1. Introduction
Monkeypox (also called Mpox by the WHO) is a viral disease that occurs mostly in central and western Africa. It was first identified in 1958 by Preben von Magnus, who recognized Mpox as a smallpox-like disease among monkeys in Denmark [
1]. The first human case of Mpox was in 1970, and another neonatal case was reported in 1972 in the Democratic Republic of Congo (DRC) [
1]. From 1970 to 1979, there were about 50 recorded human cases of Mpox, mainly from the DRC [
2]. The first outbreak of human Mpox in Africa occurred in 1986, with 400 cases and a case fatality rate (CFR) of 10% [
3]. Between 1996 and 1997, the second outbreak in the DRC occurred [
4]. The DRC recorded more than 500 human cases of Mpox in the period between 1991 and 1999 [
4]. Usually Mpox affects tropical countries, but south Sudan and the USA recorded many cases in 2003 and 2005 that came from the DRC [
5]. The DRC had recorded 2000 cases annually in the period between 2011 and 2014 [
4]. Between 2014 and 2017, there were many reported outbreaks, and in 2018, many suspected cases were reported in the DRC [
4]. The United Kingdom—UK—recorded the first case of human Mpox in May 2022 [
6] and many recorded cases were also observed in Australia, USA, and Europe [
7]. Surprisingly, the latest outbreak was not triggered by infected people or traveling to endemic areas, but by high-risk sexual behaviors; therefore, it was more sexually transmitted, particularly among homosexual men [
8,
9].
Recent studies have shown that the main cause of the spread of Mpox worldwide is the cessation of smallpox vaccination, as well as direct human transmission [
7]. Notably, to date, Mpox mainly affects children and young adults. Clinical manifestations and outcomes differ notably between children and adults. The most important feature is the distribution of the rash in children was predominantly on the trunk and face, and none of the children <12 years of age had anogenital lesions. In contrast, most adolescents are presented with anogenital lesions [
10]. All these records and observations may expose us to a potential danger of widespread resistant disease. A single confirmed case of Mpox is enough to be considered as an outbreak. Mpox recently appeared in many countries with no evident relation to endemic areas, suggesting another route of transmission; hence, we must pay much greater attention to Mpox [
7].
The clinical picture of Mpox is very similar to smallpox except for early lymphadenopathy, which is highly supportive of human Mpox. The symptoms include joint pain, fever, headache, myalgia, and three days of nausea, with an incubation period from one to three weeks [
1]. After fever and lymphadenopathy for nearly one to three days, skin lesions appear on both the periphery and the face at the same time. The affected lymph nodes are those in the groin, submandibular area, and neck [
1,
11]. The skin lesions range from small and flat spots to vesicles filled with clear and then yellow fluid, which may coalesce together forming a large lesion that can cover the whole body if the condition is severe. Skin lesions finally end with scabs that fall out, with most people having skin lesions on the soles and palms [
12,
13] Similarly to smallpox, the lesions grow simultaneously, and the lesions heal with pale marks that eventually turn dark [
14].
Also, Mpox can lead to many complications in severe cases, including acute respiratory distress, sepsis, bronchopneumonia, GIT complications, and corneal affection leading to visual loss. Human Mpox should be differentiated from smallpox and other orthopox viruses such as herpes simplex virus (HSV) 1 and 2 and varicella-zoster [
12,
14]. Transmission is mainly through respiratory droplets and direct contact, but having sex with animals may also play a role. Diarrhea, nausea, and vomiting are common complaints occurring in Mpox infection [
15,
16], and abdominal pain up to acute abdomen may also occur [
15].
Other atypical neurological symptoms may occur such as changes in consciousness, confusion, stiff neck, headache, myalgia, seizure, encephalitis, photophobia, fatigue, dizziness, visual deficit, encephalitis, coma, delirium, and encephalopathy [
4,
15,
17,
18]. Finally, other psychological symptoms such as anxiety or depression may be associated with this infection [
14]. So, we wrote this narrative review to summarize the reported neuropsychiatric manifestations of Mpox disease and its multimodality strategies of management.
