Etiopathogenesis and Treatment of Colorectal Cancer
Abstract
1. Introduction
1.1. Etiology
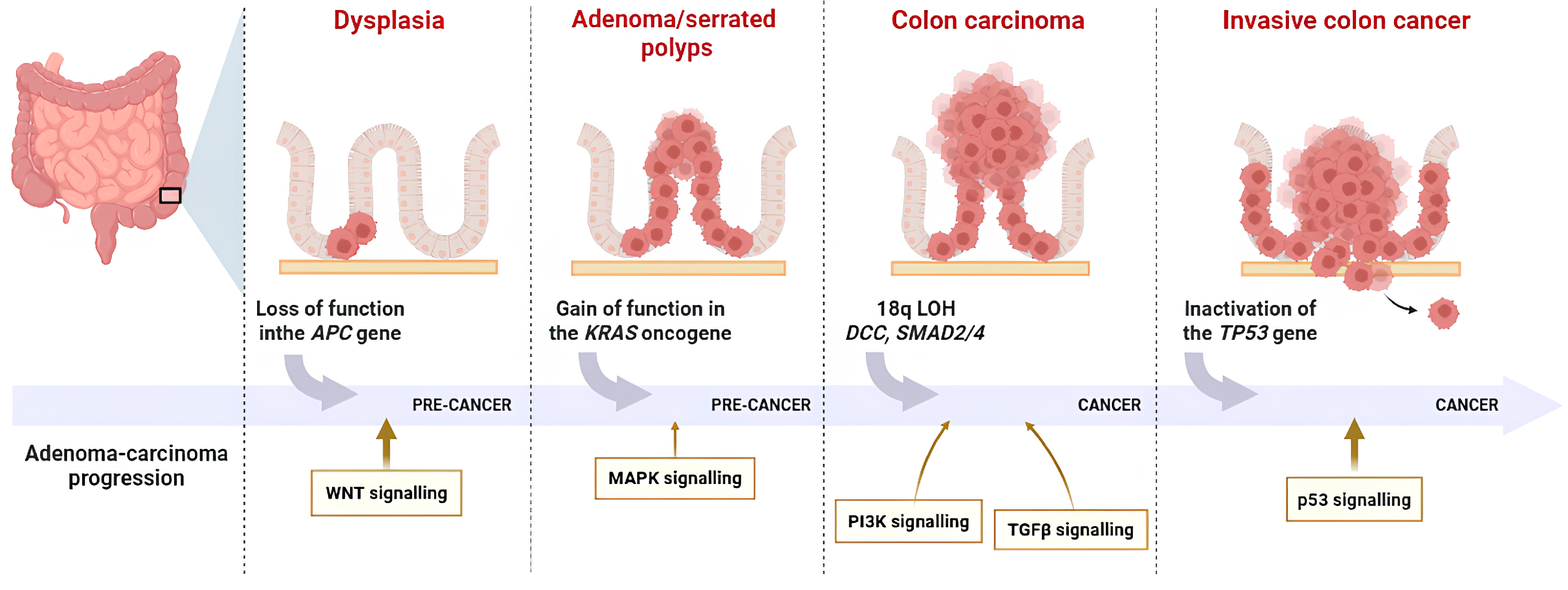
1.2. Pathophysiology of CRC
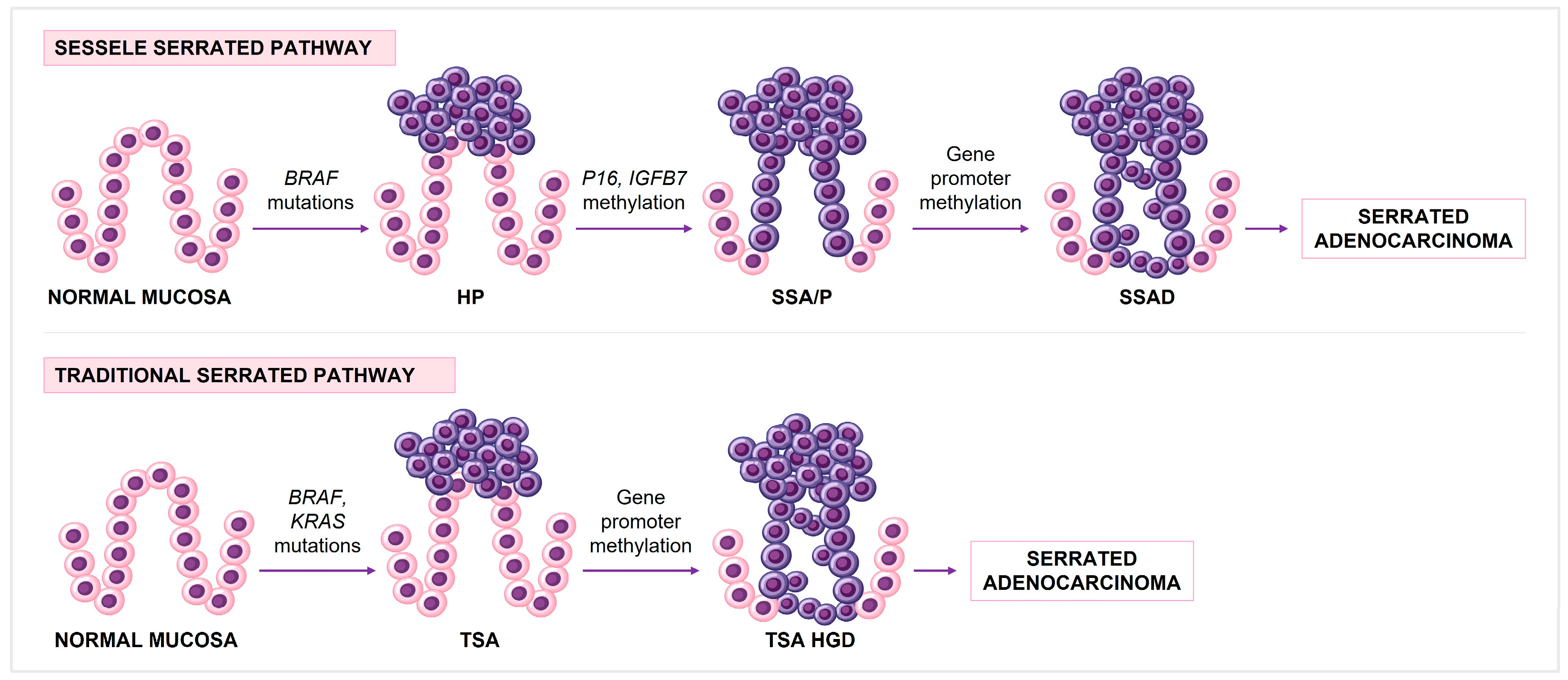
1.3. CRC Molecular Pathways
1.3.1. Chromosomal Instability (CIN)
1.3.2. Microsatellite Instability (MSI)
1.3.3. CpG Island Methylation Phenotype (CIMP)
1.4. Key Molecular Targets for CRC Targeted Therapy
1.4.1. RAS/MAPK/MEK Signaling
- EGFR and KRAS:
- BRAF:
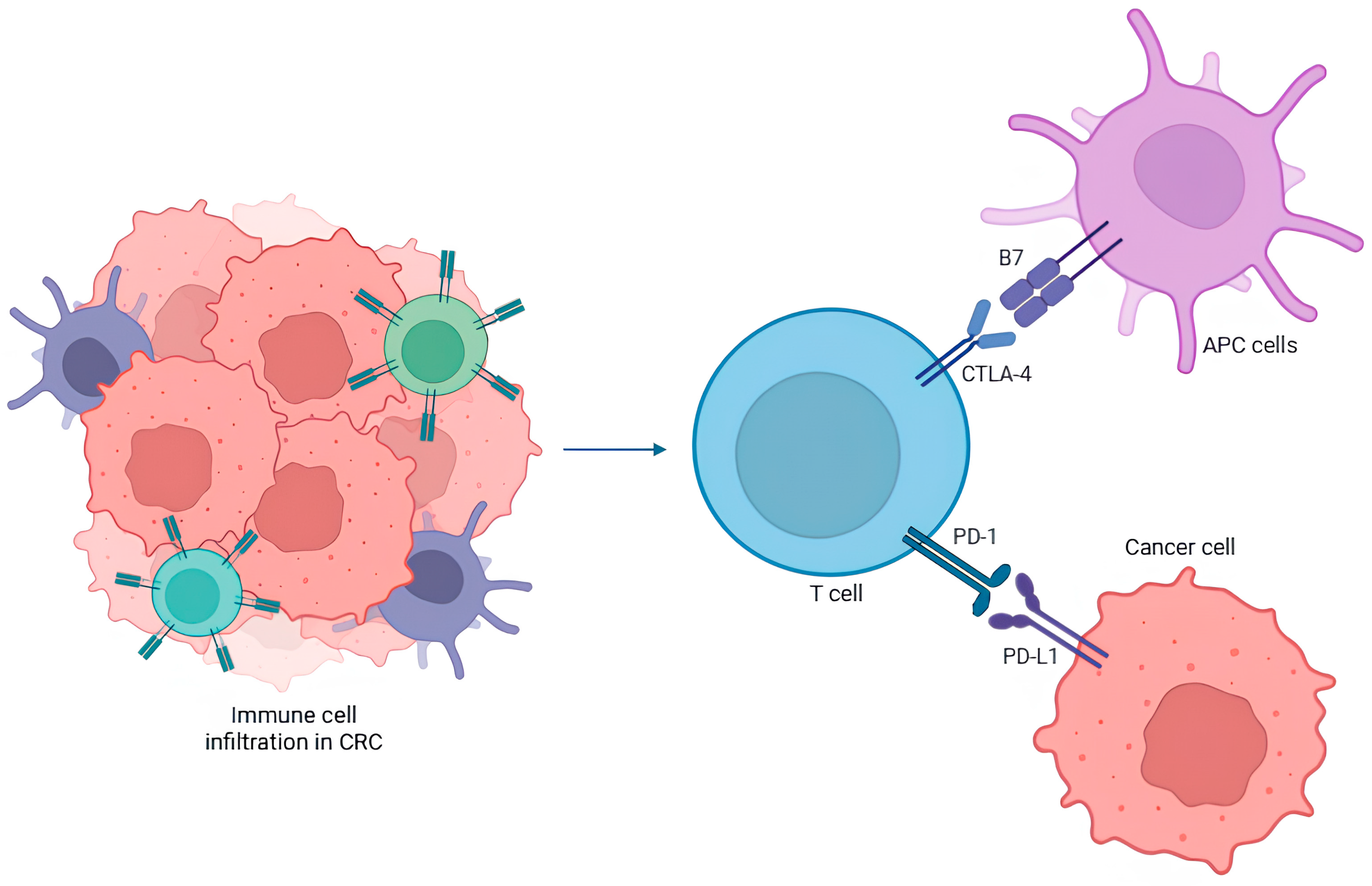
1.4.2. PD-1 and CTLA-4
1.5. Treatment
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 17p13.1 | Chromosome 17 |
| 5-FU | 5-fluorouracil |
| APCs | Antigen-presenting cells |
| APC | Adenomatous polyposis coli |
| CH3 | Methyl group |
| CIMP | CpG island methylation phenotype |
| CIN | Chromosomal instability |
| CRC | Colorectal cancer |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| DCC | Deleted in colorectal cancer |
| dels746-750 | EGFR gene are deletions in exon 19 |
| DNMTs | DNA methyltransferases |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-to-mesenchymal transition |
