Host Immune Response to Dengue Virus Infection: Friend or Foe?
Abstract
1. Introduction
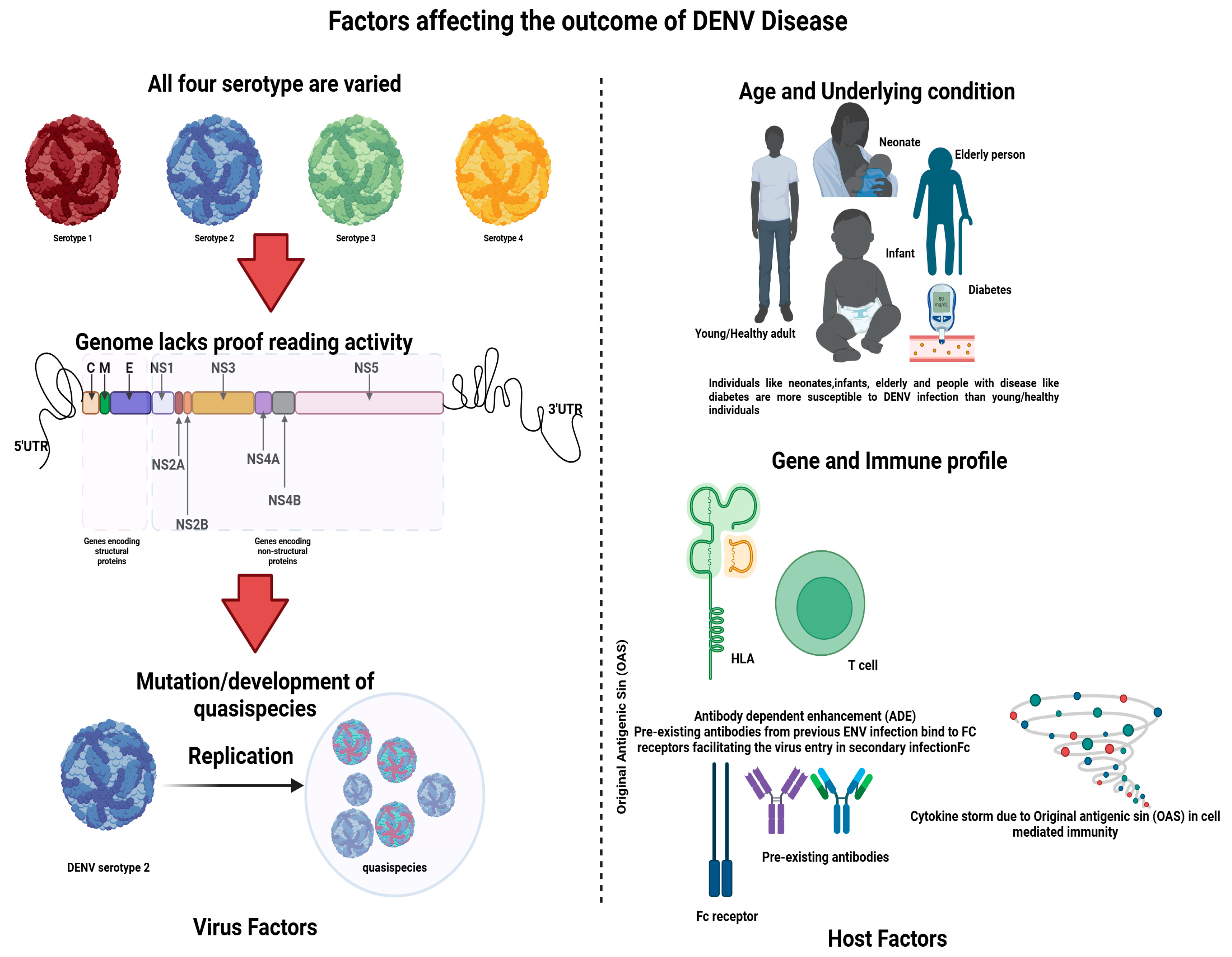
2. Dengue Virus (DENV)
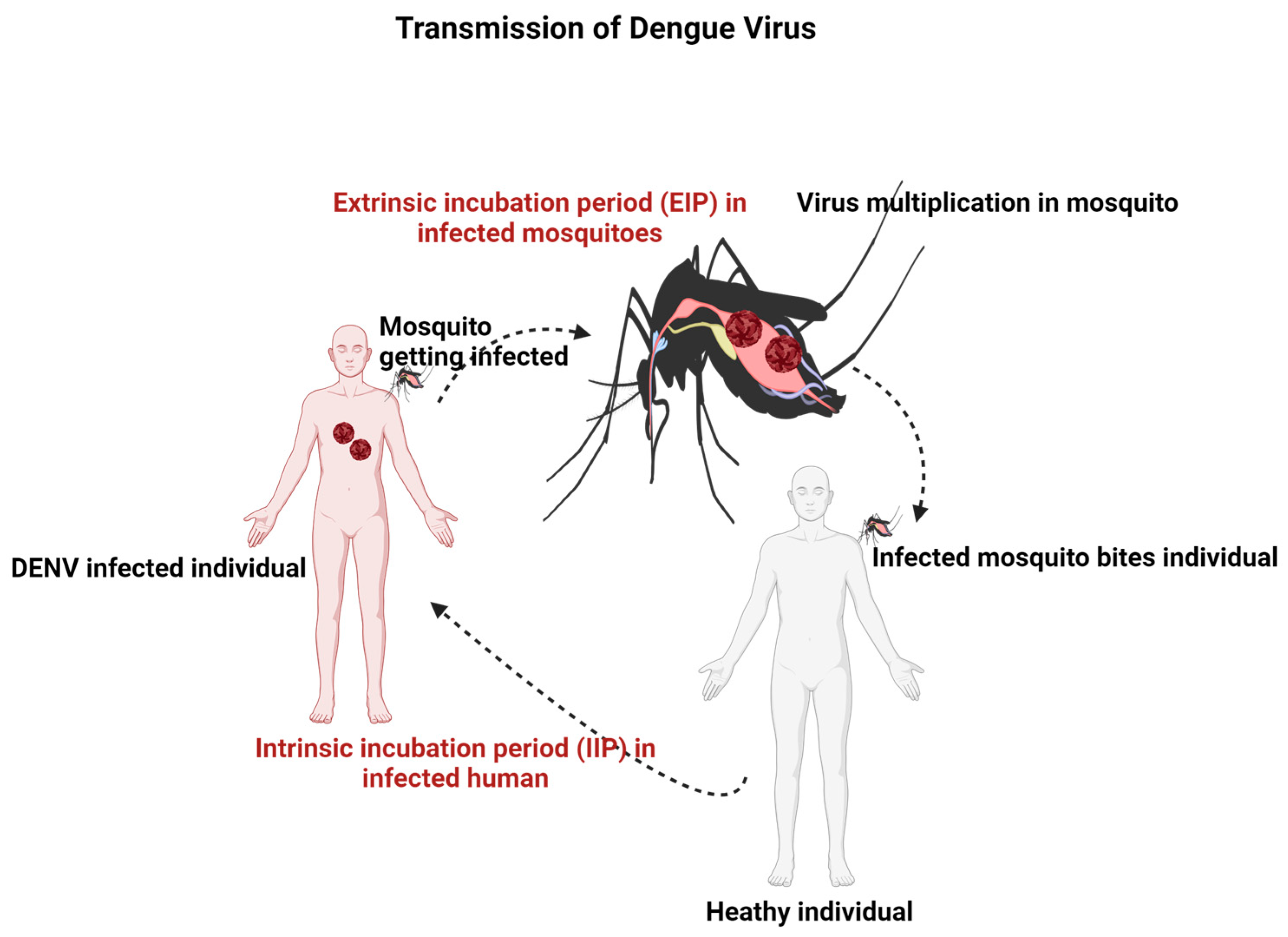
3. Pathogenesis
4. Invasion and Evasion in DENV Infection
5. Innate Immune Responses
5.1. IFNs and Activation of ISGs
5.2. Toll-Like Receptors
6. Major Histocompatibility Complex (MHC) and Natural Killer (NK) Cell Activity
7. Mast Cells
8. Complement System
9. RNA Interference (RNAi)
10. Autophagy
11. Apoptosis
12. Adaptive Immune Response
12.1. Humoral Immune Response
12.2. Cell-Mediated Immunity (CMI)
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DENV | Dengue virus |
| FV | Flavivirus |
| JEV | Japanese Encephalitis Virus |
| WNV | West Nile Virus |
| YFV | Yellow Fever Virus |
| ZV | Zika Virus |
| MVEV | Murray Valley Encephalitis Virus |
| HIV | Human Immunodeficiency Virus |
| SLEV | St. Louis Encephalitis Virus |
| TBEV | Tick Born Encephalitis Virus |
| DHF | Dengue Hemorrhagic Fever |
| DSS | Dengue shock syndrome |
| WHO | World Health Organization |
| DC | Dendritic Cells |
| RER | Rough endoplasmic reticulum |
| ER | Endoplasmic reticulum |
| EMC | Endoplasmic reticulum membrane complex |
| E | Envelope |
| C | Capsid |
| prM | peptide-Membrane |
| M | Membrane |
| NSP | Non-structural Protein |
| ED | Envelope Domain |
| FL | Fusion Loop |
| RNA | Ribonucleic Acid |
| Kb | Kilobase |
| UTRs | Untranslated regions |
| N’ | N terminus |
| RdRp | RNA-dependent RNA polymerase |
| IFN | Interferon |
| IFNAR | Interferon Receptor |
| HSP | Heparan Sulfate |
| AST | Aspartate Aminotransferase |
| ALT | Aminotransferase |
| EC | Endothelial Cells |
| CD | Cluster of Differentiation |
| pDC | Plasmocytic Dendritic Cell |
| IRF | Interferon Regulatory Factor |
| CNS | Central Nervous System |
| Phe | Phenylalanine |
| Leu | Leucine |
| NK | Natural Killer |
| MHC | Major Histocompatibility Complex |
| MBL | Mannose Binding Lectin |
| MAC | Membrane Attacking Complex |
| RISC | RNA-Induced Silencing Complex |
| Argo | Argonaute |
| ADE | Antibody-Dependent Enhancement |
| PLA2 | Phospholipase 2 |
| B cell | Bone Marrow-Derived Cell |
| T cell | Thymus Derived Cells |
| IL | Interleukin |
| FLE | Fusion Loop Epitope |
| TNF alpha | Tumor Necrosis Factor |
| CTL | Cytotoxic Lymphocytes |
| MCPT1 | Marker of Mast Cells |
| OAS | Original Antigenic Sin |
| HLA | Human Leucocyte Antigen |
| hSTING | Human STING |
| cGAS | cyclic GMP-AMP synthase a cytosolic DNA sensor |
| SARM | Selective androgen-receptor modulator |
| DAK | Dihydroxyacetone kinase |
| BAFF | B-cell activating factor BAFF |
| APRIL | Proliferation-inducing ligand |
| BACH1 | BTB domain and CNC homolog 1 |
| HO-1 | Heme-oxygenase-1 enzyme |
| RIG-1 | Retinoic acid inducible gene-1 |
| CARD | Caspase activation recruitment domains |
| MAV | Mitochondria antiviral signaling |
| IRKKe | IκB kinase |
| IRF3 | IFN regulatory factors |
| IRF7 | IFN regulatory factors |
| TBK-1 | Tank Binding Kinase-1 |
| MDA-5 | Melanoma differentiation-associated protein 5 |
| TCC | Terminal Complement Complex |
| PBMC | Peripheral blood mononuclear cells |
| C’ | C terminus |
References
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dengue Fact Sheet 2012. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 23 April 2024).