2. Methodology
Inclusion Criteria: Studies that focus on Mpox infection, its neuropsychiatric manifestations, vaccination effects, and related treatments, published in English.
Exclusion Criteria: Studies not related to human Mpox infections, those in non-English languages, or non-peer-reviewed articles.
We searched different databases such as PubMed, Scopus, WoS, and Google Scholar. The search strategy utilized Medical Subject Headings (MeSH) terms and relevant keywords to capture all the relevant literature. The following keywords were utilized “Monkeypox”, “Mpox”, “pathogenesis”, “neurological manifestation”, “psychiatric manifestation”, “neuropsychiatric manifestation”, “Mpox vaccination”, “neurological complication of vaccination”, “diagnosis”, “treatment”, “multimodalities treatment”.
3. Molecular Virology and Life Cycle
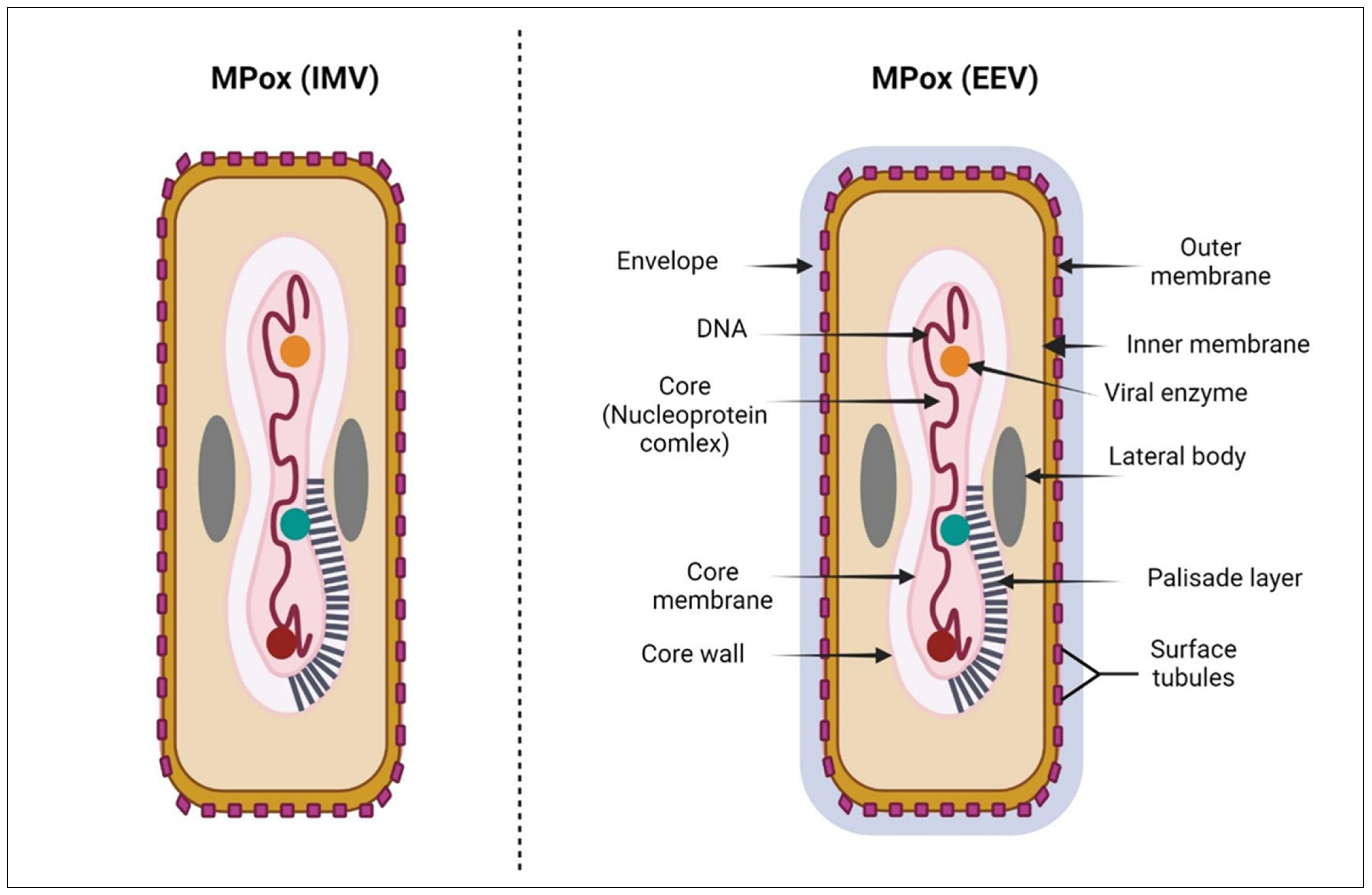
Like other orthopox viruses, Mpox is surrounded by a membrane of lipoprotein and is ovoid or brick-shaped, with a size of more than 200 nm [
19]. Mpox is composed of many components, a nucleoprotein core, double-stranded DNA, two lateral bodies, surface tubules, and an outer membrane [
20] (
Figure 1). Although Mpox is a DNA virus, it encodes RNA polymerase that helps its replication, which is a unique property. There are no known exact receptors for Mpox, but it is assumed that viral entry depends on both viral clades and the type of host cell, with the involvement of many receptors including glycosaminoglycans, chondroitin sulfate, and heparan sulfate [
