Olive Oil Polyphenols in Cancer: Molecular Mechanisms and Therapeutic Promise
Abstract
1. Introduction
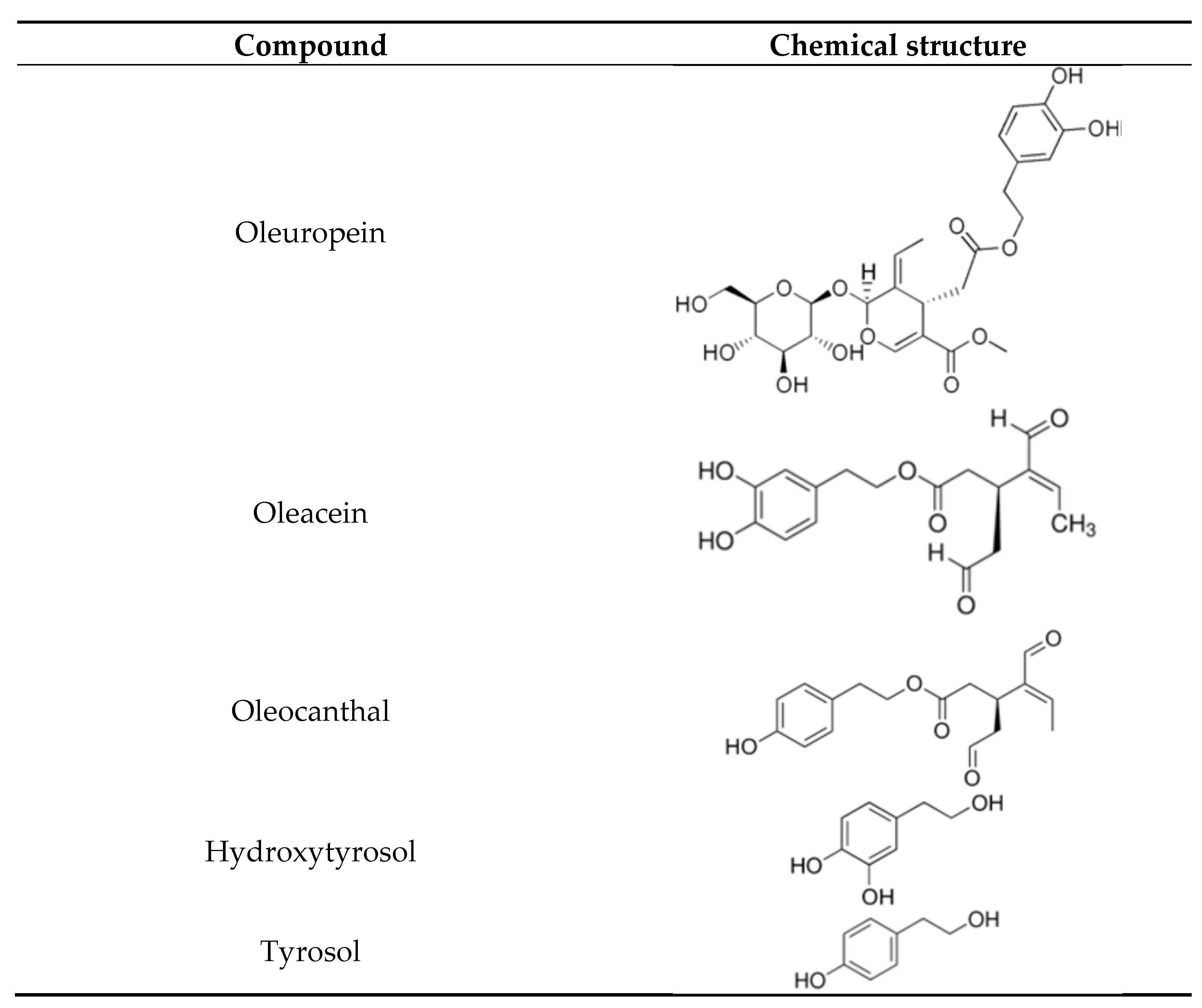
2. Olive Oil Bioactives: Tyrosol, Hydroxytyrosol, Oleuropein, Oleacein, and Oleocanthal
3. Polyphenols as Epigenetic and Immunomodulatory Agents in Cancer Prevention
3.1. Olive Polyphenols, Immune Modulation, and the Tumor Microenvironment
3.2. Molecular Mechanisms Underlying the Anticancer Effects of Olive-Derived Polyphenols
3.2.1. Oleocanthal
3.2.2. Oleocanthal as a Multi-Targeted Natural Agent in Cancer Therapy
3.3. Oleuropein
3.4. Oleacein
3.5. Tyrosol
3.6. Hydroxytyrosol
4. Challenges in Clinical Translation of Olive Oil Polyphenols
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elhawary, N.A.; Ekram, S.N.; Sembawa, H.A.; Tashkandi, E.; Bannani, S.; Azher, Z.A.; Abumansour, I.S.; Almuqati, R.M.; Attieh, R.; Sindi, I.A.; et al. Descriptive epidemiology of female breast cancer around the world: Incidence, mortality, and sociodemographic risks and disparities. Int. J. Environ. Health Res. 2025, 1, 5. [Google Scholar] [CrossRef]
- Nikolouzakis, T.K.; Chrysos, E.; Docea, A.O.; Fragkiadaki, P.; Souglakos, J.; Tsiaoussis, J.; Tsatsakis, A. Current and future trends of colorectal cancer treatment: Exploring advances in immunotherapy. Cancers 2024, 16, 1995. [Google Scholar] [CrossRef]
- Bultman, S.J. Molecular pathways: Gene–environment interactions regulating dietary fiber induction of proliferation and apoptosis via butyrate for cancer prevention. Clin. Cancer Res. 2014, 20, 799–803. [Google Scholar] [CrossRef]
- Yousef, M.; Yousef, A.; Chowdhury, S.; Fanaeian, M.M.; Knafl, M.; Peterson, J.; Zeineddine, M.; Alfaro, K.; Zeineddine, F.; Goldstein, D.; et al. Molecular, socioeconomic, and clinical factors affecting racial and ethnic disparities in colorectal cancer survival. JAMA Oncol. 2024, 10, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Farshi, E. Comprehensive overview of 31 types of cancer: Incidence, categories, treatment options, and survival rates. Int. J. Gastroenterol. Hepatol. 2024, 5, 1. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Gebretsadik, A.; Dulla, D. The top ten cancer burdens at Hawassa University’s comprehensive specialized hospital from 2013 to 2019. Ethio. J. Med. Health Sci. 2024, 4, 380–389. [Google Scholar]
- Keum, N.; Giovannucci, E.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Estupiñán Fdez de Mesa, M.; Ferguson, M.; Green, S.; Marcu, A.; Ream, E.; Whitaker, K.L. Applying the intersectionality lens to understand minority ethnic women’s experiences of the breast cancer care pathway in England: A qualitative interview study. Psycho-Oncol. 2025, 34, e70092. [Google Scholar] [CrossRef]
- Damar, M.; Damar, H.T.; Özbiçakci, Ş.; Yasli, G.; Erenay, F.S.; Özdağoğlu, G.; Pinto, A.D. Mapping intellectual structure and research hotspots of cancer studies in primary health care: A machine-learning-based analysis. Medicine 2025, 104, e41749. [Google Scholar] [CrossRef]
- Yousefi, M.; Reihani, H.; Heydari, M.; Azgomi, R.N.; Hashempur, M.H. Complementary and alternative medicine use among cancer patients in Iran: A systematic review. Prev. Med. Rep. 2024, 39, 102644. [Google Scholar] [CrossRef]
- Banda, J.C. The prevalence and correlates of probable major depressive disorder among patients living with cancer at Kamuzu Central Hospital in Malawi: A cross-sectional study. Medicon Med. Sci. 2024, 7, 11–20. [Google Scholar]
- Keinki, C.; Klein, M.A.; Käsmann, L.; Münstedt, K.; Hübner, J. Patients’ information on side effects of cancer treatment and usage of complementary and alternative medicine—A cross-sectional study. Adv. Integr. Med. 2025, 100, 497. [Google Scholar] [CrossRef]
- Baladi, A.; El Fadli, M.; Tafenzi, H.A.; El Bouaouidi, K.; Benhima, N.; Afani, L.; Essâdi, I.; Belbaraka, R. Prevalence and associated factors of herbal medicine use among breast cancer patients: A cross-sectional study in Morocco. ecancermedicalscience 2024, 18, 1786. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arye, E.; Samuels, N.; Goldstein, L.H.; Mutafoglu, K.; Omran, S.; Schiff, E.; Charalambous, H.; Dweikat, T.; Ghrayeb, I.; Bar-Sela, G.; et al. Potential risks associated with traditional herbal medicine use in cancer care: A study of Middle Eastern oncology health care professionals. Cancer 2016, 122, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Kiritsakis, A.; Keceli, T.M.; Anousakis, C.; Iorio, E.L.; Tsitsipas, C.; Shahidi, F.; Evangelou, E. Olive oil and Mediterranean diet: The importance of olive oil constituents, and mainly of its polyphenols, in human health—The redox system—Xenohormesis hypothesis. In A Review on Diverse Neurological Disorders; Academic Press: Cambridge, MA, USA, 2024; pp. 603–619. [Google Scholar]
- Saad, B. Prevention and treatment of obesity-related inflammatory diseases by edible and medicinal plants and their active compounds. Immuno 2022, 2, 609–629. [Google Scholar] [CrossRef]