- Thomas, S.J.; Endy, T.P.; Rothman, A.L. Flaviviruses: Dengue. In Viral Infections of Humans: Epidemiology and Control; Kaslow, R.A., Stanberry, L.R., Le Duc, J.W., Eds.; Springer: Boston, MA, USA, 2014; pp. 351–381. [Google Scholar]
- Twiddy, S.S.; Holmes, E.C.; Rambaut, A. Inferring the Rate and Time-Scale of Dengue Virus Evolution. Mol. Biol. Evol. 2003, 20, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Twiddy, S.S.; Farrar, J.J.; Vinh Chau, N.; Wills, B.; Gould, E.A.; Gritsun, T.; Lloyd, G.; Holmes, E.C. Phylogenetic Relationships and Differential Selection Pressures among Genotypes of Dengue-2 Virus. Virology 2002, 298, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Valenzo, E.; Danis-Lozano, R.; Velasco-Hernández, J.X.; Sánchez-Burgos, G.; Alpuche, C.; López, I.; Rosales, C.; Baronti, C.; de Lamballerie, X.; Holmes, E.C.; et al. Evolution of dengue virus in Mexico is characterized by frequent lineage replacement. Arch. Virol. 2010, 155, 1401–1412. [Google Scholar] [CrossRef]
- Goncalvez, A.P.; Escalante, A.A.; Pujol, F.H.; Ludert, J.E.; Tovar, D.; Salas, R.A.; Liprandi, F. Diversity and Evolution of the Envelope Gene of Dengue Virus Type 1. Virology 2002, 303, 110–119. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Lewis, J.G.; Gubler, D.J.; Trent, D.W. Molecular evolution and epidemiology of dengue-3 viruses. J. Gen. Virol. 1994, 75, 65–75. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Gubler, D.J.; Trent, D.W. Molecular evolution and phylogeny of dengue-4 viruses. J. Gen. Virol. 1997, 78, 2279–2284. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Dalugama, C. Dengue infection: Global importance, immunopathology and management. Clin. Med. 2022, 22, 9–13. [Google Scholar] [CrossRef]
- Nanaware, N.; Banerjee, A.; Mullick Bagchi, S.; Bagchi, P.; Mukherjee, A. Dengue Virus Infection: A Tale of Viral Exploitations and Host Responses. Viruses 2021, 13, 1967. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Ghosh, D.; Saha, R.; Sarkar, R.; Kumar, S.; Khokhar, M.; Pandey, R.K. Mechanism of Immune Evasion in Mosquito-Borne Diseases. Pathogens 2023, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Solomon, T. Pathogenic flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109. [Google Scholar] [CrossRef]
- Uno, N.; Ross, T.M. Dengue virus and the host innate immune response. Emerg. Microbes Infect. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, B.; Fu, Z.F.; Chen, H.; Cao, S. Immune evasion strategies of flaviviruses. Vaccine 2013, 31, 461–471. [Google Scholar] [CrossRef]
- de Macedo, F.C.; Nicol, A.F.; Cooper, L.D.; Yearsley, M.; Pires, A.R.; Nuovo, G.J. Histologic, viral, and molecular correlates of dengue fever infection of the liver using highly sensitive immunohistochemistry. Diagn. Mol. Pathol. 2006, 15, 223–228. [Google Scholar] [CrossRef]
- Kangwanpong, D.; Bhamarapravati, N.; Lucia, H.L. Diagnosing dengue virus infection in archived autopsy tissues by means of the in situ PCR method: A case report. Clin. Diagn. Virol. 1995, 3, 165–172. [Google Scholar] [CrossRef]
- Balsitis, S.J.; Coloma, J.; Castro, G.; Alava, A.; Flores, D.; McKerrow, J.H.; Beatty, P.R.; Harris, E. Tropism of dengue virus in mice and humans defined by viral nonstructural protein 3-specific immunostaining. Am. J. Trop. Med. Hyg. 2009, 80, 416–424. [Google Scholar] [CrossRef]
- Basílio-De-Oliveira, C.A.; Aguiar, G.R.; Baldanza, M.S.; Barth, O.M.; Eyer-Silva, W.A.; Paes, M.V. Pathologic study of a fatal case of dengue-3 virus infection in Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 2005, 9, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Gasperino, J.; Yunen, J.; Guh, A.; Tanaka, K.E.; Kvetan, V.; Doyle, H. Fulminant liver failure secondary to haemorrhagic dengue in an international traveller. Liver Int. 2007, 27, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.G.; Alvarez, M.; Rodríguez, R.; Rosario, D.; Vázquez, S.; Vald, S.L.; Cabrera, M.V.; Kourí, G. Fatal dengue hemorrhagic fever in Cuba, 1997. Int. J. Infect. Dis. 1999, 3, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Nisalak, A.; Halstead, S.B.; Singharaj, P.; Udomsakdi, S.; Nye, S.W.; Vinijchaikul, K. Observations related to pathogenesis of dengue hemorrhagic fever. 3. Virologic studies of fatal disease. Yale J. Biol. Med. 1970, 42, 293–310. [Google Scholar] [PubMed]
- Killen, H.; O’Sullivan, M.A. Detection of dengue virus by in situ hybridization. J. Virol. Methods 1993, 41, 135–146. [Google Scholar] [CrossRef]
- Miagostovich, M.P.; Ramos, R.G.; Nicol, A.F.; Nogueira, R.M.; Cuzzi-Maya, T.; Oliveira, A.V.; Marchevsky, R.S.; Mesquita, R.P.; Schatzmayr, H.G. Retrospective study on dengue fatal cases. Clin. Neuropathol. 1997, 16, 204–208. [Google Scholar]
- Teixeira, M.G.; Barreto, M.; Guerra, Z. Epidemiologia e medidas de prevenção do dengue. Inf. Epidemiol. Sus. 1999, 8, 5–33. [Google Scholar]
- Chouin-Carneiro, T.; Dos Santos, F.B. Transmission of Major Arboviruses in Brazil: The Role of Aedes aegypti and Aedes albopictus Vectors. In Biological Control of Pest and Vector Insects; InTech: London, UK, 2017. [Google Scholar]
- Chan, M.; Johansson, M.A. The Incubation Periods of Dengue Viruses. PLoS ONE 2012, 7, e50972. [Google Scholar] [CrossRef]
- Simmons, C.P.; Farrar, J.J.; Van Vinh Chau, N.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef]
- WHO. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 11 March 2020).
- Rothman, A.L. Immunity to dengue virus: A tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 2011, 11, 532–543. [Google Scholar] [CrossRef]
- Whitehorn, J.; Simmons, C.P. The pathogenesis of dengue. Vaccine 2011, 29, 7221–7228. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Pierson, T.C. Molecular Insight into Dengue Virus Pathogenesis and Its Implications for Disease Control. Cell 2015, 162, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Rothman, A.L. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol. Rev. 2008, 225, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. Molecular biology of flaviviruses. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2003; Volume 59, pp. 23–61. [Google Scholar]
- Gebhard, L.G.; Filomatori, C.V.; Gamarnik, A.V. Functional RNA elements in the dengue virus genome. Viruses 2011, 3, 1739–1756. [Google Scholar] [CrossRef] [PubMed]
- Henchal, E.A.; Putnak, J.R. The dengue viruses. Clin. Microbiol. Rev. 1990, 3, 376–396. [Google Scholar] [CrossRef]
- Clyde, K.; Kyle, J.L.; Harris, E. Recent Advances in Deciphering Viral and Host Determinants of Dengue Virus Replication and Pathogenesis. J. Virol. 2006, 80, 11418–11431. [Google Scholar] [CrossRef]
- Dwivedi, V.D.; Tripathi, I.P.; Tripathi, R.C.; Bharadwaj, S.; Mishra, S.K. Genomics, proteomics and evolution of dengue virus. Brief. Funct. Genom. 2017, 16, elw040. [Google Scholar] [CrossRef]
- Zhang, Y. Structures of immature flavivirus particles. EMBO J. 2003, 22, 2604–2613. [Google Scholar] [CrossRef]
- Byk, L.A.; Gamarnik, A.V. Properties and Functions of the Dengue Virus Capsid Protein. Annu. Rev. Virol. 2016, 3, 263–281. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. Genetic Interaction of Flavivirus Nonstructural Proteins NS1 and NS4A as a Determinant of Replicase Function. J. Virol. 1999, 73, 4611–4621. [Google Scholar] [CrossRef]
- Watterson, D.; Modhiran, N.; Young, P.R. The many faces of the flavivirus NS1 protein offer a multitude of options for inhibitor design. Antivir. Res. 2016, 130, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Scaturro, P.; Cortese, M.; Chatel-Chaix, L.; Fischl, W.; Bartenschlager, R. Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins. PLoS Pathog. 2015, 11, e1005277. [Google Scholar] [CrossRef] [PubMed]
- Winkler, G.; Randolph, V.B.; Cleaves, G.R.; Ryan, T.E.; Stollar, V. Evidence that the mature form of the flavivirus nonstructural protein NS1 is a dimer. Virology 1988, 162, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Flamand, M.; Megret, F.O.; Mathieu, M.; Lepault, J.; Rey, F.L.A.; Deubel, V. Dengue Virus Type 1 Nonstructural Glycoprotein NS1 Is Secreted from Mammalian Cells as a Soluble Hexamer in a Glycosylation-Dependent Fashion. J. Virol. 1999, 73, 6104–6110. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zou, J.; Puttikhunt, C.; Yuan, Z.; Shi, P.-Y. Two Distinct Sets of NS2A Molecules Are Responsible for Dengue Virus RNA Synthesis and Virion Assembly. J. Virol. 2015, 89, 1298–1313. [Google Scholar] [CrossRef]
- Falgout, B.; Pethel, M.; Zhang, Y.M.; Lai, C.J. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J. Virol. 1991, 65, 2467–2475. [Google Scholar] [CrossRef]
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of Dengue Virus. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef]
- Perera, R.; Kuhn, R.J. Structural proteomics of dengue virus. Curr. Opin. Microbiol. 2008, 11, 369–377. [Google Scholar] [CrossRef]