21,
22,
23]. Mpox infection takes many cellular steps as follows: viral entry, fusion, genome replication, assembly, and finally release from infected cells. There are two forms of Mpox, extracellular enveloped virion (EEV) that is responsible for dissemination and intracellular mature virion (IMV) that is released after the destruction of cells [
24] (
Figure 1). Both forms have membranes and a variable number of glycoproteins. In the binding step, the virus attaches to cells either through unknown receptors or an extracellular matrix, then at neutral or low pH, the virus enters the cell after fusion with the plasma membrane [
25]. The replication of Mpox occurs by the RNA polymerase and then the translation of proteins (early, intermediate, and late) by the host ribosome [
26]. Late proteins are essential components for the viral assembly and formation of infectious types of intracellular viruses. Microtubules transport these intracellular virions which are then enclosed by double layers of the membrane by the Golgi or endoplasmic reticulum to form the intracellular virus in an enveloped form. These intracellular forms may turn into extracellular forms when fused with the cell membrane and polymerize actin component [
27] (
Figure 2). For the virus to be replicated, other proteins are required, including Golgi4, VPS54, vascular protein sorting 52, and COG4 [
27,
28]. The known information is still not enough to fully understand virus replication and pathogenesis.
4. Immunopathogenesis and Immune Evasion of Mpox Virus
4.1. Mpox Virus–Host Immune Interaction
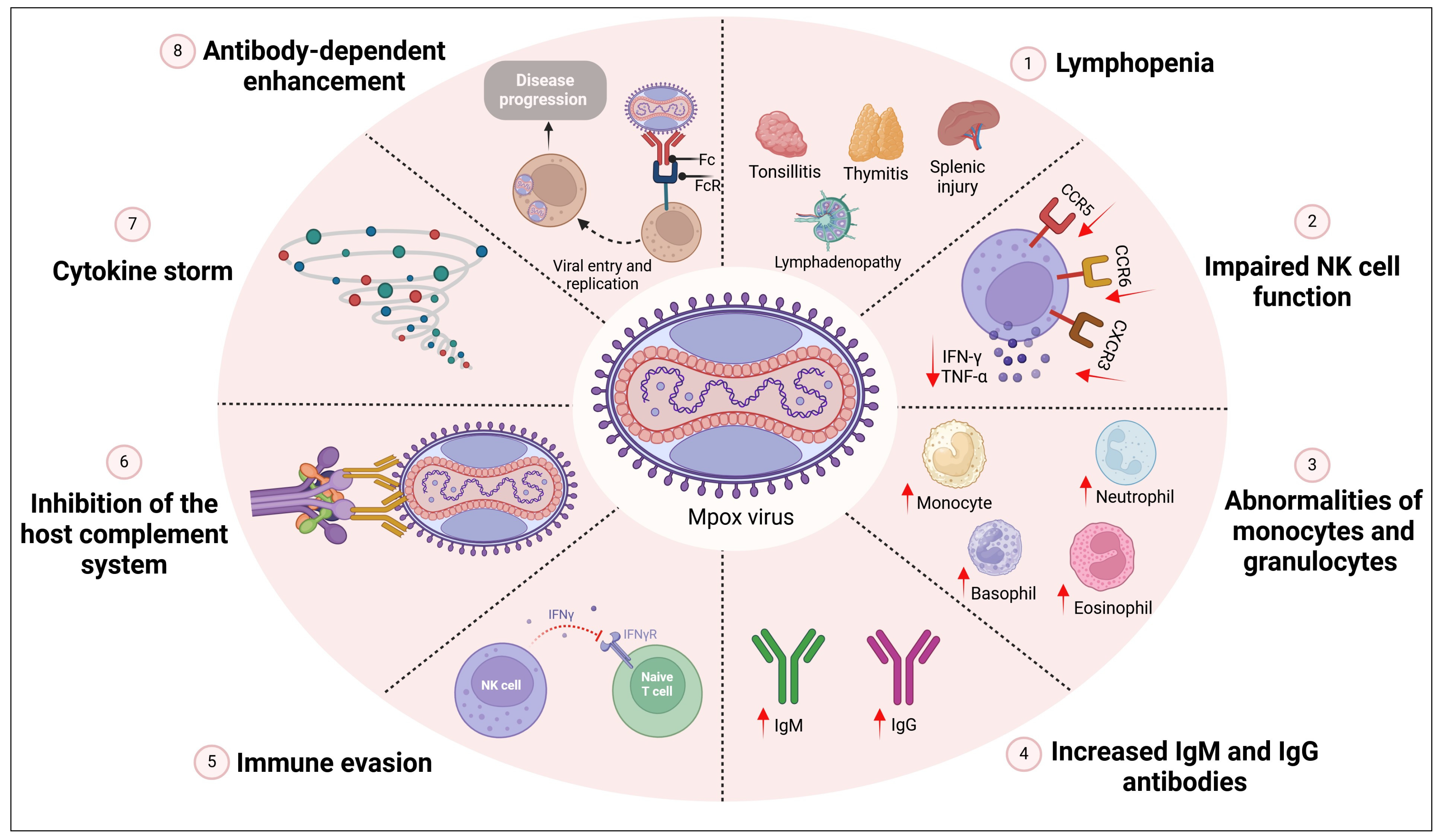
The host immune response coordinates a comprehensive defense against Mpox virus infection, which includes both innate and adaptive immune mechanisms [
30]. The virus primarily targets lymphoid tissue such as the lymph nodes, thymus, spleen, and tonsils, leading to lymphopenia. The immune reaction in Mpox infection may lead to poor prognosis. The dangers of Mpox infection are not only due to skin affection but also due to the immune response that leads to respiratory complications, deep tissue abscesses, and sepsis [
31]. Mpox infection also affects and destroys many immunological organs and causes tonsilitis, lymphadenopathy, myeloid hyperplasia, splenic injury, and thymitis [
32,
33]. Several reported studies showed that the immunopathogenesis in severe cases of Mpox infection are associated with lymphopenia, impaired function of the NK cell, monocytosis in the blood, increased antibody levels along with an increase in granulocyte and monocyte, immune evasion, complement system inhibition, cytokine storm, and antibody-dependent enhancement [
34] (
Figure 3).