- Saad, B. A review of the anti-obesity effects of wild edible plants in the Mediterranean diet and their active compounds: From traditional uses to action mechanisms and therapeutic targets. Int. J. Mol. Sci. 2023, 24, 12641. [Google Scholar] [CrossRef]
- Saad, B. History, present and prospect of Greco-Arab and Islamic herbal medicine. In History, Present and Prospect of World Traditional Medicine; World Scientific: London, UK, 2024; pp. 235–300. [Google Scholar] [CrossRef]
- Uthirapathy, S. Anticancer, anti-inflammatory, and neuroprotective effect of oleocanthal from virgin olive oil–A review. J. Angiother. 2024, 8, 1–9. [Google Scholar]
- Nsairat, H.; Jaber, A.M.; Faddah, H.; Ahmad, S. Oleuropein impact on colorectal cancer. Future Sci. OA 2024, 10, FSO. [Google Scholar] [CrossRef]
- Ahmed, Z.S.; Khan, E.; Elias, N.; Elshebiny, A.; Dou, Q. Updated review on natural polyphenols: Molecular mechanisms, biological effects, and clinical applications for cancer management. Biomolecules 2025, 15, 629. [Google Scholar] [CrossRef]
- Elbaylek, H.; Ammor, S. Adherence to the Mediterranean diet and colorectal cancer risk among Moroccan population: Hospital-based case control study. Asian Pac. J. Cancer Prev. 2024, 25, 2853. [Google Scholar] [CrossRef]
- Roşian, Ş.H.; Boarescu, I.; Boarescu, P.M. Antioxidant and anti-inflammatory effects of bioactive compounds in atherosclerosis. Int. J. Mol. Sci. 2025, 26, 1379. [Google Scholar] [CrossRef]
- Sichetti, M.; Giuseffi, M.; Giglio, E.; Marino, G.; Mecca, M. Effect of natural polyphenols on breast cancer chemoprevention and treatment. Mol. Nutr. Food Res. 2025, 69, e70055. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, B.; Paul, K.; Bakshi, P.; Bajaj, P.; Kumar, M.; Dhiman, S.; Jasrotia, S.; Kumar, P.; Dutta, R. Hydroxytyrosol in cancer research: Recent and historical insights on discoveries and mechanisms of action. Future J. Pharm. Sci. 2024, 10, 129. [Google Scholar] [CrossRef]
- Rishmawi, S.; Haddad, F.; Dokmak, G.; Karaman, R. A comprehensive review on the anti-cancer effects of oleuropein. Life 2022, 12, 1140. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Oliveira, M.B.; Alves, R.C. A comprehensive review of the antitumor activity of olive compounds: The case of olive oil, pomace, and leaf extracts, phenolic alcohols, secoiridoids, and triterpenes. Antioxidants 2025, 14, 237. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Kaddoumi, A. Extra-virgin olive oil in alzheimer’s disease: A comprehensive review of cellular, animal, and clinical studies. Int. J. Mol. Sci. 2024, 25, 1914. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.J.; Cortese, M.; Yuan, C.; Bjornevik, K.; Ascherio, A.; Wang, D.D.; Chavarro, J.E.; Stampfer, M.J.; Hu, F.B.; Willett, W.C.; et al. Consumption of olive oil and diet quality and risk of dementia-related death. JAMA Netw. Open 2024, 7, e2410021. [Google Scholar] [CrossRef]
- Chiavarini, M.; Rosignoli, P.; Giacchetta, I.; Fabiani, R. Health outcomes associated with olive oil intake: An umbrella review of meta-analyses. Foods 2024, 13, 2619. [Google Scholar] [CrossRef]
- Li, S.; Shao, H.; Sun, T.; Guo, X.; Zhang, X.; Zeng, Q.; Fang, S.; Liu, X.; Wang, F.; Liu, F.; et al. Anti-neuroinflammatory effect of hydroxytyrosol: A potential strategy for anti-depressant development. Front. Pharmacol. 2024, 15, 1366683. [Google Scholar] [CrossRef]
- Gonçalves, M.; Vale, N.; Silva, P. Neuroprotective effects of olive oil: A comprehensive review of antioxidant properties. Antioxidants 2024, 13, 762. [Google Scholar] [CrossRef] [PubMed]
- Bonvino, N.P.; Liang, J.; McCord, E.D.; Zafiris, E.; Benetti, N.; Ray, N.B.; Hung, A.; Boskou, D.; Karagiannis, T.C. OliveNet™: A Comprehensive Library of Compounds from Olea europaea. Database 2018, 2018, bay016. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Basso, C.; Uliana, G.C.; Richards, N.S.P.S. Bioactive Compounds Present in Olive Oil and Its By-Products: Bibliographic Review. Res. Soc. Dev. 2022, 11, e196111032580. [Google Scholar] [CrossRef]
- Gagour, J.; Hallouch, O.; Asbbane, A.; Bijla, L.; Laknifli, A.; Lee, L.H.; Gharby, S. A review of recent progresses on olive oil chemical profiling, extraction technology, shelf-life, and quality control. Chem. Biodivers 2024, 21, e202301697. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Gallardo-Fernández, M.; Gonzalez-Ramirez, M.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Hydroxytyrosol in foods: Analysis, food sources, EU dietary intake, and potential uses. Foods 2022, 11, 2355. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Oleocanthal: A naturally occurring anti-inflammatory agent in virgin olive oil. In Olive Oil-Constituents, Quality, Health Properties and Bioconversions; IntechOpen: Rijeka, Croatia, 2012; pp. 357–374. [Google Scholar]
- Gatt, L.; Lia, F.; Zammit-Mangion, M.; Thorpe, S.J.; Schembri-Wismayer, P. First profile of phenolic compounds from Maltese extra virgin olive oils using liquid-liquid extraction and liquid chromatography-mass spectrometry. J. Oleo Sci. 2021, 70, 145–153. [Google Scholar] [CrossRef]