- Aguirre, S.; Maestre, A.M.; Pagni, S.; Patel, J.R.; Savage, T.; Gutman, D.; Maringer, K.; Bernal-Rubio, D.; Shabman, R.S.; Simon, V.; et al. DENV Inhibits Type I IFN Production in Infected Cells by Cleaving Human STING. PLoS Pathog. 2012, 8, e1002934. [Google Scholar] [CrossRef]
- Umareddy, I.; Chao, A.; Sampath, A.; Gu, F.; Vasudevan, S.G. Dengue virus NS4B interacts with NS3 and dissociates it from single-stranded RNA. J. Gen. Virol. 2006, 87 Pt 9, 2605–2614. [Google Scholar] [CrossRef]
- Issur, M.; Geiss, B.J.; Bougie, I.; Picard-Jean, F.; Despins, S.; Mayette, J.; Hobdey, S.E.; Bisaillon, M. The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA 2009, 15, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 2003, 100, 6986–6991. [Google Scholar] [CrossRef] [PubMed]
- Crill, W.D.; Roehrig, J.T. Monoclonal Antibodies That Bind to Domain III of Dengue Virus E Glycoprotein Are the Most Efficient Blockers of Virus Adsorption to Vero Cells. J. Virol. 2001, 75, 7769–7773. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.-J.; Hsieh, M.-T.; Young, M.-J.; Kao, C.-L.; King, C.-C.; Chang, W. An External Loop Region of Domain III of Dengue Virus Type 2 Envelope Protein Is Involved in Serotype-Specific Binding to Mosquito but Not Mammalian Cells. J. Virol. 2004, 78, 378–388. [Google Scholar] [CrossRef]
- Huerta, V.; Chinea, G.; Fleitas, N.; Sarría, M.; Sánchez, J.; Toledo, P.; Padrón, G. Characterization of the interaction of domain III of the envelope protein of dengue virus with putative receptors from CHO cells. Virus Res. 2008, 137, 225–234. [Google Scholar] [CrossRef]
- Hidari, K.I.P.J.; Suzuki, T. Dengue virus receptor. Trop. Med. Health 2011, 39 (Suppl. 4), S37–S43. [Google Scholar] [CrossRef]
- Modis, Y. Relating structure to evolution in class II viral membrane fusion proteins. Curr. Opin. Virol. 2014, 5, 34–41. [Google Scholar] [CrossRef]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef]
- Zou, J.; Lee, L.T.; Wang, Q.Y.; Xie, X.; Lu, S.; Yau, Y.H.; Yuan, Z.; Geifman Shochat, S.; Kang, C.; Lescar, J.; et al. Mapping the Interactions Between the NS4B and NS3 Proteins of Dengue Virus. J. Virol. 2015, 89, 3471–3483. [Google Scholar] [CrossRef]
- Mackenzie, J. Wrapping Things Up About Virus RNA Replication. Traffic 2005, 6, 967–977. [Google Scholar] [CrossRef]
- Welsch, S.; Miller, S.; Romero-Brey, I.; Merz, A.; Bleck, C.K.E.; Walther, P.; Fuller, S.D.; Antony, C.; Krijnse-Locker, J.; Bartenschlager, R. Composition and Three-Dimensional Architecture of the Dengue Virus Replication and Assembly Sites. Cell Host Microbe 2009, 5, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Krijnse-Locker, J. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 2008, 6, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Yen, L.C.; Liao, J.T.; Lee, H.J.; Chou, W.Y.; Chen, C.W.; Lin, Y.L.; Liao, C.L. The C Terminus of the Core β-Ladder Domain in Japanese Encephalitis Virus Nonstructural Protein 1 Is Flexible for Accommodation of Heterologous Epitope Fusion. J. Virol. 2015, 90, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Falconar, A.K. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: Potential implications in haemorrhagic fever pathogenesis. Arch. Virol. 1997, 142, 897–916. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.W. Maturation of Japanese encephalitis virus glycoproteins produced by infected mammalian and mosquito cells. Virology 1989, 169, 354–364. [Google Scholar] [CrossRef]
- Winkelmann, E.R.; Widman, D.G.; Suzuki, R.; Mason, P.W. Analyses of mutations selected by passaging a chimeric flavivirus identify mutations that alter infectivity and reveal an interaction between the structural proteins and the nonstructural glycoprotein NS1. Virology 2011, 421, 96–104. [Google Scholar] [CrossRef]
- Rastogi, M.; Sharma, N.; Singh, S.K. Flavivirus NS1: A multifaceted enigmatic viral protein. Virol. J. 2016, 13, 131. [Google Scholar] [CrossRef]
- Best, S.M. The Many Faces of the Flavivirus NS5 Protein in Antagonism of Type I Interferon Signaling. J. Virol. 2017, 91, e01970-16. [Google Scholar] [CrossRef]
- Wang, B.; Thurmond, S.; Hai, R.; Song, J. Structure and function of Zika virus NS5 protein: Perspectives for drug design. Cell. Mol. Life Sci. 2018, 75, 1723–1736. [Google Scholar] [CrossRef]
- Khanam, A.; Gutiérrez-Barbosa, H.; Lyke, K.E.; Chua, J.V. Immune-Mediated Pathogenesis in Dengue Virus Infection. Viruses 2022, 14, 2575. [Google Scholar] [CrossRef]
- Guzman, M.G.; Vazquez, S. The Complexity of Antibody-Dependent Enhancement of Dengue Virus Infection. Viruses 2010, 2, 2649–2662. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Sharma, V. Dengue and its effects on liver. World J. Clin. Cases 2015, 3, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Martina, B.E.E.; Koraka, P.; Osterhaus, A.D.M.E. Dengue Virus Pathogenesis: An Integrated View. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef] [PubMed]
- Limon-Flores, A.Y.; Perez-Tapia, M.; Estrada-Garcia, I.; Vaughan, G.; Escobar-Gutierrez, A.; Calderon-Amador, J.; Herrera-Rodriguez, S.E.; Brizuela-Garcia, A.; Heras-Chavarria, M.; Flores-Langarica, A.; et al. Dengue virus inoculation to human skin explants: An effective approach to assess in situ the early infection and the effects on cutaneous dendritic cells. Int. J. Exp. Pathol. 2005, 86, 323–334. [Google Scholar] [CrossRef]
- Wu, S.J.; Grouard-Vogel, G.; Sun, W.; Mascola, J.R.; Brachtel, E.; Putvatana, R.; Louder, M.K.; Filgueira, L.; Marovich, M.A.; Wong, H.K.; et al. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 2000, 6, 816–820. [Google Scholar] [CrossRef]
- Cerny, D.; Haniffa, M.; Shin, A.; Bigliardi, P.; Tan, B.K.; Lee, B.; Poidinger, M.; Tan, E.Y.; Ginhoux, F.; Fink, K. Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus Infection. PLoS Pathog. 2014, 10, e1004548. [Google Scholar] [CrossRef]
- Durbin, A.P.; Vargas, M.J.; Wanionek, K.; Hammond, S.N.; Gordon, A.; Rocha, C.; Balmaseda, A.; Harris, E. Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology 2008, 376, 429–435. [Google Scholar] [CrossRef]
- Boonnak, K.; Slike, B.M.; Burgess, T.H.; Mason, R.M.; Wu, S.-J.; Sun, P.; Porter, K.; Rudiman, I.F.; Yuwono, D.; Puthavathana, P.; et al. Role of Dendritic Cells in Antibody-Dependent Enhancement of Dengue Virus Infection. J. Virol. 2008, 82, 3939–3951. [Google Scholar] [CrossRef]
- Ho, L.-J.; Wang, J.-J.; Shaio, M.-F.; Kao, C.-L.; Chang, D.-M.; Han, S.-W.; Lai, J.-H. Infection of Human Dendritic Cells by Dengue Virus Causes Cell Maturation and Cytokine Production. J. Immunol. 2001, 166, 1499–1506. [Google Scholar] [CrossRef]
- Kwan, W.-H.; Helt, A.-M.; Marañón, C.N.; Barbaroux, J.-B.; Hosmalin, A.; Harris, E.; Fridman, W.H.; Mueller, C.G.F. Dendritic Cell Precursors Are Permissive to Dengue Virus and Human Immunodeficiency Virus Infection. J. Virol. 2005, 79, 7291–7299. [Google Scholar] [CrossRef]
- Libraty, D.H.; Pichyangkul, S.; Ajariyakhajorn, C.; Endy, T.P.; Ennis, F.A. Human Dendritic Cells Are Activated by Dengue Virus Infection: Enhancement by Gamma Interferon and Implications for Disease Pathogenesis. J. Virol. 2001, 75, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Blackley, S.; Kou, Z.; Chen, H.; Quinn, M.; Rose, R.C.; Schlesinger, J.J.; Coppage, M.; Jin, X. Primary Human Splenic Macrophages, but Not T or B Cells, Are the Principal Target Cells for Dengue Virus Infection In Vitro. J. Virol. 2007, 81, 13325–13334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huerre, M.R.; Lan, N.T.; Marianneau, P.; Hue, N.B.; Khun, H.; Hung, N.T.; Khen, N.T.; Drouet, M.T.; Huong, V.T.; Ha, D.Q.; et al. Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch. 2001, 438, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Jessie, K.; Fong, M.Y.; Devi, S.; Lam, S.K.; Wong, K.T. Localization of Dengue Virus in Naturally Infected Human Tissues, by Immunohistochemistry and In Situ Hybridization. J. Infect. Dis. 2004, 189, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Quinn, M.; Chen, H.; Rodrigo, W.W.S.I.; Rose, R.C.; Schlesinger, J.J.; Jin, X. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J. Med. Virol. 2008, 80, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Kyle, J.L.; Beatty, P.R.; Harris, E. Dengue Virus Infects Macrophages and Dendritic Cells in a Mouse Model of Infection. J. Infect. Dis. 2007, 195, 1808–1817. [Google Scholar] [CrossRef]
- Mota, J.; Rico-Hesse, R. Humanized Mice Show Clinical Signs of Dengue Fever according to Infecting Virus Genotype. J. Virol. 2009, 83, 8638–8645. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Huang, C.-S.; Lee, C.-N.; Chang, S.-Y.; King, C.-C.; Kao, C.-L. Higher Infection of Dengue Virus Serotype 2 in Human Monocytes of Patients with G6PD Deficiency. PLoS ONE 2008, 3, e1557. [Google Scholar] [CrossRef]