| ESMO | European Society for Medical Oncology |
| FAP | Familial adenomatous polyposis |
| FDA | Food and Drug Administration |
| FOLFIRI | LV + 5-FU + irinotecan |
| FOLFIRINOX | LV + 5-FU + irinotecan + oxaplatin |
| FOLFOX | LV + 5-FU + oxaplatin |
| GDP | Guanosine diphosphate |
| GTP | Guanosine triphosphate |
| HER2 | Human Epidermal growth factor Receptor-type 2 |
| HNPCC | Hereditary non-polyposis colorectal cancer |
| HPs | Hyperplastic polyps |
| HRAS | Harvey rat sarcoma viral oncogene homolog |
| IGFBP7 | Insulin-like growth factor binding protein 7 |
| IL-6 | Interleukin-6 |
| INCA | National Cancer Institute |
| KRAS | Kirsten rat sarcoma |
| L858R | Leucine for arginine in codon 858 of exon 21 |
| LOH | Loss of heterozygosity |
| LV | Leucovorin |
| MAPK | Mitogen-activated protein kinase |
| MGMT | Methylguanine-DNA Methyltransferase |
| MMR | Mismatch repair |
| MP | Mixed polyps |
| MSS | Microsatellites stability |
| MSI | Microsatellite instability |
| NCCN | National Comprehensive Cancer Network |
| NGS | Next-generation sequencing |