- Ussia, S.; Ritorto, G.; Mollace, R.; Serra, M.; Tavernese, A.; Altomare, C.; Macrì, R. Exploring the benefits of extra virgin olive oil on cardiovascular health enhancement and disease prevention: A systematic review. Nutrients 2025, 17, 1843. [Google Scholar] [CrossRef]
- Blasi, F.; Ianni, F.; Cossignani, L. Phenolic profiling for geographical and varietal authentication of extra virgin olive oil. Trends Food Sci. Technol. 2024, 147, 104444. [Google Scholar] [CrossRef]
- López-Huertas, E.; Lozano-Sánchez, J.; Segura-Carretero, A. Olive oil varieties and ripening stages containing the antioxidants hydroxytyrosol and derivatives in compliance with EFSA health claim. Food Chem. 2021, 342, 128291. [Google Scholar] [CrossRef]
- Safarzadeh Markhali, F. Effect of processing on phenolic composition of olive oil products and olive mill by-products and possibilities for enhancement of sustainable processes. Processes 2021, 9, 953. [Google Scholar] [CrossRef]
- Rey-Giménez, R.; Vázquez Ayala, S.; Laya Reig, D.; Sánchez-Gimeno, A.C. Chemometric and Physico-Chemical Characterization of Fruit and Olive Oils from Autochthonous Cultivars Grown in Aragon (Spain). Foods 2023, 12, 803. [Google Scholar] [CrossRef]
- Boronat, A.; Soldevila-Domenech, N.; Rodríguez-Morató, J.; Martínez-Huélamo, M.; Lamuela-Raventós, R.M.; De la Torre, R. Beer phenolic composition of simple phenols, prenylated flavonoids and alkylresorcinols. Molecules 2020, 25, 2582. [Google Scholar] [CrossRef]
- Khalil, A.A.; Rahman, M.M.; Rauf, A.; Islam, M.R.; Manna, S.J.; Khan, A.A.; Ullah, S.; Akhtar, M.N.; Aljohani, A.S.; Abdulmonem, W.A.; et al. Oleuropein: Chemistry, extraction techniques and nutraceutical perspectives-An update. Crit. Rev. Food Sci. Nutr. 2024, 64, 9933–9987. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors influencing phenolic compounds in table olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef] [PubMed]
- López-Yerena, A.; Ninot, A.; Jiménez-Ruiz, N.; Lozano-Castellón, J.; Pérez, M.; Escribano-Ferrer, E.; Vallverdú-Queralt, A. Influence of the ripening stage and extraction conditions on the phenolic fingerprint of ‘Corbella’ extra-virgin olive oil. Antioxidants 2021, 10, 877. [Google Scholar] [CrossRef]
- Tsimidou, M.Z. A critical appraisal of the separation protocols proposed for the implementation of the health claim on “olive oil polyphenols” (EC regulation 432/2012). Separations 2022, 9, 351. [Google Scholar] [CrossRef]
- De Medina, V.S.; Miho, H.; Melliou, E.; Magiatis, P.; Priego-Capote, F.; de Castro, M.D.L. Quantitative method for determination of oleocanthal and oleacein in virgin olive oils by liquid chromatography–tandem mass spectrometry. Talanta 2017, 162, 24–31. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds–nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as potent epigenetics agents for cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Rakib, A.; Mandal, M.; Khandaker, M.U.; Reza, H.M.; AlAjmi, M.F.; Alruwaili, N.K.; Dhama, K. Polyphenols: Role in modulating immune function and obesity. Biomolecules 2024, 14, 221. [Google Scholar] [CrossRef]
- Krawczyk, M.; Burzynska-Pedziwiatr, I.; Wozniak, Ł.A.; Bukowiecka-Matusiak, M. Impact of polyphenols on inflammatory and oxidative stress factors in diabetes mellitus. Biomolecules 2023, 13, 1402. [Google Scholar] [CrossRef]
- Milenkovic, D.; Jude, B.; Morand, C. miRNA as molecular target of polyphenols underlying their biological effects. Free Radic. Biol. Med. 2013, 64, 40–51. [Google Scholar] [CrossRef]
- Wang, J.; Yan, T.; Long, C.; Cai, W. Oncogenic function and prognostic significance of Abelson interactor 1 in hepatocellular carcinoma. Int. J. Oncol. 2017, 50, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Torres, E.; Hernández-Oliveras, A.; Meneses-Morales, I.; Rodríguez, G.; Fuentes-García, G.; Zarain-Herzberg. Á Resveratrol up-regulates ATP2A3 gene expression in breast cancer cell lines through epigenetic mechanisms. Int. J. Biochem. Cell Biol. 2019, 113, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, I.; Ortíz-Flores, R.; Badía, R.; Serrano-Moratalla, B.; Martínez-Hervás, S.; Ascaso, J.F.; Valls, R.M.; Caimari, A. Rich oleocanthal and oleacein extra virgin olive oil and inflammatory and antioxidant status in people with obesity and prediabetes. Clin. Nutr. 2023, 42, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Boronat, A.; De la Torre, R.; Pali-Casanova, R. Cardiovascular and metabolic benefits of extra virgin olive oil phenolic compounds: Mechanistic insights from in vivo studies. Cells 2024, 13, 1555. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kalailingam, P.; Delcour, J.A.; Fogliano, V.; Thanabalu, T. Olive-Derived Antioxidant Dietary Fiber Modulates Gut Microbiota Composition and Attenuates Atopic Dermatitis Like Inflammation in Mice. Mol. Nutr. Food Res. 2023, 67, 2200127. [Google Scholar] [CrossRef]