- Rothman, A.L.; Medin, C.L.; Friberg, H.; Currier, J.R. Immunopathogenesis Versus Protection in Dengue Virus Infections. Curr. Trop. Med. Rep. 2014, 1, 13–20. [Google Scholar] [CrossRef]
- Friberg, H.; Bashyam, H.; Toyosaki-Maeda, T.; Potts, J.A.; Greenough, T.; Kalayanarooj, S.; Gibbons, R.V.; Nisalak, A.; Srikiatkhachorn, A.; Green, S.; et al. Cross-Reactivity and Expansion of Dengue-Specific T cells During Acute Primary and Secondary Infections in Humans. Sci. Rep. 2011, 1, 51. [Google Scholar] [CrossRef]
- Mongkolsapaya, J.; Dejnirattisai, W.; Xu, X.N.; Vasanawathana, S.; Tangthawornchaikul, N.; Chairunsri, A.; Sawasdivorn, S.; Duangchinda, T.; Dong, T.; Rowland-Jones, S.; et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003, 9, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Dung, N.T.; Duyen, H.T.; Thuy, N.T.; Ngoc, T.V.; Chau, N.V.; Hien, T.T.; Rowland-Jones, S.L.; Dong, T.; Farrar, J.; Wills, B.; et al. Timing of CD8+ T cell responses in relation to commencement of capillary leakage in children with dengue. J. Immunol. 2010, 184, 7281–7287. [Google Scholar] [CrossRef] [PubMed]
- Anders, K.L.; Nguyet, N.M.; Chau, N.V.; Hung, N.T.; Thuy, T.T.; Lien Le, B.; Farrar, J.; Wills, B.; Hien, T.T.; Simmons, C.P. Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg. 2011, 84, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Khor, C.C.; Chau, T.N.; Pang, J.; Davila, S.; Long, H.T.; Ong, R.T.; Dunstan, S.J.; Wills, B.; Farrar, J.; Van Tram, T.; et al. Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat. Genet. 2011, 43, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Rico-Hesse, R.; Harrison, L.M.; Salas, R.A.; Tovar, D.; Nisalak, A.; Ramos, C.; Boshell, J.; de Mesa, M.T.; Nogueira, R.M.; da Rosa, A.T. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology 1997, 230, 244–251. [Google Scholar] [CrossRef]
- Marchette, N.J.; Halstead, S.B.; Nash, D.R.; Stenhouse, A.C. Recovery of Dengue Viruses from Tissues of Experimentally Infected Rhesus Monkeys. Appl. Microbiol. 1972, 24, 328–333. [Google Scholar] [CrossRef]
- Prestwood, T.R.; May, M.M.; Plummer, E.M.; Morar, M.M.; Yauch, L.E.; Shresta, S. Trafficking and Replication Patterns Reveal Splenic Macrophages as Major Targets of Dengue Virus in Mice. J. Virol. 2012, 86, 12138–12147. [Google Scholar] [CrossRef]
- Dhole, P.; Nakayama, E.E.; Saito, A.; Limkittikul, K.; Phanthanawiboon, S.; Shioda, T.; Kurosu, T. Sequence diversity of dengue virus type 2 in brain and thymus of infected interferon receptor ko mice: Implications for dengue virulence. Virol. J. 2016, 13, 199. [Google Scholar] [CrossRef][Green Version]
- Fink, K.; Ng, C.; Nkenfou, C.; Vasudevan, S.G.; Van Rooijen, N.; Schul, W. Depletion of macrophages in mice results in higher dengue virus titers and highlights the role of macrophages for virus control. Eur. J. Immunol. 2009, 39, 2809–2821. [Google Scholar] [CrossRef]
- Yam-Puc, J.C.; García-Cordero, J.; Calderón-Amador, J.; Donis-Maturano, L.; Cedillo-Barrón, L.; Flores-Romo, L. Germinal Center Reaction Following Cutaneous Dengue Virus Infection in Immune-Competent Mice. Front. Immunol. 2015, 6, 188. [Google Scholar] [CrossRef]
- Trung, D.T.; Thao Le, T.T.; Hien, T.T.; Hung, N.T.; Vinh, N.N.; Hien, P.T.; Chinh, N.T.; Simmons, C.; Wills, B. Liver involvement associated with dengue infection in adults in Vietnam. Am. J. Trop. Med. Hyg. 2010, 83, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Prestwood, T.R.; Shresta, S. Enhanced Infection of Liver Sinusoidal Endothelial Cells in a Mouse Model of Antibody-Induced Severe Dengue Disease. Cell Host Microbe 2010, 7, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.; Couvelard, A.; Degott, C.; Lagorce-Pagès, C.; Bruneel, F.; Bouvet, E.; Vachon, F. Dengue virus induced hepatitis with chronic calcific changes. Gut 2001, 49, 864–865. [Google Scholar] [CrossRef] [PubMed]
- Paes, M.V.; Pinhão, A.T.; Barreto, D.F.; Costa, S.M.; Oliveira, M.P.; Nogueira, A.C.; Takiya, C.M.; Farias-Filho, J.C.; Schatzmayr, H.G.; Alves, A.M.B.; et al. Liver injury and viremia in mice infected with dengue-2 virus. Virology 2005, 338, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Wahid, S.F.; Sanusi, S.; Zawawi, M.M.; Ali, R.A. A comparison of the pattern of liver involvement in dengue hemorrhagic fever with classic dengue fever. Southeast Asian J. Trop. Med. Public Health 2000, 31, 259–263. [Google Scholar] [PubMed]
- Póvoa, T.F.; Alves, A.M.B.; Oliveira, C.A.B.; Nuovo, G.J.; Chagas, V.L.A.; Paes, M.V. The Pathology of Severe Dengue in Multiple Organs of Human Fatal Cases: Histopathology, Ultrastructure and Virus Replication. PLoS ONE 2014, 9, e83386. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, S.; Imbulpitiya, I.; Abeysekera, R.; Waduge, R.; Rajapakse, R.; Weerakoon, K. Extensive haemorrhagic necrosis of liver is an unpredictable fatal complication in dengue infection: A postmortem study. BMC Infect. Dis. 2014, 14, 141. [Google Scholar] [CrossRef]
- Dalrymple, N.; Mackow, E.R. Productive Dengue Virus Infection of Human Endothelial Cells Is Directed by Heparan Sulfate-Containing Proteoglycan Receptors. J. Virol. 2011, 85, 9478–9485. [Google Scholar] [CrossRef]
- Balsitis, S.J.; Harris, E. Animal Models of Dengue Virus Infection and Disease: Applications, Insights, and Frontiers. In Frontiers in Dengue Virus Research; Hanley, K.A., Weaver, S.C., Eds.; Caister Academic Press: Wymondham, UK, 2010; pp. 103–115. [Google Scholar]
- Balsitis, S.J.; Williams, K.L.; Lachica, R.; Flores, D.; Kyle, J.L.; Mehlhop, E.; Johnson, S.; Diamond, M.S.; Beatty, P.R.; Harris, E. Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification. PLoS Pathog. 2010, 6, e1000790. [Google Scholar] [CrossRef]
- Arturo, C.-H.; Duncan, R.S. Mammalian Dengue Virus Receptors; Report No.: 0250-8362; WHO Regional Office for South-East Asia: New Delhi, India, 2005; pp. 119–135. [Google Scholar]
- Chen, H.-C.; Hofman, F.M.; Kung, J.T.; Lin, Y.-D.; Wu-Hsieh, B.A. Both Virus and Tumor Necrosis Factor Alpha Are Critical for Endothelium Damage in a Mouse Model of Dengue Virus-Induced Hemorrhage. J. Virol. 2007, 81, 5518–5526. [Google Scholar] [CrossRef]
- Diamond, M.S.; Edgil, D.; Roberts, T.G.; Lu, B.; Harris, E. Infection of Human Cells by Dengue Virus Is Modulated by Different Cell Types and Viral Strains. J. Virol. 2000, 74, 7814–7823. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Saito, M.; Mapua, C.A.; Natividad, F.F. Dengue illness: Clinical features and pathogenesis. J. Infect. Chemother. 2007, 13, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Boonpucknavig, S.; Boonpucknavig, V.; Bhamarapravati, N.; Nimmannitya, S. Immunofluorescence study of skin rash in patients with dengue hemorrhagic fever. Arch. Pathol. Lab. Med. 1979, 103, 463–466. [Google Scholar] [PubMed]
- Sahaphong, S.; Riengrojpitak, S.; Bhamarapravati, N.; Chirachariyavej, T. Electron microscopic study of the vascular endothelial cell in dengue hemorrhagic fever. Southeast Asian J. Trop. Med. Public Health 1980, 11, 194–204. [Google Scholar] [PubMed]
- Couvelard, A.; Marianneau, P.; Bedel, C.; Drouet, M.T.; Vachon, F.; Hénin, D.; Deubel, V. Report of a fatal case of dengue infection with hepatitis: Demonstration of dengue antigens in hepatocytes and liver apoptosis. Hum. Pathol. 1999, 30, 1106–1110. [Google Scholar] [CrossRef]
- Salgado, D.M.; Eltit, J.M.; Mansfield, K.; Panqueba, C.; Castro, D.; Vega, M.R.; Xhaja, K.; Schmidt, D.; Martin, K.J.; Allen, P.D.; et al. Heart and Skeletal Muscle Are Targets of Dengue Virus Infection. Pediatr. Infect. Dis. J. 2010, 29, 238–242. [Google Scholar] [CrossRef]
- Avirutnan, P.; Zhang, L.; Punyadee, N.; Manuyakorn, A.; Puttikhunt, C.; Kasinrerk, W.; Malasit, P.; Atkinson, J.P.; Diamond, M.S. Secreted NS1 of Dengue Virus Attaches to the Surface of Cells via Interactions with Heparan Sulfate and Chondroitin Sulfate E. PLoS Pathog. 2007, 3, e183. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef]
- Azizan, A.; Sweat, J.; Espino, C.; Gemmer, J.; Stark, L.; Kazanis, D. Differential proinflammatory and angiogenesis-specific cytokine production in human pulmonary endothelial cells, HPMEC-ST1.6R infected with dengue-2 and dengue-3 virus. J. Virol. Methods 2006, 138, 211–217. [Google Scholar] [CrossRef]
- Cruz-Oliveira, C.; Freire, J.M.; Conceição, T.M.; Higa, L.M.; Castanho, M.A.R.B.; Da Poian, A.T. Receptors and routes of dengue virus entry into the host cells. FEMS Microbiol. Rev. 2015, 39, 155–170. [Google Scholar] [CrossRef]
- Back, A.T.; Lundkvist, A. Dengue viruses—An overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar]
- Smit, J.M.; Moesker, B.; Rodenhuis-Zybert, I.; Wilschut, J. Flavivirus cell entry and membrane fusion. Viruses 2011, 3, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Sabeena, S.P.; Varma, M.; Arunkumar, G. Current Understanding of the Pathogenesis of Dengue Virus Infection. Curr. Microbiol. 2021, 78, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef] [PubMed]