| NRAS | Neuroblastoma rat sarcoma viral oncogene homolog |
| PCR | Polymerase chain reaction |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PIK3 | Phosphatidylinositol 3-kinase |
| RTK | Receptor with tyrosine kinase |
| S-IIP | Switch-II pocket |
| SSA/P | Sessile serrated adenomas/polyps |
| SSAD | Sessile serrated adenoma with high-grade dysplasia |
| TCR | T cell receptor |
| TGFβ | Transforming growth factor-beta |
| TNF-α | Tumor necrosis factor-alpha |
| TP53 | Gene that encodes the tumor protein p53 |
| TSA | Traditional serrated adenomas |
| VEGF | Vascular endothelial growth factor |
| XELOX | Capecitabine + oxaplatin |
References
- Ministério da Saúde. Estimativa 2023: Incidência de Câncer no Brasil, 1st ed.; Instituto Nacional de Câncer: Rio de Janeiro, Brazil, 2022; pp. 1–162. Available online: https://www.inca.gov.br/sites/ufu.sti.inca.local/files/media/document/estimativa-2023.pdf (accessed on 20 June 2025).
- International Agency for Research on Cancer. Globocan 2020: Cancer Fact Sheets-Colorectal Cancer. Available online: https://gco.iarc.fr/en (accessed on 23 June 2025).