- Tamburini, B.; Di Liberto, D.; Pratelli, G.; Rizzo, C.; Barbera, L.L.; Lauricella, M.; Carlisi, D.; Maggio, A.; Palumbo Piccionello, A.; D’Anneo, A.; et al. Extra Virgin Olive Oil Polyphenol-Enriched Extracts Exert Antioxidant and Anti-Inflammatory Effects on Peripheral Blood Mononuclear Cells from Rheumatoid Arthritis Patients. Antioxidants 2025, 14, 171. [Google Scholar] [CrossRef]
- Rosillo, M.Á.; Villegas, I.; Vázquez-Román, V.; Fernández-Santos, J.M.; Ortega-Vidal, J.; Salido, S.; González-Rodríguez, M.L.; Alarcón-de-la-Lastra, C. Dietary oleacein, a secoiridoid from extra virgin olive oil, prevents collagen-induced arthritis in mice. Food Funct. 2024, 15, 838–852. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Jiang, L.; Zhong, L. Suppression of inflammation and oxidative stress in macrophages by hydroxytyrosol via activation of AMPK/Nrf2 signaling pathway. J. Agric. Food Chem. 2019, 67, 3984–3993. [Google Scholar]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef]
- Elnagar, A.Y.; Sylvester, P.W.; El Sayed, K.A. (-)-Oleocanthal as a c-Met inhibitor for the control of metastatic breast and prostate cancers. Planta Med. 2011, 77, 1013–1019. [Google Scholar] [CrossRef]
- Wang, R.; Ganbold, M.; Ferdousi, F.; Tominaga, K.; Isoda, H. A rare olive compound oleacein improves lipid and glucose metabolism, and inflammatory functions: A comprehensive whole-genome transcriptomics analysis in adipocytes differentiated from healthy and diabetic adipose stem cells. Int. J. Mol. Sci. 2023, 24, 10419. [Google Scholar] [CrossRef]
- Marrero, A.D.; Ortega-Vidal, J.; Salido, S.; Castilla, L.; Vidal, I.; Quesada, A.R.; Altarejos, J.; Martínez-Poveda, B.; Medina, M.Á. Anti-angiogenic effects of oleacein and oleocanthal: New bioactivities of compounds from extra virgin olive oil. Biomed. Pharmacother. 2023, 165, 115234. [Google Scholar] [CrossRef]
- González-Rodríguez, M.; Ait Edjoudi, D.; Cordero-Barreal, A.; Farrag, M.; Varela-García, M.; Torrijos-Pulpón, C.; Ruiz-Fernández, C.; Capuozzo, M.; Ottaiano, A.; Lago, F.; et al. Oleocanthal, an antioxidant phenolic compound in extra virgin olive oil (EVOO): A comprehensive systematic review of its potential in inflammation and cancer. Antioxidants 2023, 12, 2112. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Abbattista, R.; Losito, I.; Calvano, C.D.; Cataldi, T.R.I. Exploring the isomeric precursors of olive oil major secoiridoids: An insight into olive leaves and drupes by liquid-chromatography and Fourier-transform tandem mass spectrometry. Foods 2021, 10, 2050. [Google Scholar] [CrossRef] [PubMed]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef]
- De Stefanis, D.; Schembari, A.; Muscat, A.; Melaiu, O.; Dominici, C.; Gallì, R.; Ferretti, E. Antiproliferative effects of EVOO phenolics in liver cancer cells. Cancers 2019, 11, 1640. [Google Scholar] [CrossRef]
- Qusa, M.H.; Kumar, D.; Ghosh, S.; Alazzam, M.; Alshammari, F. Oleocanthal xylitol-based solid dispersion for breast cancer. Int. J. Pharm. 2019, 569, 118596. [Google Scholar] [CrossRef]
- Muñoz-García, R.; Marín-Aguilar, F.; García-Fuentes, E.; Varela-López, A.; Giampieri, F.; Alvarez-Suarez, J.M.; Cordero, M.D.; Battino, M. Oleocanthal diet ameliorates lupus in mice. Antioxidants 2023, 12, 1303. [Google Scholar] [CrossRef]
- Montoya, T.; Vicente, J.; Domínguez, C.; Bautista-Lorite, J.; Reyes-Zurita, F.J.; Sánchez-Quesada, C.; Sánchez-Fidalgo, S. Oleocanthal reduces inflammation in arthritis mice. Antioxidants 2021, 10, 650. [Google Scholar] [CrossRef]
- Patnaik, R.; Varghese, R.; Jannati, S.; Naidoo, N.; Banerjee, Y. Targeting PAR2-mediated inflammation in osteoarthritis: A comprehensive in vitro evaluation of oleocanthal’s potential as a functional food intervention for chondrocyte protection and anti-inflammatory effects. BMC Musculoskelet. Disord. 2024, 25, 769. [Google Scholar] [CrossRef]
- Montoya, T.; Sánchez-Hidalgo, M.; Castejón, M.L.; Vazquéz-Román, M.V.; de Sotomayor, M.A.; Ortega-Vidal, J.; González, M.L.; Alarcón-de-la-Lastra, C. Oleocanthal supplemented diet improves renal damage and endothelial dysfunction in pristane-induced systemic lupus erythematosus in mice. Food Res. Int. 2023, 163, 112140. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ayoub, N.M.; Tajmim, A.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. (-)-Oleocanthal prevents breast cancer locoregional recurrence in orthotopic mouse models. Cancers 2019, 11, 637. [Google Scholar] [CrossRef]
- Xiang, C.; Chen, J.; Fu, P. HGF/Met signaling in cancer invasion: Impact on cytoskeleton remodeling. Cancers 2017, 9, 44. [Google Scholar] [CrossRef]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.-Y.; El Sayed, K.A. Olive phenolics as c-Met inhibitors: (-)-Oleocanthal attenuates tumor growth in breast cancer models. PLoS ONE 2014, 9, e95954. [Google Scholar] [CrossRef]
- Siddique, A.B.; Kilgore, P.C.S.R.; Tajmim, A.; Singh, S.S.; Meyer, S.A.; El Sayed, K.A.; Rao, P.P.N. (-)-Oleocanthal as a dual c-MET/COX-2 inhibitor for lung cancer control. Nutrients 2020, 12, 1749. [Google Scholar] [CrossRef]