- Avirutnan, P.; Punyadee, N.; Noisakran, S.; Komoltri, C.; Thiemmeca, S.; Auethavornanan, K.; Jairungsri, A.; Kanlaya, R.; Tangthawornchaikul, N.; Puttikhunt, C.; et al. Vascular Leakage in Severe Dengue Virus Infections: A Potential Role for the Nonstructural Viral Protein NS1 and Complement. J. Infect. Dis. 2006, 193, 1078–1088. [Google Scholar] [CrossRef]
- Nascimento, E.J.M.; Silva, A.M.; Cordeiro, M.T.; Brito, C.A.; Gil, L.H.V.G.; Braga-Neto, U.; Marques, E.T.A. Alternative Complement Pathway Deregulation Is Correlated with Dengue Severity. PLoS ONE 2009, 4, e6782. [Google Scholar] [CrossRef]
- Aguirre, S.; Luthra, P.; Sanchez-Aparicio, M.T.; Maestre, A.M.; Patel, J.; Lamothe, F.; Fredericks, A.C.; Tripathi, S.; Zhu, T.; Pintado-Silva, J.; et al. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat. Microbiol. 2017, 2, 17037. [Google Scholar] [CrossRef]
- Chan, Y.K.; Gack, M.U. Viral evasion of intracellular DNA and RNA sensing. Nat. Rev. Microbiol. 2016, 14, 360–373. [Google Scholar] [CrossRef]
- He, Z.; Zhu, X.; Wen, W.; Yuan, J.; Hu, Y.; Chen, J.; An, S.; Dong, X.; Lin, C.; Yu, J.; et al. Dengue Virus Subverts Host Innate Immunity by Targeting Adaptor Protein MAVS. J. Virol. 2016, 90, 7219–7230. [Google Scholar] [CrossRef]
- Shah, P.S.; Link, N.; Jang, G.M.; Sharp, P.P.; Zhu, T.; Swaney, D.L.; Johnson, J.R.; Von Dollen, J.; Ramage, H.R.; Satkamp, L.; et al. Comparative Flavivirus-Host Protein Interaction Mapping Reveals Mechanisms of Dengue and Zika Virus Pathogenesis. Cell 2018, 175, 1931–1945.e1918. [Google Scholar] [CrossRef]
- Garcia, M.; Wehbe, M.; Lévêque, N.; Bodet, C. Skin innate immune response to flaviviral infection. Eur. Cytokine Netw. 2017, 28, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Goubau, D.; Schlee, M.; Deddouche, S.; Pruijssers, A.J.; Zillinger, T.; Goldeck, M.; Schuberth, C.; Van der Veen, A.G.; Fujimura, T.; Rehwinkel, J.; et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 2014, 514, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef]
- Sun, P.; Kochel, T.J. The Battle Between Infection and Host Immune Responses of Dengue Virus and Its Implication in Dengue Disease Pathogenesis. Sci. World J. 2013, 2013, 843469. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Rivera, A.; Parker, D.; Durbin, J.E. Type III IFNs: Beyond antiviral protection. Semin. Immunol. 2019, 43, 101303. [Google Scholar] [CrossRef]
- Sadler, A.J.; Williams, B.R.G. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef]
- Bowie, A.G.; Unterholzner, L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat. Rev. Immunol. 2008, 8, 911–922. [Google Scholar] [CrossRef]
- Der, S.D.; Zhou, A.; Williams, B.R.G.; Silverman, R.H. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 1998, 95, 15623–15628. [Google Scholar] [CrossRef] [PubMed]
- Shuai, K.; Stark, G.R.; Kerr, I.M.; Darnell, J.E., Jr. A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science 1993, 261, 1744–1746. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; García-Sastre, A. STAT2 signaling and dengue virus infection. JAK-STAT 2014, 3, e27715. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Harris, E. Interferon inhibits dengue virus infection by preventing translation of viral RNA through a PKR-independent mechanism. Virology 2001, 289, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Kurane, I.; Innis, B.L.; Nimmannitya, S.; Nisalak, A.; Ennis, F.A.; Meager, A. High Levels of Interferon Alpha in the Sera of Children with Dengue Virus Infection. Am. J. Trop. Med. Hyg. 1993, 48, 222–229. [Google Scholar] [CrossRef]
- Sudiro, T.M.; Zivny, J.; Ishiko, H.; Green, S.; Vaughn, D.W.; Kalayanarooj, S.; Nisalak, A.; Norman, J.E.; Ennis, F.A.; Rothman, A.L. Analysis of plasma viral RNA levels during acute dengue virus infection using quantitative competitor reverse transcription-polymerase chain reaction. J. Med. Virol. 2001, 63, 29–34. [Google Scholar] [CrossRef]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue Viremia Titer, Antibody Response Pattern, and Virus Serotype Correlate with Disease Severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef]
- Muñoz-Jordán, J.L.; Sánchez-Burgos, G.G.; Laurent-Rolle, M.; García-Sastre, A. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 2003, 100, 14333–14338. [Google Scholar] [CrossRef]
- Jones, M.; Davidson, A.; Hibbert, L.; Gruenwald, P.; Schlaak, J.; Ball, S.; Foster, G.R.; Jacobs, M. Dengue Virus Inhibits Alpha Interferon Signaling by Reducing STAT2 Expression. J. Virol. 2005, 79, 5414–5420. [Google Scholar] [CrossRef]
- Ashour, J.; Laurent-Rolle, M.; Shi, P.-Y.; García-Sastre, A. NS5 of Dengue Virus Mediates STAT2 Binding and Degradation. J. Virol. 2009, 83, 5408–5418. [Google Scholar] [CrossRef]
- Burdette, D.L.; Vance, R.E. STING and the innate immune response to nucleic acids in the cytosol. Nat. Immunol. 2013, 14, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING Regulates Intracellular DNA-Mediated, Type I Interferon-Dependent Innate Immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Yang, Y.; Li, S.; Wang, Y.Y.; Li, Y.; Diao, F.; Lei, C.; He, X.; Zhang, L.; Tien, P.; et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 2008, 29, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Olagnier, D.; Lin, R. Host and Viral Modulation of RIG-I-Mediated Antiviral Immunity. Front. Immunol. 2016, 7, 662. [Google Scholar] [CrossRef]
- Li, J.; Lim, S.P.; Beer, D.; Patel, V.; Wen, D.; Tumanut, C.; Tully, D.C.; Williams, J.A.; Jiricek, J.; Priestle, J.P.; et al. Functional profiling of recombinant NS3 proteases from all four serotypes of dengue virus using tetrapeptide and octapeptide substrate libraries. J. Biol. Chem. 2005, 280, 28766–28774. [Google Scholar] [CrossRef]
- Stabell, A.C.; Meyerson, N.R.; Gullberg, R.C.; Gilchrist, A.R.; Webb, K.J.; Old, W.M.; Perera, R.; Sawyer, S.L. Dengue viruses cleave STING in humans but not in nonhuman primates, their presumed natural reservoir. eLife 2018, 7, e31919. [Google Scholar] [CrossRef]
- Ran, Y.; Shu, H.-B.; Wang, Y.-Y. MITA/STING: A central and multifaceted mediator in innate immune response. Cytokine Growth Factor Rev. 2014, 25, 631–639. [Google Scholar] [CrossRef]
- Abe, T.; Harashima, A.; Xia, T.; Konno, H.; Konno, K.; Morales, A.; Ahn, J.; Gutman, D.; Barber, G.N. STING recognition of cytoplasmic DNA instigates cellular defense. Mol. Cell 2013, 50, 5–15. [Google Scholar] [CrossRef]
- Seth, R.B.; Sun, L.; Ea, C.-K.; Chen, Z.J. Identification and Characterization of MAVS, a Mitochondrial Antiviral Signaling Protein that Activates NF-κB and IRF3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef]
- Zhou, J.; Zhuang, Z.; Li, J.; Feng, Z. Significance of the cGAS-STING Pathway in Health and Disease. Int. J. Mol. Sci. 2023, 24, 13316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Fernandez-Sesma, A. Innate Immune DNA Sensing of Flaviviruses. Viruses 2020, 12, 979. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; MacDuff, D.A.; Imanaka, N.; Gainey, M.D.; Shrestha, B.; Eitson, J.L.; Mar, K.B.; Richardson, R.B.; Ratushny, A.V.; Litvak, V.; et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 2014, 505, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.Y.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 2011, 472, 481–485. [Google Scholar] [CrossRef]
- Holm, C.K.; Jensen, S.B.; Jakobsen, M.R.; Cheshenko, N.; Horan, K.A.; Moeller, H.B.; Gonzalez-Dosal, R.; Rasmussen, S.B.; Christensen, M.H.; Yarovinsky, T.O.; et al. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat. Immunol. 2012, 13, 737–743. [Google Scholar] [CrossRef]
- Loo, Y.M.; Gale, M., Jr. Immune signaling by RIG-I-like receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54 Pt 1, 1–13. [Google Scholar] [CrossRef]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef]
- Verma, R.; Pandey, A.K.; Chakraborty, R.; Prakash, S.; Jain, A. Toll-Like receptor 3 genetic polymorphism in dengue encephalitis. J. Fam. Med. Prim. Care 2024, 13, 2397–2403. [Google Scholar] [CrossRef]
- Gill, N.; Deacon, P.M.; Lichty, B.; Mossman, K.L.; Ashkar, A.A. Induction of innate immunity against herpes simplex virus type 2 infection via local delivery of Toll-like receptor ligands correlates with beta interferon production. J. Virol. 2006, 80, 9943–9950. [Google Scholar] [CrossRef]