- Matsuda, T.; Fujimoto, A.; Igarashi, Y. Colorectal Cancer: Epidemiology, risk factors, and public health strategies. Digestion 2025, 106, 91–99. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.Q.; Luo, Q.; Wang, L.; Song, G.B.; Sheng, J.P.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. NCCN guidelines insights: Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Singh, M.; Morris, V.K.; Bandey, I.N.; Hong, D.S.; Kopetz, S. Advancements in combining targeted therapy and immunotherapy for colorectal cancer. Trends Cancer 2024, 10, 598–609. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Saha, S.; Ghosh, S.; Ghosh, S.; Nandi, S.; Nayak, A. Unraveling the complexities of colorectal cancer and its promising therapies —An updated review. Int. Immunopharmacol. 2024, 143, 113325. [Google Scholar] [CrossRef] [PubMed]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef]
- Jankowski, W.M.; Fichna, J.; Tarasiuk-Zawadzka, A. The interplay between diet and the enteric nervous system in the pathophysiology of colorectal cancer. Folia Med. Cracov. 2024, 64, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Kleis, L.; Depetris-Chauvin, A.; Jaskulski, S.; Damerell, V.; Michels, K.B.; Gigic, B.; Nöthlings, U.; Panagiotou, G. Beneficial microbiome and diet interplay in early-onset colorectal cancer. EMBO Mol. Med. 2025, 17, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Esplin, E.D.; Hanson, C.; Wu, S.; Horning, A.M.; Barapour, N.; Nevins, S.A.; Jiang, L.; Contrepois, K.; Lee, H.; Guha, T.K.; et al. Multiomic analysis of familial adenomatous polyposis reveals molecular pathways associated with early tumorigenesis. Nat. Cancer 2024, 5, 1737–1753. [Google Scholar] [CrossRef]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppälä, T.T.; Ten Broeke, S.W.; Plazzer, J.P.; Nakken, S.; Engel, C.; Aretz, S.; Jenkins, M.A.; Sunde, L.; et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020, 22, 1569. [Google Scholar] [CrossRef]
- Roht, L.; Laidre, P.; Tooming, M.; Tõnisson, N.; Nõukas, M.; Nurm, M.; Estonian Biobank Research Team; Roomere, H.; Rekker, K.; Toome, K.; et al. The prevalence and molecular landscape of lynch syndrome in the affected and general population. Cancers 2023, 15, 3663. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.A.; Morreau, H.; de Miranda, N.F.C.C.; van Wezel, T. The missing heritability of familial colorectal cancer. Mutagenesis 2020, 35, 221–231. [Google Scholar] [CrossRef]
- Nassar, D.; Blanpain, C. Cancer stem cells: Basic concepts and therapeutic implications. Annu. Rev. Pathol. 2016, 23, 47–76. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Morán, A.; Ortega, P.; de Juan, C.; Fernández-Marcelo, T.; Frías, C.; Sánchez-Pernaute, A.; Torres, A.J.; Díaz-Rubio, E.; Iniesta, P.; Benito, M. Differential colorectal carcinogenesis: Molecular basis and clinical relevance. World J. Gastrointest. Oncol. 2010, 2, 151–158. [Google Scholar] [CrossRef]
- Müller, M.F.; Ibrahim, A.E.; Arends, M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016, 469, 125–134. [Google Scholar] [CrossRef]
- Mäkinen, M.J.; George, S.M.; Jernvall, P.; Mäkelä, J.; Vihko, P.; Karttunen, T.J. Colorectal carcinoma associated with serrated adenoma--prevalence, histological features, and prognosis. J. Pathol. 2001, 193, 286–294. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Yang, S.; Mack, C.; Xu, H.; Huang, C.S.; Mulcahy, E.; Amorosino, M.; Farraye, F.A. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am. J. Surg. Pathol. 2006, 30, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Kriegl, L.; Neumann, J.; Vieth, M.; Greten, F.R.; Reu, S.; Jung, A.; Kirchner, T. Up and downregulation of p16(Ink4a) expression in BRAF-mutated polyps/adenomas indicates a senescence barrier in the serrated route to colon cancer. Mod. Pathol. 2011, 24, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- De Palma, F.D.E.; D’Argenio, V.; Pol, J.; Kroemer, G.; Maiuri, M.C.; Salvatore, F. The molecular hallmarks of the serrated pathway in colorectal cancer. Cancers 2019, 11, 1017. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of colorectal carcinogenesis. Gastroenterology 2020, 158, 291–302. [Google Scholar] [CrossRef]
- Raskov, H.; Søby, J.H.; Troelsen, J.; Bojesen, R.D.; Gögenur, I. Driver gene mutations and epigenetics in colorectal cancer. Ann. Surg. 2020, 271, 75–85. [Google Scholar] [CrossRef]
