state or prognosis.
Looking forward, this single-ligand approach serves as a foundation for a more comprehensive diagnostic platform. We envision the development of a biochip array utilizing a series of novel, specific ligands to quantitatively map the full spectrum of macrophage subpopulations (M1, M2, M0, and their subtypes). Such a platform would generate a high-dimensional dataset, providing approximately 10 additional “coordinates” for typing BALF samples. This quantitative, multi-marker analysis is expected to yield a far richer dataset than existing semi-quantitative methods based on a limited range of known antibody targets, thereby enabling the characterization of previously unidentified macrophage subsets.
The goal of this biochip-based diagnostic is to advance personalized medicine for inflammatory lung diseases. By providing a precise and quantitative assessment of the macrophage balance, which is a critical determinant of disease severity and outcome, clinicians can develop tailored therapeutic tactics. This strategy will focus on modulating the M1/M2 axis to steer the immune response towards a favorable resolution.
Furthermore, the ligands developed for this diagnostic platform have direct therapeutic potential. The same macrophage subtype-specific ligands can be engineered into targeted delivery systems for anti-inflammatory agents. These systems could be used to deliver both standard pharmaceuticals and novel drug candidates, such as natural compounds that have shown significant macrophage-associated anti-inflammatory activity in our preliminary experiments and in the literature. This “theranostic” approach, combining precise diagnostics with targeted therapy, holds significant promise for improving outcomes for patients with chronic and acute inflammatory lung diseases.