- Wong, J.P.; Christopher, M.E.; Viswanathan, S.; Dai, X.; Salazar, A.M.; Sun, L.Q.; Wang, M. Antiviral role of toll-like receptor-3 agonists against seasonal and avian influenza viruses. Curr. Pharm. Des. 2009, 15, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Becker, Y. A point of view: HIV-1/AIDS is an allergy but CpG ODN treatments may inhibit virus replication and reactivate the adaptive immunity—Hypothesis and implications. Virus Genes. 2005, 30, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. Recent Insights into the Molecular Mechanism of Toll-Like Receptor Response to Dengue Virus Infection. Front. Microbiol. 2021, 12, 744233. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Santos, C.; Azeredo, E.L.D. Innate Immune Response to Dengue Virus: Toll-like Receptors and Antiviral Response. Viruses 2022, 14, 992. [Google Scholar] [CrossRef]
- Lobigs, M.; Müllbacher, A.; Lee, E. Evidence that a mechanism for efficient flavivirus budding upregulates MHC class I. Immunol. Cell Biol. 2004, 82, 184–188. [Google Scholar] [CrossRef]
- Hershkovitz, O.; Zilka, A.; Bar-Ilan, A.; Abutbul, S.; Davidson, A.; Mazzon, M.; Kümmerer, B.M.; Monsoengo, A.; Jacobs, M.; Porgador, A. Dengue Virus Replicon Expressing the Nonstructural Proteins Suffices to Enhance Membrane Expression of HLA Class I and Inhibit Lysis by Human NK Cells. J. Virol. 2008, 82, 7666–7676. [Google Scholar] [CrossRef]
- King, C.A.; Marshall, J.S.; Alshurafa, H.; Anderson, R. Release of vasoactive cytokines by antibody-enhanced dengue virus infection of a human mast cell/basophil line. J. Virol. 2000, 74, 7146–7150. [Google Scholar] [CrossRef]
- St. John, A.L.; Rathore, A.P.S.; Yap, H.; Ng, M.-L.; Metcalfe, D.D.; Vasudevan, S.G.; Abraham, S.N. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc. Natl. Acad. Sci. USA 2011, 108, 9190–9195. [Google Scholar] [CrossRef]
- Avirutnan, P.; Matangkasombut, P. Unmasking the role of mast cells in dengue. eLife 2013, 2, e00767. [Google Scholar] [CrossRef]
- Avirutnan, P.; Hauhart, R.E.; Marovich, M.A.; Garred, P.; Atkinson, J.P.; Diamond, M.S. Complement-Mediated Neutralization of Dengue Virus Requires Mannose-Binding Lectin. mBio 2011, 2, e00276-11-e. [Google Scholar] [CrossRef]
- Pokidysheva, E.; Zhang, Y.; Battisti, A.J.; Bator-Kelly, C.M.; Chipman, P.R.; Xiao, C.; Gregorio, G.G.; Hendrickson, W.A.; Kuhn, R.J.; Rossmann, M.G. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell 2006, 124, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Thiel, S.; Vorup-Jensen, T.; Stover, C.M.; Schwaeble, W.; Laursen, S.B.; Poulsen, K.; Willis, A.C.; Eggleton, P.; Hansen, S.; Holmskov, U.; et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature 1997, 386, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Matsushita, M.; Endo, Y. The lectin-complement pathway—Its role in innate immunity and evolution. Immunol. Rev. 2004, 198, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Libraty, D.H.; Young, P.R.; Pickering, D.; Endy, T.P.; Kalayanarooj, S.; Green, S.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A.L. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 2002, 186, 1165–1168. [Google Scholar] [CrossRef]
- Young, P.R.; Hilditch, P.A.; Bletchly, C.; Halloran, W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 2000, 38, 1053–1057. [Google Scholar] [CrossRef]
- Alcon, S.; Talarmin, A.; Debruyne, M.; Falconar, A.; Deubel, V.; Flamand, M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 2002, 40, 376–381. [Google Scholar] [CrossRef]
- Avirutnan, P.; Fuchs, A.; Hauhart, R.E.; Somnuke, P.; Youn, S.; Diamond, M.S.; Atkinson, J.P. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J. Exp. Med. 2010, 207, 793–806. [Google Scholar] [CrossRef]
- Avirutnan, P.; Hauhart, R.E.; Somnuke, P.; Blom, A.M.; Diamond, M.S.; Atkinson, J.P. Binding of Flavivirus Nonstructural Protein NS1 to C4b Binding Protein Modulates Complement Activation. J. Immunol. 2011, 187, 424–433. [Google Scholar] [CrossRef]
- Kurosu, T.; Chaichana, P.; Yamate, M.; Anantapreecha, S.; Ikuta, K. Secreted complement regulatory protein clusterin interacts with dengue virus nonstructural protein 1. Biochem. Biophys. Res. Commun. 2007, 362, 1051–1056. [Google Scholar] [CrossRef]
- Brandt, W.E.; Chiewslip, D.; Harris, D.L.; Russell, P.K. Partial purification and characterization of a dengue virus soluble complement-fixing antigen. J. Immunol. 1970, 105, 1565–1568. [Google Scholar] [CrossRef]
- Schlesinger, J.J.; Brandriss, M.W.; Walsh, E.E. Protection of mice against dengue 2 virus encephalitis by immunization with the dengue 2 virus non-structural glycoprotein NS1. J. Gen. Virol. 1987, 68 Pt 3, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Henchal, E.A.; Henchal, L.S.; Schlesinger, J.J. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J. Gen. Virol. 1988, 69 Pt 8, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Falgout, B.; Bray, M.; Schlesinger, J.J.; Lai, C.J. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J. Virol. 1990, 64, 4356–4363. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Chen, W.; Maguire, T.; Austin, F. Immunoreactivity and protective effects in mice of a recombinant dengue 2 Tonga virus NS1 protein produced in a baculovirus expression system. J. Gen. Virol. 1993, 74 Pt 1, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bossi, F.; Fischetti, F.; Pellis, V.; Bulla, R.; Ferrero, E.; Mollnes, T.E.; Regoli, D.; Tedesco, F. Platelet-activating factor and kinin-dependent vascular leakage as a novel functional activity of the soluble terminal complement complex. J. Immunol. 2004, 173, 6921–6927. [Google Scholar] [CrossRef] [PubMed]
- Jeang, K.-T. RNAi in the regulation of mammalian viral infections. BMC Biol. 2012, 10, 58. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Sánchez-Vargas, I.; Scott, J.C.; Poole-Smith, B.K.; Franz, A.W.E.; Barbosa-Solomieu, V.; Wilusz, J.; Olson, K.E.; Blair, C.D. Dengue Virus Type 2 Infections of Aedes aegypti Are Modulated by the Mosquito’s RNA Interference Pathway. PLoS Pathog. 2009, 5, e1000299. [Google Scholar] [CrossRef]
- Kakumani, P.K.; Ponia, S.S.; Shanmugam, R.K.; Sood, V.; Chinnappan, M.; Banerjea, A.C.; Medigeshi, G.R.; Malhotra, P.; Mukherjee, S.K.; Bhatnagar, R.K. Role of RNA Interference (RNAi) in Dengue Virus Replication and Identification of NS4B as an RNAi Suppressor. J. Virol. 2013, 87, 8870–8883. [Google Scholar] [CrossRef]
- Su, Y.C.; Huang, Y.F.; Wu, Y.W.; Chen, H.F.; Wu, Y.H.; Hsu, C.C.; Hsu, Y.C.; Lee, J.C. MicroRNA-155 inhibits dengue virus replication by inducing heme oxygenase-1-mediated antiviral interferon responses. FASEB J. 2020, 34, 7283–7294. [Google Scholar] [CrossRef]
- Smith, J.L.; Jeng, S.; McWeeney, S.K.; Hirsch, A.J. A MicroRNA Screen Identifies the Wnt Signaling Pathway as a Regulator of the Interferon Response during Flavivirus Infection. J. Virol. 2017, 91, e02388-16. [Google Scholar] [CrossRef] [PubMed]
- Murphy Schafer, A.R.; Smith, J.L.; Pryke, K.M.; DeFilippis, V.R.; Hirsch, A.J. The E3 Ubiquitin Ligase SIAH1 Targets MyD88 for Proteasomal Degradation During Dengue Virus Infection. Front. Microbiol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.K.; Lin, C.K.; Wu, Y.H.; Chen, Y.H.; Chen, W.C.; Young, K.C.; Lee, J.C. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci. Rep. 2016, 6, 32176. [Google Scholar] [CrossRef] [PubMed]
- Kuma, A.; Hatano, M.; Matsui, M.; Yamamoto, A.; Nakaya, H.; Yoshimori, T.; Ohsumi, Y.; Tokuhisa, T.; Mizushima, N. The role of autophagy during the early neonatal starvation period. Nature 2004, 432, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef]
- Panyasrivanit, M.; Greenwood, M.P.; Murphy, D.; Isidoro, C.; Auewarakul, P.; Smith, D.R. Induced autophagy reduces virus output in dengue infected monocytic cells. Virology 2011, 418, 74–84. [Google Scholar] [CrossRef]
- Lee, Y.R.; Lei, H.Y.; Liu, M.T.; Wang, J.R.; Chen, S.H.; Jiang-Shieh, Y.F.; Lin, Y.S.; Yeh, T.M.; Liu, C.C.; Liu, H.S. Autophagic machinery activated by dengue virus enhances virus replication. Virology 2008, 374, 240–248. [Google Scholar] [CrossRef]
- Mateo, R.; Nagamine, C.M.; Spagnolo, J.; Méndez, E.; Rahe, M.; Gale, M.; Yuan, J.; Kirkegaard, K. Inhibition of Cellular Autophagy Deranges Dengue Virion Maturation. J. Virol. 2013, 87, 1312–1321. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Mettling, C.; Wu, S.-R.; Yu, C.-Y.; Perng, G.-C.; Lin, Y.-S.; Lin, Y.-L. Autophagy-associated dengue vesicles promote viral transmission avoiding antibody neutralization. Sci. Rep. 2016, 6, 32243. [Google Scholar] [CrossRef]