- Mohyeldin, M.M.; Busnena, B.A.; Akl, M.R.; El Sayed, K.A. Semisynthetic analogs enhance oleocanthal’s c-Met inhibition in breast cancer. Eur. J. Med. Chem. 2016, 118, 299–315. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ayoub, N.M.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. Oleocanthal attenuates castration-resistant prostate cancer by targeting SMYD2. Cancers 2022, 14, 3542. [Google Scholar] [CrossRef]
- Khanfar, M.A.; Bardaweel, S.K.; Akl, M.R.; El Sayed, K.A. Oleocanthal as a potent mTOR inhibitor: Biological and modeling studies. Phytother. Res. 2015, 29, 1776–1782. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, J.; Peng, L. (-)-Oleocanthal inhibits STAT3 signaling in melanoma cells. Oncol. Rep. 2017, 37, 483–491. [Google Scholar] [CrossRef]
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R.; Sun, B.; Pan, S.; Liang, D.; Liu, L. (-)-Oleocanthal blocks STAT3 activation in hepatocellular carcinoma. Oncotarget 2016, 7, 43475–43491. [Google Scholar] [CrossRef]
- Kugić, A.; Dabelić, S.; Brala, C.J.; Barbarić, M.; Urlić, I.; Roje, M.; Smolko, A.; Samaržija, I. Secoiridoids modulate metabolic activity in melanoma cells. Molecules 2022, 27, 3310. [Google Scholar] [CrossRef] [PubMed]
- Polini, B.; Digiacomo, M.; Carpi, S.; Bertini, S.; Gado, F.; Saccomanni, G.; Macchia, M.; Nieri, P.; Manera, C.; Fogli, S. Oleocanthal and oleacein inhibit Erk/Akt and B-Raf in non-melanoma skin cancer cells. Toxicol. Vitr. 2018, 52, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Tarun, M.T.I.; Elsayed, H.E.; Ebrahim, H.Y.; El Sayed, K.A. The olive oil phenolic (-)-oleocanthal suppresses colorectal cancer progression and recurrence by modulating SMYD2–EZH2 and c-Met activation. Nutrients 2025, 17, 397. [Google Scholar] [CrossRef]
- Alhajri, H.; Alterary, S.; Alrfaei, B.M.; Al-Qahtani, W.S. Therapeutic potential evaluation of green synthesized silver nanoparticles derived from olive (Olea europaea L.) leaf extracts against breast cancer cells. J. Nanophotonics 2021, 15, 036003. [Google Scholar] [CrossRef]
- Ci, Y.; Qiao, J.; Han, M. Oleuropein suppresses the metastasis of MCF-7 human breast cancer cells via inhibition of the PI3K/AKT/NF-κB pathway. Molecules 2016, 21, 1634. [Google Scholar] [CrossRef]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Sethi, G. Oleuropein induces apoptosis in breast cancer cells via the p53 pathway. J. Cell Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- Bossio, S.; Perri, A.; Malivindi, R.; Giordano, F.; Rago, V.; Mirabelli, M.; Salatino, A.; Brunetti, A.; Greco, E.A.; Aversa, A. Oleuropein counteracts both the proliferation and migration of intra- and extragonadal seminoma cells. Nutrients 2022, 14, 2323. [Google Scholar] [CrossRef]
- Aggarwal, V.; Kumar, G.; Aggarwal, D.; Yerer, M.B.; Cumaoğlu, A.; Kumar, M.; Sak, K.; Mittal, S.; Tuli, H.S.; Sethi, G. Cancer preventive role of olives and olive oil via modulation of apoptosis and nuclear factor-kappa B activation. In Olives and Olive Oil in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2021; pp. 377–388. [Google Scholar]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Cerdá, B.; Gálvez, J.; De la Fuente, J.; Nieto, M.L. Olive oil polyphenols modulate cannabinoid CB1 receptor gene expression in colon cancer. Mol. Nutr. Food Res. 2016, 60, 242–255. [Google Scholar] [CrossRef]
- Bhattacherjee, D.; Raina, K.; Mandal, T.K.; Thummer, R.P.; Bhabak, K.P. Targeting Wnt/β-catenin signaling pathway in triple-negative breast cancer by benzylic organotrisulfides: Contribution of the released hydrogen sulfide towards potent anti-cancer activity. Free Radic. Biol. Med. 2022, 191, 82–96. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, X.; Zhao, Y.; Wang, H.; Chen, L. The Wnt/β-catenin signaling pathway in the tumor microenvironment of hepatocellular carcinoma. Cancer Biol. Med. 2022, 19, 305–318. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Predes, D.; Borges, H.L.; Abreu, J.G. Therapeutic potential of naturally occurring small molecules to target the Wnt/β-catenin signaling pathway in colorectal cancer. Cancers 2022, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Bawari, S.; Sharma, S.; DeLiberto, L.K.; Bishayee, A. Targeting the crosstalk between canonical Wnt/β-catenin and inflammatory signaling cascades: A novel strategy for cancer prevention and therapy. Pharmacol. Ther. 2021, 227, 107876. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lv, T.; Jin, B.; Li, Y.; Fan, Z. Regulatory role of PPAR in colorectal cancer. Cell Death Discov. 2025, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Ercolano, G.; Tenore, G.C.; Ianaro, A. Olive leaf extract inhibits metastatic melanoma spread through suppression of epithelial to mesenchymal transition. Phytother. Res. 2022, 36, 4002–4013. [Google Scholar] [CrossRef]