- Hultcrantz, R. Aspects of colorectal cancer screening, methods, age and gender. J. Intern. Med. 2021, 289, 493–507. [Google Scholar] [CrossRef]
- Hong, S.N. Genetic and epigenetic alterations of colorectal cancer. Intestig. Res. 2018, 16, 327–337. [Google Scholar] [CrossRef]
- Jung, G.; Hernández-Illán, E.; Moreira, L.; Balaguer, F.; Goel, A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 111–130. [Google Scholar] [CrossRef]
- Aaltonen, L.A.; Peltomäki, P.; Mecklin, J.P.; Järvinen, H.; Jass, J.R.; Green, J.S.; Lynch, H.T.; Watson, P.; Tallqvist, G.; Juhola, M. Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res. 1994, 54, 1645–1648. [Google Scholar] [PubMed]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef]
- Jimeno, A.; Messersmith, W.A.; Hirsch, F.R.; Franklin, W.A.; Eckhardt, S.G. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: Practical application of patient selection. J. Clin. Oncol. 2009, 27, 1130–1136. [Google Scholar] [CrossRef]
- Lièvre, A.; Bachet, J.B.; Boige, V.; Cayre, A.; Le Corre, D.; Buc, E.; Ychou, M.; Bouché, O.; Landi, B.; Louvet, C.; et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. 2008, 26, 374–379. [Google Scholar] [CrossRef]
- Jung, B.; Staudacher, J.J.; Beauchamp, D. Transforming growth factor β superfamily signaling in development of colorectal cancer. Gastroenterology 2017, 152, 36–52. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Tian, T. TGF-β Signaling in Metastatic Colorectal Cancer (mCRC): From Underlying Mechanism to Potential Applications in Clinical Development. Int. J. Mol. Sci. 2022, 23, 14436. [Google Scholar] [CrossRef]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.; Iglesias, M.; Céspedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.; et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Usset, J.; Rosendahl Huber, A.; Andrianova, M.A.; Batlle, E.; Carles, J.; Cuppen, E.; Elez, E.; Felip, E.; Gómez-Rey, M.; Giacco, D.L.; et al. Five latent factors underlie response to immunotherapy. Nat. Genet. 2024, 56, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Armaghany, T.; Wilson, J.D.; Chu, Q.; Mills, G. Genetic alterations in colorectal cancer. Gastrointest. Cancer. Res. 2012, 5, 19–27. [Google Scholar] [PubMed]
- Lane, D.; Levine, A. p53 Research: The past thirty years and the next thirty years. Cold Spring Harb. Perspect. Biol. 2010, 2, a000893. [Google Scholar] [CrossRef]
- Malki, A.; ElRuz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.E. Molecular mechanisms of colon cancer progression and metastasis: Recent insights and advancements. Int. J. Mol. Sci. 2020, 22, 130. [Google Scholar] [CrossRef]
- Lynch, H.T.; de la Chapelle, A. Hereditary colorectal cancer. N. Engl. J. Med. 2003, 348, 919–932. [Google Scholar] [CrossRef]
- Pino, M.S.; Chung, D.C. The chromosomal instability pathway in colon cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef]
- Wankhede, D.; Yuan, T.; Kloor, M.; Halama, N.; Brenner, H.; Hoffmeister, M. Clinical significance of combined tumour-infiltrating lymphocytes and microsatellite instability status in colorectal cancer: A systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 2024, 9, 609–619. [Google Scholar] [CrossRef]
- Afrăsânie, V.A.; Marinca, M.V.; Alexa-Stratulat, T.; Gafton, B.; Păduraru, M.; Adavidoaiei, A.M.; Miron, L.; Rusu, C. KRAS, NRAS, BRAF, HER2 and microsatellite instability in metastatic colorectal cancer—Practical implications for the clinician. Radiol. Oncol. 2019, 53, 265–274. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, e240029. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar]
- Ryan, E.; Sheahan, K.; Creavin, B.; Mohan, H.M.; Winter, D.C. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit. Rev. Oncol. Hematol. 2017, 116, 38–57. [Google Scholar] [CrossRef]
- Vila, A.P.S.; Rodrigues, G.H.; Marzochi, L.L.; Oliveira-Cucolo, J.G.; Galbiatti-Dias, A.L.S.; Andrade, R.F.M.; de Santi Neto, D.; Gomes Netinho, J.; Castiglioni, L.; Pavarino, É.C.; et al. Epidemiological and molecular evaluation of BRAF, KRAS, NRAS genes and MSI in the development of colorectal cancer. Gene 2023, 20, 147395. [Google Scholar] [CrossRef] [PubMed]