- Lennemann, N.J.; Coyne, C.B. Dengue and Zika viruses subvert reticulophagy by NS2B3-mediated cleavage of FAM134B. Autophagy 2017, 13, 322–332. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Netsawang, J.; Noisakran, S.; Puttikhunt, C.; Kasinrerk, W.; Wongwiwat, W.; Malasit, P.; Yenchitsomanus, P.T.; Limjindaporn, T. Nuclear localization of dengue virus capsid protein is required for DAXX interaction and apoptosis. Virus Res. 2010, 147, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Shafee, N.; AbuBakar, S. Dengue virus type 2 NS3 protease and NS2B-NS3 protease precursor induce apoptosis. J. Gen. Virol. 2003, 84 Pt 8, 2191–2195. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M.P.; Chambers, J.A.; Pankhong, P.; Chattergoon, M.; Attatippaholkun, W.; Dang, K.; Shah, N.; Weiner, D.B. Host cell killing by the West Nile Virus NS2B-NS3 proteolytic complex: NS3 alone is sufficient to recruit caspase-8-based apoptotic pathway. Virology 2006, 345, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-C.; Lin, S.-C.; Chen, W.-Y.; Yen, Y.-T.; Lai, C.-W.; Tao, M.-H.; Lin, Y.-L.; Miaw, S.-C.; Wu-Hsieh, B.A. Dengue Viral Protease Interaction with NF-κB Inhibitor α/β Results in Endothelial Cell Apoptosis and Hemorrhage Development. J. Immunol. 2014, 193, 1258–1267. [Google Scholar] [CrossRef]
- Jan, J.-T.; Chen, B.-H.; Ma, S.-H.; Liu, C.-I.; Tsai, H.-P.; Wu, H.-C.; Jiang, S.-Y.; Yang, K.-D.; Shaio, M.-F. Potential Dengue Virus-Triggered Apoptotic Pathway in Human Neuroblastoma Cells: Arachidonic Acid, Superoxide Anion, and NF-κB Are Sequentially Involved. J. Virol. 2000, 74, 8680–8691. [Google Scholar] [CrossRef]
- Limonta, D.; Capó, V.; Torres, G.; Pérez, A.B.; Guzmán, M.G. Apoptosis in tissues from fatal dengue shock syndrome. J. Clin. Virol. 2007, 40, 50–54. [Google Scholar] [CrossRef]
- Vatti, A.; Monsalve, D.M.; Pacheco, Y.; Chang, C.; Anaya, J.-M.; Gershwin, M.E. Original antigenic sin: A comprehensive review. J. Autoimmun. 2017, 83, 12–21. [Google Scholar] [CrossRef]
- Li, X.; Kantola, K.; Hedman, L.; Arku, B.; Hedman, K.; Söderlund-Venermo, M. Original antigenic sin with human bocaviruses 1-4. J. Gen. Virol. 2015, 96, 3099–3108. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.-M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Furukawa, M.; Matsui, M.; Katsuki, K.; Inouye, S. ‘Original antigenic sin’ phenomenon in neutralizing antibody responses in children with enterovirus meningitis. J. Clin. Virol. 2000, 19, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Elmastour, F.; Jaidane, H.; Aguech-Oueslati, L.; Benkahla, M.A.; Aouni, M.; Gharbi, J.; Sane, F.; Hober, D. Immunoglobulin G-dependent enhancement of the infection with Coxsackievirus B4 in a murine system. Virulence 2016, 7, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Bopegamage, S. Enterovirus infections: Pivoting role of the adaptive immune response. Virulence 2016, 7, 495–497. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoon, I.K.; Srikiatkhachorn, A.; Hermann, L.; Buddhari, D.; Scott, T.W.; Jarman, R.G.; Aldstadt, J.; Nisalak, A.; Thammapalo, S.; Bhoomiboonchoo, P.; et al. Characteristics of mild dengue virus infection in Thai children. Am. J. Trop. Med. Hyg. 2013, 89, 1081–1087. [Google Scholar] [CrossRef]
- Ripoll, D.R.; Wallqvist, A.; Chaudhury, S. Molecular Simulations Reveal the Role of Antibody Fine Specificity and Viral Maturation State on Antibody-Dependent Enhancement of Infection in Dengue Virus. Front. Cell. Infect. Microbiol. 2019, 9, 200. [Google Scholar] [CrossRef]
- Wahala, W.M.P.B.; Huang, C.; Butrapet, S.; White, L.J.; De Silva, A.M. Recombinant Dengue Type 2 Viruses with Altered E Protein Domain III Epitopes Are Efficiently Neutralized by Human Immune Sera. J. Virol. 2012, 86, 4019–4023. [Google Scholar] [CrossRef]
- Halstead, S.B.; O’Rourke, E.J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 1977, 146, 201–217. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef]
- Muhammad Azami, N.A.; Takasaki, T.; Kurane, I.; Moi, M.L. Non-Human Primate Models of Dengue Virus Infection: A Comparison of Viremia Levels and Antibody Responses During Primary and Secondary Infection among Old World and New World Monkeys. Pathogens 2020, 9, 247. [Google Scholar] [CrossRef]
- Watanabe, S.; Chan, K.W.; Wang, J.; Rivino, L.; Lok, S.M.; Vasudevan, S.G. Dengue Virus Infection with Highly Neutralizing Levels of Cross-Reactive Antibodies Causes Acute Lethal Small Intestinal Pathology Without a High Level of Viremia in Mice. J. Virol. 2015, 89, 5847–5861. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Salje, H.; Cummings, D.A.T.; Rodriguez-Barraquer, I.; Katzelnick, L.C.; Lessler, J.; Klungthong, C.; Thaisomboonsuk, B.; Nisalak, A.; Weg, A.; Ellison, D.; et al. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature 2018, 557, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Ngono, A.E.; Shresta, S. Immune Response to Dengue and Zika. Annu. Rev. Immunol. 2018, 36, 279–308. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.F.; Voon, G.Z.; Lim, H.X.; Chua, M.L.; Poh, C.L. Innate and adaptive immune evasion by dengue virus. Front. Cell. Infect. Microbiol. 2022, 12, 1004608. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; Jeewandara, C.; Ogg, G.S. Dysfunctional Innate Immune Responses and Severe Dengue. Front. Cell. Infect. Microbiol. 2020, 10, 590004. [Google Scholar] [CrossRef]
- Beltramello, M.; Williams, K.L.; Simmons, C.P.; Macagno, A.; Simonelli, L.; Quyen, N.T.; Sukupolvi-Petty, S.; Navarro-Sanchez, E.; Young, P.R.; de Silva, A.M.; et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 2010, 8, 271–283. [Google Scholar] [CrossRef]
- Slon Campos, J.L.; Mongkolsapaya, J.; Screaton, G.R. The immune response against flaviviruses. Nat. Immunol. 2018, 19, 1189–1198. [Google Scholar] [CrossRef]
- Cockburn, J.J.; Navarro Sanchez, M.E.; Fretes, N.; Urvoas, A.; Staropoli, I.; Kikuti, C.M.; Coffey, L.L.; Arenzana Seisdedos, F.; Bedouelle, H.; Rey, F.A. Mechanism of dengue virus broad cross-neutralization by a monoclonal antibody. Structure 2012, 20, 303–314. [Google Scholar] [CrossRef]
- Pierson, T.C.; Kuhn, R.J. Capturing a Virus while It Catches Its Breath. Structure 2012, 20, 200–202. [Google Scholar] [CrossRef][Green Version]
- Fibriansah, G.; Ng, T.S.; Kostyuchenko, V.A.; Lee, J.; Lee, S.; Wang, J.; Lok, S.M. Structural changes in dengue virus when exposed to a temperature of 37 °C. J. Virol. 2013, 87, 7585–7592. [Google Scholar] [CrossRef] [PubMed]
- van der Schaar, H.M.; Rust, M.J.; Waarts, B.L.; van der Ende-Metselaar, H.; Kuhn, R.J.; Wilschut, J.; Zhuang, X.; Smit, J.M. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J. Virol. 2007, 81, 12019–12028. [Google Scholar] [CrossRef] [PubMed]
- Zybert, I.A.; van der Ende-Metselaar, H.; Wilschut, J.; Smit, J.M. Functional importance of dengue virus maturation: Infectious properties of immature virions. J. Gen. Virol. 2008, 89 Pt 12, 3047–3051. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B.; Mahalingam, S.; Marovich, M.A.; Ubol, S.; Mosser, D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: Disease regulation by immune complexes. Lancet Infect. Dis. 2010, 10, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Rodenhuis-Zybert, I.A.; Van Der Schaar, H.M.; Da Silva Voorham, J.M.; Van Der Ende-Metselaar, H.; Lei, H.-Y.; Wilschut, J.; Smit, J.M. Immature Dengue Virus: A Veiled Pathogen? PLoS Pathog. 2010, 6, e1000718. [Google Scholar] [CrossRef]
- Smith, S.A.; Zhou, Y.; Olivarez, N.P.; Broadwater, A.H.; de Silva, A.M.; Crowe, J.E., Jr. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J. Virol. 2012, 86, 2665–2675. [Google Scholar] [CrossRef]
- Parameswaran, P.; Charlebois, P.; Tellez, Y.; Nunez, A.; Ryan, E.M.; Malboeuf, C.M.; Levin, J.Z.; Lennon, N.J.; Balmaseda, A.; Harris, E.; et al. Genome-wide patterns of intrahuman dengue virus diversity reveal associations with viral phylogenetic clade and interhost diversity. J. Virol. 2012, 86, 8546–8558. [Google Scholar] [CrossRef]
- Parameswaran, P.; Wang, C.; Trivedi, S.B.; Eswarappa, M.; Montoya, M.; Balmaseda, A.; Harris, E. Intrahost Selection Pressures Drive Rapid Dengue Virus Microevolution in Acute Human Infections. Cell Host Microbe 2017, 22, 400–410.e405. [Google Scholar] [CrossRef]
- Beasley, D.W.C.; Barrett, A.D.T. Identification of Neutralizing Epitopes within Structural Domain III of the West Nile Virus Envelope Protein. J. Virol. 2002, 76, 13097–13100. [Google Scholar] [CrossRef]
- Jennings, A.D.; Gibson, C.A.; Miller, B.R.; Mathews, J.H.; Mitchell, C.J.; Roehrig, J.T.; Wood, D.J.; Taffs, F.; Sil, B.K.; Whitby, S.N.; et al. Analysis of a yellow fever virus isolated from a fatal case of vaccine-associated human encephalitis. J. Infect. Dis. 1994, 169, 512–518. [Google Scholar] [CrossRef]
- Lin, B.; Parrish, C.R.; Murray, J.M.; Wright, P.J. Localization of a neutralizing epitope on the envelope protein of dengue virus type 2. Virology 1994, 202, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Lok, S.M.; Ng, M.L.; Aaskov, J. Amino acid and phenotypic changes in dengue 2 virus associated with escape from neutralisation by IgM antibody. J. Med. Virol. 2001, 65, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.K.; Selvanesan, S.; Sivalingam, B.; Chem, Y.K.; Norizah, I.; Zuridah, H.; Kumarasamy, V.; Chua, K.B. Isolation of monoclonal antibodies-escape variant of dengue virus serotype 1. Singapore Med. J. 2006, 47, 940–946. [Google Scholar] [PubMed]