- Huang, Q.; Liang, X.; Ren, T.; Huang, Y.; Zhang, H.; Yu, Y.; Chen, C.; Wang, W.; Niu, J.; Lou, J.; et al. The role of tumor-associated macrophages in osteosarcoma progression—Therapeutic implications. Cell. Oncol. 2021, 44, 525–539. [Google Scholar] [CrossRef]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and cycling hypoxia: Drivers of cancer chronic inflammation through HIF-1 and NF-κB activation—A review of the molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Z.; Yang, X.; Liu, L.; Ahn, K.S. An updated review on the potential antineoplastic actions of oleuropein. Phytother. Res. 2022, 36, 365–379. [Google Scholar] [CrossRef]
- Öner, Ç.; Çolak, E. Novel natural compounds for hepatocellular carcinoma treatment. Front. Nat. Prod. Chem. 2023, 11, 32–72. [Google Scholar]
- Kwon, M.J. Matrix metalloproteinases as therapeutic targets in breast cancer. Front. Oncol. 2023, 12, 1108695. [Google Scholar] [CrossRef] [PubMed]
- Ansari, B.; Küpeli Akkol, E.; Khan, H.; Shah, M.A. Olive leaf (Oleuropein) and its role in cancer: Therapeutic updates. In Nutraceuticals and Cancer Signaling: Clinical Aspects and Mode of Action; Springer: Berlin/Heidelberg, Germany, 2021; pp. 367–400. [Google Scholar]
- Albogami, S.; Hassan, A.M. Assessment of the efficacy of olive leaf (Olea europaea L.) extracts in the treatment of colorectal cancer and prostate cancer using in vitro cell models. Molecules 2021, 26, 4069. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.H.; Al-Samydai, A.; Sulaibi, M.A.; Alqaraleh, M.; Abed, A.I.; Shalan, N.; Alsanabrah, A.; Alsotari, S.T.; Nsairat, H.; Alshaer, W. Development of pegylated nano-phytosome formulation with oleuropein and rutin to compare anti-colonic cancer activity with Olea europaea leaves extract. Chem. Biodivers. 2023, 20, e202300534. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, G.; Özdemir, F. Novel anti-tumor strategy for breast cancer: Synergistic role of oleuropein with paclitaxel therapeutic in MCF-7 cells. Anti-Cancer Agents Med. Chem. 2024, 24, 224–234. [Google Scholar] [CrossRef]
- Flore, G.; Deledda, A.; Lombardo, M.; Armani, A.; Velluzzi, F. Effects of functional and nutraceutical foods in the context of the Mediterranean diet in patients diagnosed with breast cancer. Antioxidants 2023, 12, 1845. [Google Scholar] [CrossRef]
- Karousi, P.; Kontos, C.K.; Papakotsi, P.; Kostakis, I.K.; Skaltsounis, A.L.; Scorilas, A. Next-generation sequencing reveals altered gene expression and enriched pathways in triple-negative breast cancer cells treated with oleuropein and oleocanthal. Funct. Integr. Genom. 2023, 23, 299. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Mohyeldin, M.M.; Ebrahim, H.Y.; McGehee, O.C.; Tarun, M.T.; El Sayed, K.A. (-)-Oleuropein as a novel metastatic castration-resistant prostate cancer progression and recurrence suppressor via targeting PCSK9-LDLR axis. Nutrients 2025, 17, 1445. [Google Scholar] [CrossRef]
- Gervasi, F.; Pojero, F. Use of oleuropein and hydroxytyrosol for cancer prevention and treatment: Considerations about how bioavailability and metabolism impact their adoption in clinical routine. Biomedicines 2025, 12, 502. [Google Scholar] [CrossRef]
- Pruthi, S.; Heisey, R.E.; Bevers, T.B. Chemoprevention for breast cancer. Ann. Surg. Oncol. 2015, 22, 3230–3235. [Google Scholar] [CrossRef]
- Flora, S.; Ferguson, L. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005, 591, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Carpi, S.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Scoditti, E.; Nieri, P. miRNA modulation and antitumor activity by the extra-virgin olive oil polyphenol oleacein in human melanoma cells. Front. Pharmacol. 2020, 11, 574317. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Celano, M.; Lombardo, G.E.; Maggisano, V.; Procopio, A.; Russo, D.; Navarra, M. Oleacein inhibits STAT3, activates the apoptotic machinery, and exerts anti-metastatic effects in the SH-SY5Y human neuroblastoma cells. Food Funct. 2020, 11, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Uli, G.; Oliverio, M.; Bellizzi, D.; Gallo Cantafio, M.E.; Grillone, K.; Passarino, G.; Colica, C.; Nardi, M.; Rossi, M.; Procopio, A.; et al. Anti-tumor activity and epigenetic impact of the polyphenol oleacein in multiple myeloma. Cancers 2019, 11, 990. [Google Scholar] [CrossRef]
- Kusuma, I.Y.; Habibie, H.; Bahar, M.A.; Budán, F.; Csupor, D. Anticancer Effects of Secoiridoids—A Scoping Review of the Molecular Mechanisms behind the Chemopreventive Effects of the Olive Tree Components Oleocanthal, Oleacein, and Oleuropein. Nutrients 2024, 16, 2755. [Google Scholar] [CrossRef]
- Rojas Gil, A.P.; Kodonis, I.; Ioannidis, A.; Nomikos, T.; Dimopoulos, I.; Kosmidis, G.; Katsa, M.E.; Melliou, E.; Magiatis, P. The effect of dietary intervention with high-oleocanthal and oleacein olive oil in patients with early-stage chronic lymphocytic leukemia: A pilot randomized trial. Front. Oncol. 2022, 11, 810249. [Google Scholar] [CrossRef]
- Wang, W.; Du, L.; Wei, Q.; Lu, M.; Xu, D.; Li, Y. Synthesis and health effects of phenolic compounds: A focus on tyrosol, hydroxytyrosol, and 3,4-dihydroxyacetophenone. Antioxidants 2025, 14, 476. [Google Scholar] [CrossRef]