- van Engeland, M.; Derks, S.; Smits, K.M.; Meijer, G.A.; Herman, J.G. Colorectal cancer epigenetics: Complex simplicity. J. Clin. Oncol. 2011, 29, 1382–1391. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Gallois, C.; Pernot, S.; Zaanan, A.; Taieb, J. Colorectal cancer: Why does side matter? Drugs 2018, 78, 789–798. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Chakravarti, D.; Shalapour, S.; DePinho, R.A. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021, 35, 787–820. [Google Scholar] [CrossRef]
- Kim, J.E.; Hong, Y.S.; Kim, H.J.; Kim, K.P.; Lee, J.L.; Park, S.J.; Lim, S.B.; Park, I.J.; Kim, C.W.; Yoon, Y.S.; et al. Defective mismatch repair status was not associated with DFS and OS in stage II colon cancer treated with adjuvant chemotherapy. Ann. Surg. Oncol. 2015, 22, S630–S6307. [Google Scholar] [CrossRef] [PubMed]
- Protásio, B.M.; Matutino, A.; Lage, L.V.; Santana, I.; Ramos, R.; Sabbaga, J.; Capareli, F.; Saragiotto, D.; Riechelmann, R.; Hoff, P.M. Safety and efficacy of a modified FLOX adjuvant regimen for patients with stage III colorectal cancer treated in the community. Clin. Color. Cancer 2017, 16, 65–72. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Nordlinger, B.; Adam, R.; Köhne, C.H.; Pozzo, C.; Poston, G.; Ychou, M.; Rougier, P.; European Colorectal Metastases Treatment Group. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer 2006, 42, 2212–2221. [Google Scholar] [CrossRef]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B., III; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef]
- Potocki, P.M.; Wójcik, P.; Chmura, Ł.; Goc, B.; Fedewicz, M.; Bielańska, Z.; Swadźba, J.; Konopka, K.; Kwinta, Ł.; Wysocki, P.J. Clinical Characterization of Targetable Mutations (BRAF V600E and KRAS G12C) in Advanced Colorectal Cancer—A Nation-Wide Study. Int. J. Mol. Sci. 2023, 24, 9073. [Google Scholar] [CrossRef]
- Riely, G.J.; Politi, K.A.; Miller, V.A.; Pao, W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin. Cancer Res. 2006, 12, 7232–7241. [Google Scholar] [CrossRef]
- Segatto, O.; Anastasi, S.; Alemà, S. Regulation of epidermal growth factor receptor signalling by inducible feedback inhibitors. J. Cell Sci. 2011, 124, 1785–1793. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Yatabe, Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010, 277, 301–308. [Google Scholar] [CrossRef]
- Jurišić, V.; Obradovic, J.; Pavlović, S.; Djordjevic, N. Epidermal Growth Factor Receptor Gene in Non- Small-Cell Lung Cancer: The Importance of Promoter Polymorphism Investigation. Anal. Cell. Pathol. 2018, 14, 6192187. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Peeters, M.; Siena, S.; Humblet, Y.; Hendlisz, A.; Neyns, B.; Canon, J.L.; Van Laethem, J.L.; Maurel, J.; Richardson, G.; et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 2007, 25, 1658–1664. [Google Scholar] [CrossRef]
- Baselga, J. The EGFR as a target for anticancer therapy-focus on cetuximab. Eur. J. Cancer 2001, 37, S16–S22. [Google Scholar] [CrossRef]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef]
- Tveit, K.M.; Guren, T.; Glimelius, B.; Pfeiffer, P.; Sorbye, H.; Pyrhonen, S.; Sigurdsson, F.; Kure, E.; Ikdahl, T.; Skovlund, E.; et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: The NORDIC-VII study. J. Clin. Oncol. 2012, 30, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. RAS oncogenes: The first 30 years. Nat. Rev. Cancer 2003, 3, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Porru, M.; Pompili, L.; Caruso, C.; Biroccio, A.; Leonetti, C. Targeting KRAS in metastatic colorectal cancer: Current strategies and emerging opportunities. J. Exp. Clin. Cancer Res. 2018, 37, 57. [Google Scholar] [CrossRef]
- McCormick, F. KRAS as a therapeutic target. Clin. Cancer Res. 2015, 21, 1797–1801. [Google Scholar] [CrossRef]
- Huang, D.; Sun, W.; Zhou, Y.; Li, P.; Chen, F.; Chen, H.; Xia, D.; Xu, E.; Lai, M.; Wu, Y.; et al. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. 2018, 37, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Haigis, K.M. KRAS alleles: The devil is in the detail. Trends Cancer 2017, 3, 686–697. [Google Scholar] [CrossRef]
- Sameer, A.S.; ul Rehman, S.; Pandith, A.A.; Syeed, N.; Shah, Z.A.; Chowdhri, N.A.; Wani, K.A.; Siddiqi, M.A. Molecular gate keepers succumb to gene aberrations in colorectal cancer in Kashmiri population, revealing a high incidence area. Saudi J. Gastroenterol. 2009, 15, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS mutation: From undruggable to druggable in cancer. Signal Transduct. Target. Ther. 2021, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guan, X.; Zhang, X.; Luan, X.; Song, Z.; Cheng, X.; Zhang, W.; Qin, J.J. Targeting KRAS mutant cancers: From druggable therapy to drug resistance. Mol. Cancer 2022, 21, 159. [Google Scholar] [CrossRef]