- Weiskopf, D.; Angelo, M.A.; De Azeredo, E.L.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; De Silva, A.D.; De Silva, A.M.; et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2053. [Google Scholar] [CrossRef] [PubMed]
- Klenerman, P.; Zinkernagel, R.M. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature 1998, 394, 482–485. [Google Scholar] [CrossRef]
- Kurane, I.; Dai, L.C.; Livingston, P.G.; Reed, E.; Ennis, F.A. Definition of an HLA-DPw2-restricted epitope on NS3, recognized by a dengue virus serotype-cross-reactive human CD4+ CD8− cytotoxic T-cell clone. J. Virol. 1993, 67, 6285–6288. [Google Scholar] [CrossRef]
- Kurane, I.; Okamoto, Y.; Dai, L.C.; Zeng, L.L.; Brinton, M.A.; Ennis, F.A. Flavivirus-cross-reactive, HLA-DR15-restricted epitope on NS3 recognized by human CD4+ CD8− cytotoxic T lymphocyte clones. J. Gen. Virol. 1995, 76 Pt 9, 2243–2249. [Google Scholar] [CrossRef]
- Livingston, P.G.; Kurane, I.; Dai, L.C.; Okamoto, Y.; Lai, C.J.; Men, R.; Karaki, S.; Takiguchi, M.; Ennis, F.A. Dengue virus-specific, HLA-B35-restricted, human CD8+ cytotoxic T lymphocyte (CTL) clones. Recognition of NS3 amino acids 500 to 508 by CTL clones of two different serotype specificities. J. Immunol. 1995, 154, 1287–1295. [Google Scholar] [CrossRef]
- Mathew, A.; Kurane, I.; Green, S.; Stephens, H.A.F.; Vaughn, D.W.; Kalayanarooj, S.; Suntayakorn, S.; Chandanayingyong, D.; Ennis, F.A.; Rothman, A.L. Predominance of HLA-Restricted Cytotoxic T-Lymphocyte Responses to Serotype-Cross-Reactive Epitopes on Nonstructural Proteins following Natural Secondary Dengue Virus Infection. J. Virol. 1998, 72, 3999–4004. [Google Scholar] [CrossRef]
- Zivny, J.; Kurane, I.; Leporati, A.M.; Ibe, M.; Takiguchi, M.; Zeng, L.L.; Brinton, M.A.; Ennis, F.A. A single nine-amino acid peptide induces virus-specific, CD8+ human cytotoxic T lymphocyte clones of heterogeneous serotype specificities. J. Exp. Med. 1995, 182, 853–863. [Google Scholar] [CrossRef]
- Mathew, A.; Townsley, E.; Ennis, F.A. Elucidating the role of T cells in protection against and pathogenesis of dengue virus infections. Future Microbiol. 2014, 9, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Kurane, I.; Rothman, A.L.; Zeng, L.L.; Brinton, M.A.; Ennis, F.A. Dominant recognition by human CD8+ cytotoxic T lymphocytes of dengue virus nonstructural proteins NS3 and NS1.2a. J. Clin. Investig. 1996, 98, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Kurane, I.; Brinton, M.A.; Samson, A.L.; Ennis, F.A. Dengue virus-specific, human CD4+ CD8− cytotoxic T-cell clones: Multiple patterns of virus cross-reactivity recognized by NS3-specific T-cell clones. J. Virol. 1991, 65, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Kurane, I.; Meager, A.; Ennis, F.A. Dengue virus-specific human T cell clones. Serotype crossreactive proliferation, interferon gamma production, and cytotoxic activity. J. Exp. Med. 1989, 170, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Kurane, I.; Zeng, L.; Brinton, M.A.; Ennis, F.A. Definition of an Epitope on NS3 Recognized by Human CD4+ Cytotoxic T Lymphocyte Clones Cross-Reactive for Dengue Virus Types 2, 3, and 4. Virology 1998, 240, 169–174. [Google Scholar] [CrossRef]
- Rivino, L.; Kumaran, E.A.P.; Jovanovic, V.; Nadua, K.; Teo, E.W.; Pang, S.W.; Teo, G.H.; Gan, V.C.H.; Lye, D.C.; Leo, Y.S.; et al. Differential Targeting of Viral Components by CD4+ versus CD8+ T Lymphocytes in Dengue Virus Infection. J. Virol. 2013, 87, 2693–2706. [Google Scholar] [CrossRef]
- Lindow, J.C.; Borochoff-Porte, N.; Durbin, A.P.; Whitehead, S.S.; Fimlaid, K.A.; Bunn, J.Y.; Kirkpatrick, B.D. Primary Vaccination with Low Dose Live Dengue 1 Virus Generates a Proinflammatory, Multifunctional T Cell Response in Humans. PLoS Negl. Trop. Dis. 2012, 6, e1742. [Google Scholar] [CrossRef][Green Version]
- Guy, B.; Nougarede, N.; Begue, S.; Sanchez, V.; Souag, N.; Carre, M.; Chambonneau, L.; Morrisson, D.N.; Shaw, D.; Qiao, M.; et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008, 26, 5712–5721. [Google Scholar] [CrossRef]
- Tricou, V.; Yu, D.; Reynales, H.; Biswal, S.; Saez-Llorens, X.; Sirivichayakul, C.; Lopez, P.; Borja-Tabora, C.; Bravo, L.; Kosalaraksa, P.; et al. Long-term efficacy and safety of a tetravalent dengue vaccine (TAK-003): 4·5-year results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2024, 12, e257–e270. [Google Scholar] [CrossRef]
- Rothman, A.L.; Kurane, I.; Lai, C.J.; Bray, M.; Falgout, B.; Men, R.; Ennis, F.A. Dengue virus protein recognition by virus-specific murine CD8+ cytotoxic T lymphocytes. J. Virol. 1993, 67, 801–806. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; Grifoni, A.; O’Rourke, P.H.; Sidney, J.; Paul, S.; De Silva, A.D.; Phillips, E.; Mallal, S.; Premawansa, S.; et al. HLA-DRB1 Alleles Are Associated with Different Magnitudes of Dengue Virus–Specific CD4+ T-Cell Responses. J. Infect. Dis. 2016, 214, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
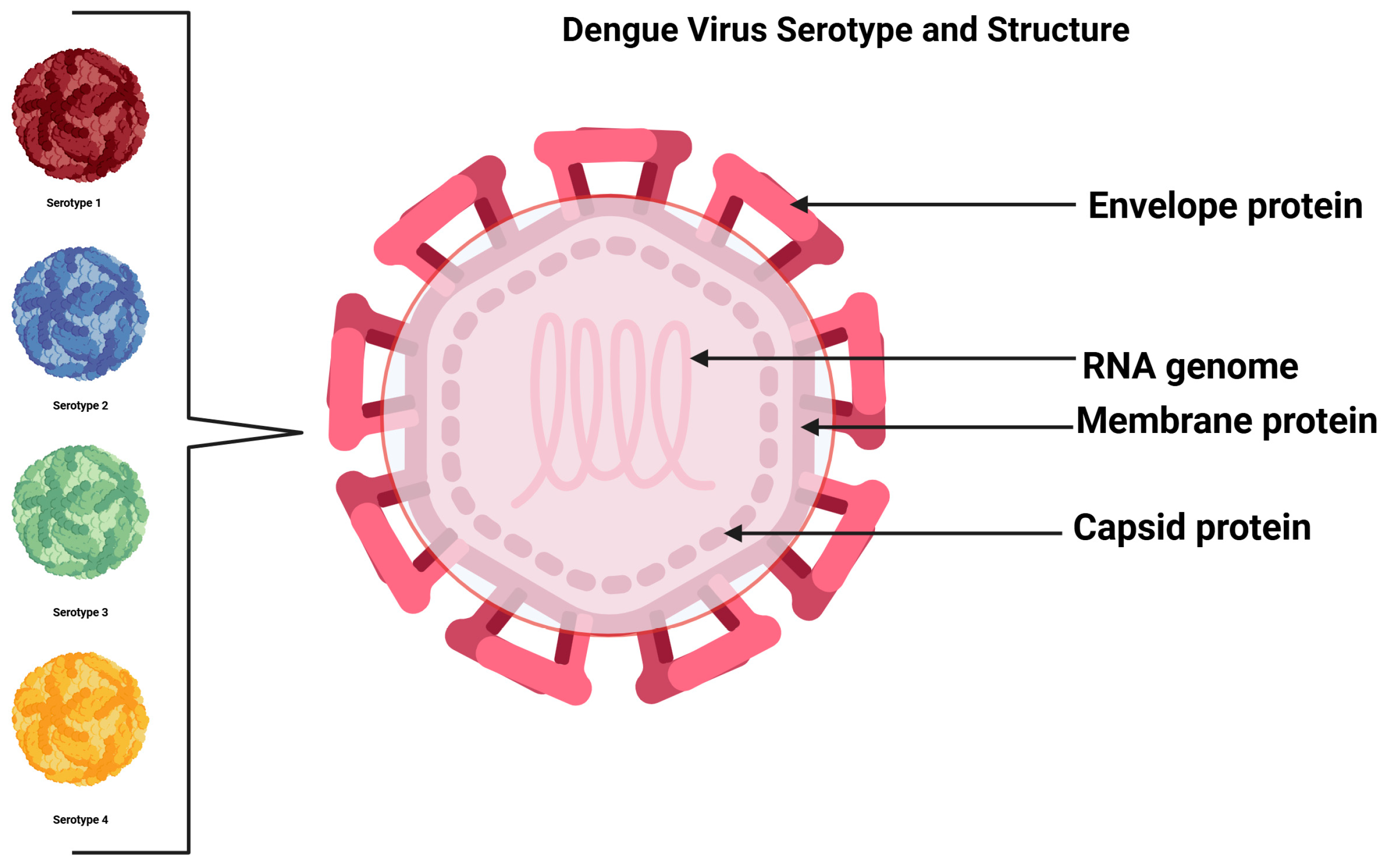

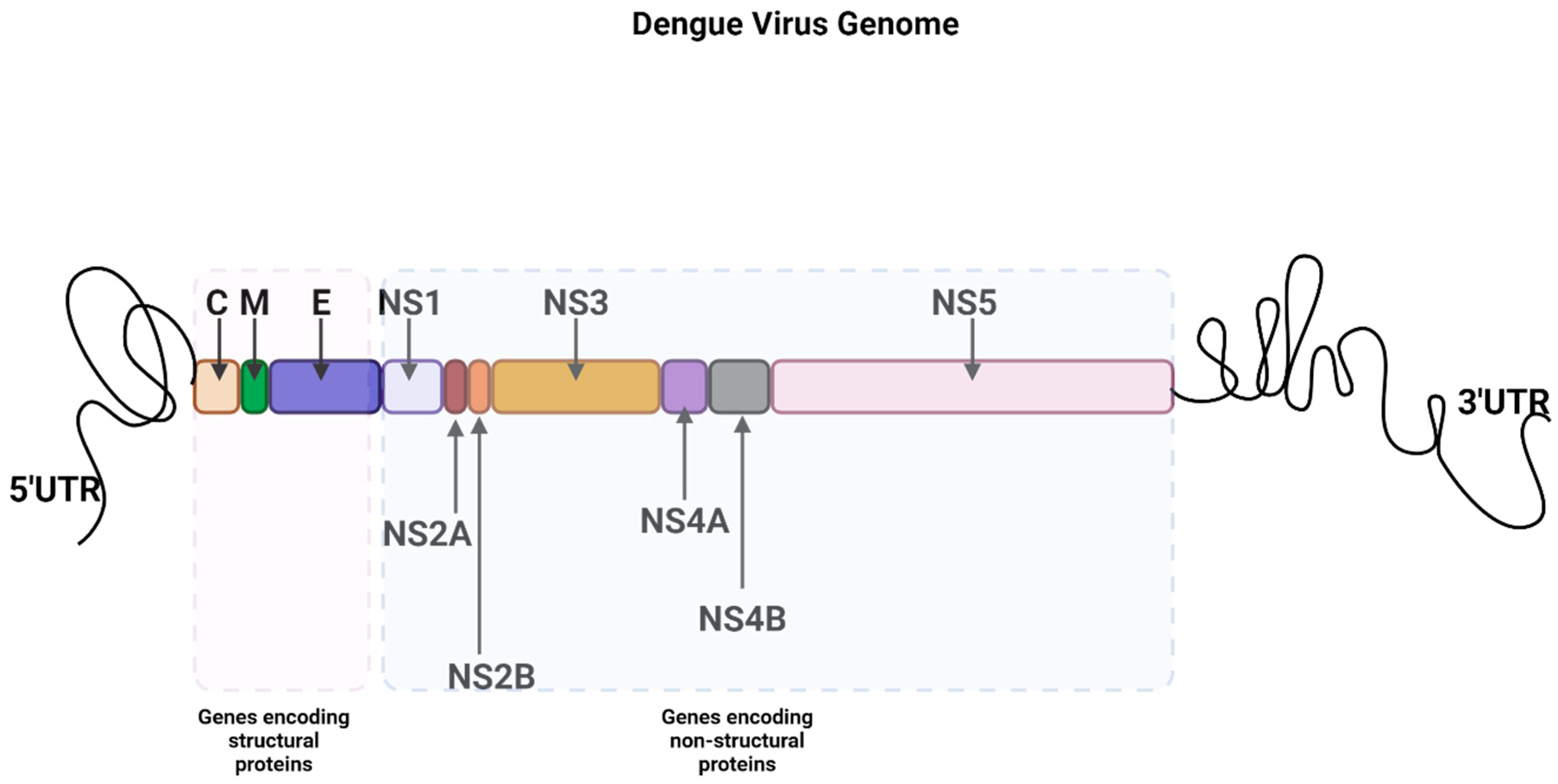

| Proteins | Functions | References | |
|---|---|---|---|
| Structural | E | DENV receptor binding and fusion site | [43] |
| consists of three domains (I, II, III) | host range, tropism, virulence | ||
| prM | forms protruding trimers with E giving a spiky appearance | [44] | |
| part of immature virions | form a cap that prevents the premature fusion of E before virus release | ||
| M | arrangement/maturation | [43] | |
| sits below the E protein | [44] | ||
| C | Nucleocapsid formation | [45] | |
| Homo-dimeric protein | encapsidation of virus | ||
| Non-Structural | NS1 | viral replication | [46] |
| dimer in the initial stage and hexamer in the latter stage | interacts with NS4A, and NS4B to form a virus replication complex (RC) | [46,47] | |
| occurs in many different forms (membrane-bound, soluble form, NS1′) | interacts with E and prM to facilitate the production of infectious virions | [48] | |
| [49] | |||
| [50] | |||
| NS2A | RNA synthesis | [51] | |
| transmembrane protein | RNA packaging/virion assembly | [49] | |
| [50] | |||
| NS2B | interacts with NS3 to make it functional | [52] | |
| membrane-associated protein | a cofactor in the structural activation of the DENV serine protease of NS3 | [53] | |
| [53,54] | |||
| NS2B3 protease complex | cleaves hSTING | [55] | |
| NS3 | polyprotein and host protein cleavage at specific site | [52] | |
| bifunctional with protease activity at the N terminus while helicase activity at the C terminus | unwinding the RNA duplex during replication | [56] | |
| NS4A | membrane alterations necessary for viral replication | [53] | |
| integral membrane protein | [54] | ||
| NS4B | interacts with NS3 helicase domain | [56] | |
| integral membrane protein | [53] | ||
| [54] | |||
| NS5 | 5′ capping of new viral genomes | [57] | |
| bifunctional with the N terminus as methyl transferase and C terminus as RNA-dependent RNA polymerase | [54] |
| Functions | References | |
|---|---|---|
| Proteins | ||
| NS1 | vascular hyperpermeability | [125,132,133] |
| Interaction with TLR4 | suppression of the complement pathways | [134] |
| NS2A | IFN antagonist | [53] |
| [54] | ||
| NS2B | inhibits type I IFN production by targeting cyclic GMP-AMP synthase (cGAS) for lysosomal degradation and prevents mitochondrial DNA sensing | [135,136] |
| NS3 | NS3 interacts with chaperone protein 14-3-3e to inhibit the translocation of RIG-I to the adaptor protein MAVS. | [136] |
| NS4A | NS4A inhibits the interaction of RIG-I with the adaptor protein MAVS by binding to the N-terminal CARD-like domain and C-terminal transmembrane domain of MAVS, resulting in the suppression of IRF3 activation and IFN production | [137] |
| NS4B | Blocks IFN-induced signal transduction | [53] |
| integral membrane protein | [54] | |
| NS5 | Blocks production of ISGs by interrupting the transcription complex PAF1C | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhole, P.; Zaidi, A.; Nariya, H.K.; Sinha, S.; Jinesh, S.; Srivastava, S. Host Immune Response to Dengue Virus Infection: Friend or Foe? Immuno 2024, 4, 549-577. https://doi.org/10.3390/immuno4040033
Dhole P, Zaidi A, Nariya HK, Sinha S, Jinesh S, Srivastava S. Host Immune Response to Dengue Virus Infection: Friend or Foe? Immuno. 2024; 4(4):549-577. https://doi.org/10.3390/immuno4040033
Chicago/Turabian StyleDhole, Priya, Amir Zaidi, Hardik K. Nariya, Shruti Sinha, Sandhya Jinesh, and Shivani Srivastava. 2024. "Host Immune Response to Dengue Virus Infection: Friend or Foe?" Immuno 4, no. 4: 549-577. https://doi.org/10.3390/immuno4040033
APA StyleDhole, P., Zaidi, A., Nariya, H. K., Sinha, S., Jinesh, S., & Srivastava, S. (2024). Host Immune Response to Dengue Virus Infection: Friend or Foe? Immuno, 4(4), 549-577. https://doi.org/10.3390/immuno4040033