- Riolo, R.; De Rosa, R.; Simonetta, I.; Tuttolomondo, A. Olive oil in the Mediterranean diet and its biochemical and molecular effects on cardiovascular health through an analysis of genetics and epigenetics. Int. J. Mol. Sci. 2022, 23, 16002. [Google Scholar] [CrossRef]
- Katsa, M.E.; Nomikos, T. Olive oil phenolics and platelets—From molecular mechanisms to human studies. Rev. Cardiovasc. Med. 2022, 23, 255. [Google Scholar] [CrossRef]
- Calahorra, J.; Araujo-Abad, S.; Granadino-Roldán, J.M.; Naranjo, Á.; Martínez-Lara, E.; Siles, E. Tyrosol: Repercussion of the lack of a hydroxyl group in the response of MCF-7 cells to hypoxia. J. Med. Food 2023, 26, 511–520. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The olive biophenols oleuropein and hydroxytyrosol selectively reduce proliferation, influence the cell cycle, and induce apoptosis in pancreatic cancer cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Li, Y.; Wang, H.; Cui, Y.; Feng, Z.; Li, H.; Li, Y.; Wang, Y.; Wurtz, K.; Weber, P.; et al. Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells. Curr. Cancer Drug Targets 2013, 13, 625–639. [Google Scholar] [CrossRef]
- Bouallagui, Z.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol-rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Jin, Z. Tyrosol induces increased expression of NQO1 enzyme gene and inhibits cell proliferation in hepatocellular carcinoma cells. Chin. J. Pathophysiol. 2002, 8, 5–9. [Google Scholar]
- El-Azem, N.; Pulido-Moran, M.; Ramirez-Tortosa, C.L.; Quiles, J.L.; Cara, F.E.; Sanchez-Rovira, P.; Granados-Principal, S.; Ramirez-Tortosa, M. Modulation by hydroxytyrosol of oxidative stress and antitumor activities of paclitaxel in breast cancer. Eur. J. Nutr. 2019, 58, 1203–1211. [Google Scholar] [CrossRef]
- López de Las Hazas, M.C.; Piñol, C.; Macià, A.; Motilva, M.J. Hydroxytyrosol and the colonic metabolites derived from virgin olive oil intake induce cell cycle arrest and apoptosis in colon cancer cells. J. Agric. Food Chem. 2017, 65, 6467–6476. [Google Scholar] [CrossRef]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef]
- Toteda, G.; Lupinacci, S.; Vizza, D.; Bonofiglio, R.; Perri, E.; Bonofiglio, M.; Lofaro, D.; La Russa, A.; Leone, F.; Gigliotti, P.; et al. High doses of hydroxytyrosol induce apoptosis in papillary and follicular thyroid cancer cells. J. Endocrinol. Investig. 2017, 40, 153–162. [Google Scholar] [CrossRef]
- Sarsour, E.H.; Goswami, M.; Kalen, A.L.; Lafin, J.T.; Goswami, P.C. Hydroxytyrosol inhibits chemokine C-C motif ligand 5 mediated aged quiescent fibroblast-induced stimulation of breast cancer cell proliferation. Age 2014, 36, 9645. [Google Scholar] [CrossRef]
- Terzuoli, E.; Nannelli, G.; Frosini, M.; Giachetti, A.; Ziche, M.; Donnini, S. Inhibition of cell cycle progression by the hydroxytyrosol-cetuximab combination yields enhanced chemotherapeutic efficacy in colon cancer cells. Oncotarget 2017, 8, 83207–83224. [Google Scholar] [CrossRef]
- Parra-Perez, A.M.; Pérez-Jiménez, A.; Gris-Cárdenas, I.; Bonel-Pérez, G.C.; Carrasco-Díaz, L.M.; Mokhtari, K.; García-Salguero, L.; Lupiáñez, J.A.; Rufino-Palomares, E.E.; Reyes-Zurita, F.J. Involvement of the PI3K/AKT intracellular signaling pathway in the anti-cancer activity of hydroxytyrosol, a polyphenol from Olea europaea, in hematological cells and implication of HSP60 levels in its anti-inflammatory activity. Int. J. Mol. Sci. 2022, 23, 7053. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.; Camacho-Corencia, P.; Sanchez-Rovira, P.; Vera-Ramirez, L.; Ramirez-Tortosa, M.C. Hydroxytyrosol inhibits growth and cell proliferation and promotes high expression of sfrp4 in rat mammary tumors. Mol. Nutr. Food Res. 2011, 55 (Suppl. S1), S117–S126. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Luo, M.; Liu, T. Hydroxytyrosol promotes mitochondrial apoptosis and inhibits the proliferation of human colon cancer cells via reactive oxygen species generation. Food Funct. 2014, 5, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arroyo, S.; Salvadó, M.J.; Mena, P.; García-Villalba, R.; Espín, J.C.; Perona, J.S. Modulatory effects of hydroxytyrosol and oleuropein on autophagy in breast cancer cells. Antioxidants 2020, 9, 376. [Google Scholar]
- Han, J.; Talorete, T.P.N.; Yamada, P.; Isoda, H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. J. Breast Cancer 2009, 12, 85–90. [Google Scholar] [CrossRef]
- Bernini, R.; Gilardini Montani, M.S.; Romani, A.; D’Urso, G.; Agamennone, M.; Cossignani, L.; Amatori, S.; Ciriolo, M.R. Oleocanthal and oleacein inhibit angiogenesis by targeting endothelial cells: A comparative study. Biomed. Pharmacother. 2023, 164, 115115. [Google Scholar] [CrossRef]
- Bender, C.; Candi, I.; Rogel, E.; Salvi, A.; Rossi, F.; Moretti, D.; Biasi, F.; Arciello, M.; Cattaruzza, M.S.; Toti, E.; et al. Efficacy of hydroxytyrosol-rich food supplements on reducing lipid oxidation in humans. Int. J. Mol. Sci. 2023, 24, 5521. [Google Scholar] [CrossRef]