- Desai, J.; Alonso, G.; Kim, S.H.; Cervantes, A.; Karasic, T.; Medina, L.; Shacham-Shmueli, E.; Cosman, R.; Falcon, A.; Gort, E.; et al. Divarasib plus cetuximab in KRAS G12C-positive colorectal cancer: A phase 1b trial. Nat. Med. 2024, 30, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Weiss, J.; Pelster, M.S.; Spira, A.I.; Barve, M.; Ou, S.I.; Leal, T.A.; Bekaii-Saab, T.S.; Paweletz, C.P.; Heavey, G.A.; et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 388, 44–54. [Google Scholar] [CrossRef]
- Garcia-Carbonero, N.; Martinez-Useros, J.; Li, W.; Orta, A.; Perez, N.; Carames, C.; Hernandez, T.; Moreno, I.; Serrano, G.; Garcia-Foncillas, J. KRAS and BRAF Mutations as Prognostic and Predictive Biomarkers for Standard Chemotherapy Response in Metastatic Colorectal Cancer: A Single Institutional Study. Cells 2020, 9, 219. [Google Scholar] [CrossRef]
- Xing, M. BRAF mutation in thyroid cancer. Endocr. Relat. Cancer 2005, 12, 245–262. [Google Scholar] [CrossRef]
- Wan, P.T.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; Barford, D.; et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef]
- Caputo, F.; Santini, C.; Bardasi, C.; Cerma, K.; Casadei-Gardini, A.; Spallanzani, A.; Andrikou, K.; Cascinu, S.; Gelsomino, F. BRAF-mutated colorectal cancer: Clinical and molecular insights. Int. J. Mol. Sci. 2019, 20, 5369. [Google Scholar] [CrossRef]
- Cohen, R.; Cervera, P.; Svrcek, M.; Pellat, A.; Dreyer, C.; de Gramont, A.; André, T. BRAF-mutated colorectal cancer: What is the optimal strategy for treatment? Curr. Treat. Options Oncol. 2017, 18, 9. [Google Scholar] [CrossRef]
- Yaeger, R.; Kotani, D.; Mondaca, S.; Parikh, A.R.; Bando, H.; Van Seventer, E.E.; Taniguchi, H.; Zhao, H.; Thant, C.N.; de Stanchina, E.; et al. Response to anti-EGFR therapy in patients with BRAF non-V600-mutant metastatic colorectal cancer. Clin. Cancer Res. 2019, 25, 7089–7097. [Google Scholar] [CrossRef] [PubMed]
- Ursem, C.; Atreya, C.E.; Van Loon, K. Emerging treatment options for BRAF-mutant colorectal cancer. Gastrointest. Cancer 2018, 8, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bylsma, L.C.; Gillezeau, C.; Garawin, T.A.; Kelsh, M.A.; Fryzek, J.P.; Sangaré, L.; Lowe, K.A. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta-analysis. Cancer Med. 2020, 9, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, L.J.; Peng, X.F.; Deng, L.; Wang, Y.; Li, J.J.; Guo, D.L.; Niu, X.H. Anti-PD-1/PD-L1 therapy for colorectal cancer: Clinical implications and future considerations. Transl. Oncol. 2024, 40, 101851. [Google Scholar] [CrossRef]
- Edner, N.M.; Carlesso, G.; Rush, J.S.; Walker, L.S.K. Targeting co-stimulatory molecules in autoimmune disease. Nat. Rev. Drug Discov. 2020, 19, 860–883. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.X.; Istl, A.C.; Quan, D.; Skaro, A.; Tang, E.; Zheng, X. PD-1 and PD-L1 inhibitors in cold colorectal cancer: Challenges and strategies. Cancer Immunol. Immunother. 2023, 72, 3875–3893. [Google Scholar] [CrossRef]
- Kuno, S.; Pakpian, N.; Muanprasat, C. The potential role of PD-1/PD-L1 small molecule inhibitors in colorectal cancer with different mechanisms of action. Eur. J. Pharmacol. 2025, 992, 177351. [Google Scholar] [CrossRef]
- Sangani, P.S.; Yazdani, S.; Khalili-Tanha, G.; Ghorbani, E.; Al-Hayawi, I.S.; Fiuji, H.; Khazaei, M.; Hassanian, S.M.; Kiani, M.; Ghayour-Mobarhan, M.; et al. The therapeutic impact of programmed death-1 in the treatment of colorectal cancer. Pathol. Res. Pract. 2024, 259, 155345. [Google Scholar] [CrossRef]
- Tai, X.; Van Laethem, F.; Pobezinsky, L.; Guinter, T.; Sharrow, S.O.; Adams, A.; Granger, L.; Kruhlak, M.; Lindsten, T.; Thompson, C.B.; et al. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood 2012, 119, 5155–5163. [Google Scholar] [CrossRef]
- Ciombor, K.K.; Strickler, J.H.; Bekaii-Saab, T.S.; Yaeger, R. BRAF-mutated advanced colorectal cancer: A rapidly changing therapeutic landscape. J. Clin. Oncol. 2022, 40, 2706–2715. [Google Scholar] [CrossRef]
- Grothey, A.; Fakih, M.; Tabernero, J. Management of BRAF-mutant metastatic colorectal cancer: A review of treatment options and evidence-based guidelines. Ann. Oncol. 2021, 32, 959–967. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; Alcaide-Garcia, J.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy in microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer: 5-year follow-up from the randomized phase III KEYNOTE-177 study. Ann. Oncol. 2025, 36, 277–284. [Google Scholar] [CrossRef]
- André, T.; Elez, E.; Lenz, H.J.; Jensen, L.H.; Touchefeu, Y.; Van Cutsem, E.; Garcia-Carbonero, R.; Tougeron, D.; Mendez, G.A.; Schenker, M.; et al. Nivolumab plus ipilimumab versus nivolumab in microsatellite instability-high metastatic colorectal cancer (CheckMate 8HW): A randomised, open-label, phase 3 trial. Lancet 2025, 405, 383–395. [Google Scholar] [CrossRef]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: Updated survival results and subgroup analyses from the BEACON study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef]
- Frank, M.H.; Wilson, B.J.; Gold, J.S.; Frank, N.Y. Clinical implications of colorectal cancer stem cells in the age of single-cell omics and targeted therapies. Gastroenterology 2021, 160, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, T.; Cai, X.; Dong, J.; Xia, C.; Zhou, Y.; Ding, R.; Yang, R.; Tan, J.; Zhang, L.; et al. Neoadjuvant immunotherapy for MSI-H/dMMR locally advanced colorectal cancer: New strategies and unveiled opportunities. Front. Immunol. 2022, 17, 795972. [Google Scholar] [CrossRef]
- Martianov, A.S.; Mitiushkina, N.V.; Ershova, A.N.; Martynenko, D.E.; Bubnov, M.G.; Amankwah, P.; Yanus, G.A.; Aleksakhina, S.N.; Tiurin, V.I.; Venina, A.R.; et al. KRAS, NRAS, BRAF, HER2 and MSI status in a large consecutive series of colorectal carcinomas. Int. J. Mol. Sci. 2023, 24, 4868. [Google Scholar] [CrossRef] [PubMed]
- Picard, E.; Verschoor, C.P.; Ma, G.W.; Pawelec, G. Relationships between immune landscapes, genetic subtypes and responses to immunotherapy in colorectal cancer. Front. Immunol. 2020, 6, 369. [Google Scholar] [CrossRef] [PubMed]

| Treatment Line | Tumor Characteristics | Treatment |
|---|---|---|
| First-Line Treatment | RAS-wt, BRAF-wt, MSS: | |
| FOLFOX or FOLFIRI + Cetuximab or Panitumumab | |
| FOLFOX, FOLFIRI or FOLFOXIRI + Bevacizumab | |
| RAS-mut | FOLFOX, FOLFIRI or FOLFOXIRI + Bevacizumab | |
| BRAF-mut | FOLFOX + Cetuximab + Encorafenib | |
| MMR/MSI-high or MMR/MSI-high + BRAF-mut | Pembrolizumab Nivolumab + Ipilimumab | |
| Second-Line Treatment | RAS-mut, Anti-EGFR naïve: | |
| FOLFIRI or Irinotecan + Cetuximab or Panitumumab | |
| 5-FU or Capecitabine-based ChT + Bevacizumab, Ramucirumabe or Aflibercept | |
| BRAF V600E-mut | Encorafenib + Cetuximab If not previously used | |
| MMR/MSI-high | Nivolumab + Ipilimumab (Patients not receiving immunotherapy) Pembrolizumab | |
| KRAS p.G12C | Cetuximab + Adagradib Panitumumab + Sotorasib | |
| Third-Line Treatment | RAS-wt, BRAF-wt, HER2+ | Trastuzumab + Tucatinib Trastuzumab deruxtecan |
| RAS-wt, BRAF-wt | Trifluridine/Tipiracil + Bevacizumab Cetuximab or Panitumumab (If not previously used) Irinotecan + Cetuximab Regorafenib Trifluridine/Tipiracil or Fruquintinib | |
| RAS-mut | Trifluridine/Tipiracil + Bevacizumab Regorafenib Trifluridine/Tipiracil or Fruquintinib | |
| KRAS p.G12C | Cetuximab + Adagradib Panitumumab + Sotorasib If not previously used | |
| BRAF-mut | Encorafenib + Cetuximab (If not previously used) Trifluridine/Tipiracil + Bevacizumab Regorafenib Trifluridine/Tipiracil Fruquintinib |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocchi, M.; Fernandes, E.V.; Pereira, N.d.S.; Amarante, M.K. Etiopathogenesis and Treatment of Colorectal Cancer. Immuno 2025, 5, 31. https://doi.org/10.3390/immuno5030031
Bocchi M, Fernandes EV, Pereira NdS, Amarante MK. Etiopathogenesis and Treatment of Colorectal Cancer. Immuno. 2025; 5(3):31. https://doi.org/10.3390/immuno5030031
Chicago/Turabian StyleBocchi, Mayara, Eduardo Vignoto Fernandes, Nathália de Sousa Pereira, and Marla Karine Amarante. 2025. "Etiopathogenesis and Treatment of Colorectal Cancer" Immuno 5, no. 3: 31. https://doi.org/10.3390/immuno5030031
APA StyleBocchi, M., Fernandes, E. V., Pereira, N. d. S., & Amarante, M. K. (2025). Etiopathogenesis and Treatment of Colorectal Cancer. Immuno, 5(3), 31. https://doi.org/10.3390/immuno5030031