- Nikou, T.; Sakavitsi, M.E.; Kalampokis, E.; Perivolioti, E.; Halabalaki, M. Metabolism and bioavailability of olive bioactive constituents based on in vitro, in vivo and human studies. Nutrients 2022, 14, 3773. [Google Scholar] [CrossRef]
- Nikou, T.; Karampetsou, K.V.; Koutsoni, O.S.; Skaltsounis, A.L.; Dotsika, E.; Halabalaki, M. Pharmacokinetics and metabolism investigation of oleocanthal. J. Nat. Prod. 2024, 87, 530–543. [Google Scholar] [CrossRef]
- Costa, V.; Costa, M.; Videira, R.A.; Rocha, S.; Serra, A.T.; Paiva-Martins, F. Anti-inflammatory activity of olive oil polyphenols—The role of oleacein and its metabolites. Biomedicines 2022, 10, 2990. [Google Scholar] [CrossRef]
- Marrero, A.D.; López, A.; Ortega, M.; Suárez, M.; Rodríguez, Y.; García, C.; Fernández-Cabezudo, M.J. Nanoformulation approaches for enhancing the stability and bioactivity of olive polyphenols. Nutrients 2024, 16, 1283. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Toti, E.; De Amicis, R.; Dellavia, C.; Marino, M.; Menna, R.; Cresci, A. Olive polyphenols and health outcomes: An umbrella review of systematic reviews and meta-analyses. Nutrients 2023, 15, 1471. [Google Scholar]
- Saumet, A.; Fernandez-Cabezudo, M.J.; El-Serafi, A.T.; Bashir, G.; Bashir, M.A.; Suhail, M.; Bashir, H. Early-phase pilot clinical trial evaluating EVOO enriched with oleocanthal and oleacein in chronic lymphocytic leukemia. J. Nutr. Biochem. 2024, 115, 109408. [Google Scholar]
- Saad, B. Management of Obesity-Related Inflammatory and Cardiovascular Diseases by Medicinal Plants: From Traditional Uses to Therapeutic Targets. Biomedicines 2023, 11, 2204. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; Vallverdú-Queralt, A.; Rinaldi de Alvarenga, J.F.; Denoya, G.I.; Lamuela-Raventós, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Kalua, C.M.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D. Changes in virgin olive oil composition with malaxation temperature and time. Food Chem. 2006, 99, 385–391. [Google Scholar]
- Blasi, F.; Ceccarini, M.R.; Bistarelli, S.; Galli, F.; Cossignani, L.; Bartolini, D.; Ianni, F. Impact of Extra-Virgin Olive Oil Storage Conditions on Phenolic Content and Wound-Healing Properties. Foods 2025, 14, 2104. [Google Scholar] [CrossRef]
- Francioso, A.; Federico, R.; Maggiore, A.; Fontana, M.; Boffi, A.; D’Erme, M.; Mosca, L. Green Route for the Isolation and Purification of Hydroxytyrosol, Tyrosol, Oleacein and Oleocanthal from Extra Virgin Olive Oil. Molecules. 2020, 25, 3654. [Google Scholar] [CrossRef]



| Mechanism | Compound | Molecular/Cellular Targets |
|---|---|---|
| Inhibition of cell proliferation | Oleocanthal | CDK6↓, cyclin D1↓, TRPC6↓, P21↑, P27↑ |
| Oleacein | CDK2↓ | |
| Oleuropein | Cyclin D1↓, p21↑, p53↑, CDK↓ | |
| Tyrosol | Cyclin D1↓, PCNA↓, CDK4↓, CDK6↓, p21↑, p27↑ | |
| Induction of apoptosis | Oleocanthal | caspase 3, 8, 9↑, Bcl-xL↓, Bcl-2↓, Mcl-1↓, survivin↓ |
| Oleacein | Bcl-2↓, Mcl-1↓, BAX↑ | |
| Oleuropein | Bax↑, Bid↑, Bad↑, Bcl-2↓, p62↓, p70S6K↓, mGlo2↑, LC3-II/LC3-I↑, Beclin-1↑ | |
| Tyrosol | Bax↑, Bcl-2↓, caspase-3↑, PARP cleavage↑, ROS↑ | |
| Inhibition of angiogenesis | Oleocanthal | AKT↓, ERK1/2↓, c-Met↓ |
| Oleuropein | MMP-2, -9↓, uPA↓, VEGF-A↓, D↓, HIF-1α↓ | |
| Tyrosol | VEGF↓, HIF-1α↓, MMP-2↓ | |
| Inhibition of metastasis | Oleocanthal | E-cadherin↑, N-cadherin↓, vimentin↓, STAT3↓, Brk/paxillin/Rac1↓, c-Met↓ |
| Oleuropein | SIRT-1↓, HDAC4↓, miR-194↑, PD-L1↓, XIST↑ | |
| Tyrosol | MMP-2↓, MMP-9↓, N-cadherin↓, vimentin↓, E-cadherin↑ | |
| Modulation of cancer-linked pathways | Oleocanthal | ERK1/2↓, AKT↓, STAT3↓, SMYD2↓, Brk↓ |
| Oleacein | c-KIT↓, K-RAS↓, PIK3R3↓, STAT3↓ | |
| Oleuropein | NF-κB↓, cyclin D1↓, ERK p44/42↓, ERK1/2↓, AKT↓ | |
| Tyrosol | NF-κB↓, AKT↓, ERK1/2↓, JNK↓, PI3K↓, MAPK↓ |
| Anticancer Mechanism | Ty | HT | OP | OC | OT |
|---|---|---|---|---|---|
| Induction of apoptosis | √ | √ | √ | √ | √ |
| Anti-inflammatory | √ | √ | √ | √ | √ |
| Anti-oxidant | √ | √ | √ | √ | √ |
| Anti-metastasis | √ | √ | √ | ||
| Inhibition of cell proliferation | √ | √ | √ | √ | |
| Inhibition of angiogenesis | √ | √ | √ | ||
| Induction of autophagy | √ | √ | √ | ||
| Inhibition of cell migration | √ | √ | √ | ||
| Targeting cell signaling pathways | √ | √ | √ | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, B.; Kmail, A. Olive Oil Polyphenols in Cancer: Molecular Mechanisms and Therapeutic Promise. Immuno 2025, 5, 36. https://doi.org/10.3390/immuno5030036
Saad B, Kmail A. Olive Oil Polyphenols in Cancer: Molecular Mechanisms and Therapeutic Promise. Immuno. 2025; 5(3):36. https://doi.org/10.3390/immuno5030036
Chicago/Turabian StyleSaad, Bashar, and Abdalsalam Kmail. 2025. "Olive Oil Polyphenols in Cancer: Molecular Mechanisms and Therapeutic Promise" Immuno 5, no. 3: 36. https://doi.org/10.3390/immuno5030036
APA StyleSaad, B., & Kmail, A. (2025). Olive Oil Polyphenols in Cancer: Molecular Mechanisms and Therapeutic Promise. Immuno, 5(3), 36. https://doi.org/10.3390/immuno5030036