Upon the attachment and entrance of the Mpox virus to the human host cell, innate immune cells such as natural killer (NK) cells, dendritic cells (DC), and macrophages are quickly activated and trigger inflammatory cascades. This response is characterized by the secretion of proinflammatory cytokines and chemokines, including IL-1, TNF-α, and IFNs, which activate and recruit more immune cells to the site of the infection [
35]. The innate immune response also includes other pathways such as DNA sensing, the IFN-PKR pathway, TLRs and NOD-like receptor signaling pathways, the inflammasome activation pathway, NK cell pathway, apoptosis pathway, and complement system pathway, which are discussed in detail in [
34,
36,
37]. DC, the key guards of the immune system, are critical in linking innate and adaptive immune responses and beginning the adaptive immune response to the Mpox virus [
38]. They process the viral antigens and transport them to lymphoid organs, where they present them to T cells. This mechanism activates and expands antigen-specific T cells, such as CD4+ helper T cells and CD8+ cytotoxic T cells (CTLs). CD4+ T cells play an important role in helping B cells differentiate into antibody-secreting plasma cells and produce antigen-specific neutralizing antibodies, which are known to be associated with cell-mediated immunity [
39]. At the same time, CD8+ T cells directly target and kill infected cells by releasing cytotoxic granules containing perforin and granzymes, restricting viral propagation within the host. A crucial part of the host defense against the Mpox virus is humoral immunity, which is mediated by B cells and antibodies, in addition to cellular immunity. When B cells come into contact with viral antigens, they activate and differentiate into plasma cells, which produce virus-specific antibodies [
40]. These antibodies can neutralize the virus by adhering to its surface proteins, inhibiting viral attachment and penetration into host cells. Moreover, antibodies can identify viral particles for phagocytosis by neutrophils and macrophages, assisting in the removal of the particles through opsonization. The adaptive immune response established against Mpox virus infection also results in the development of immunological memory, offering long-term protection against reinfection [
30]. Following the recovery from acute infection, memory T and B cells remain in the host, allowing for a quick and strong response when exposed to the virus again. When exposed to viral antigens, memory T cells can quickly multiply and develop into CTLs, exhibiting improved effector activities and offering prompt protection against viral propagation [
41].
Understanding the dynamic interaction between the Mpox virus and the host immune system is critical for developing effective treatments and vaccines for Mpox virus infection. Through the clarification of the molecular mechanisms behind immune evasion and the identification of crucial targets for intervention, researchers can develop tactics for enhancing host immunity and suppressing viral dissemination. Furthermore, understanding the host immune response to Mpox virus infection can help create next-generation vaccines that can elicit strong and long-lasting immunity against this emerging infectious disease.
4.2. Tactics of Immune Evasion
The Mpox virus has developed complex evasion tactics to avoid immune recognition and create a prolonged infection in the host [
42,
43]. These evasion strategies target different components of the host immune response, allowing the virus to circumvent antiviral defenses and boost its survival and spread.
One tactic used by the Mpox virus is the modulation of the host’s innate immune signaling pathways. Upon infection, the virus produces a number of immunomodulatory proteins that inhibit the host’s complement system and disrupt the production and signaling of host antiviral cytokines, such as IFNs and proinflammatory cytokines [
44]. For example, the Mpox virus creates soluble cytokine fake receptors that competitively bind to host cytokines, blocking their interaction with cellular receptors and suppressing the immune response [
45]. For example, secreted IFN decoy receptors, like IFN-γ receptor and IFN-α/β-binding protein (IFN-α/βBP), that bind IFNs with great affinity prevent their interaction with cellular receptors [
34,
45,
46]. It was reported that the extremely pathogenic Mpox virus can encode IFN-α/βBPs and suppress the type I IFN-induced immune response [
47].
Furthermore, the virus secretes ortholog proteins that inhibit the activation of pattern recognition receptors (PRRs) and block the subsequent signaling pathways activated by the cytokine–receptor interaction, reducing antiviral gene expression and boosting viral replication [
41]. For example, Mpox viruses have orthologs of BCL-2-like proteins (A47, B13, P1, C6, and D11) that suppress IFNs and NF-κB activation, allowing the virus to bypass the host’s innate immune response [
36]. Additionally, the Mpox virus encodes the F3 protein, which is necessary for the Mpox IFN-resistant phenotype [
34]. Amazingly, even in the presence of IFN-γ, this virus can still replicate as it encodes F3 protein. Double-stranded RNA (dsRNA) is produced in high amounts during Mpox infection [
48]. So, F3 protein of the virus binds to these RNAs, hiding them from PRRs and evading innate immunity [
49]. The F3 subunit of the virus also inhibits eIF2α (eukaryotic translation inhibition factor2) and PKR phosphorylation (through a dsRNA-dependent manner) and so blocks translation with a reduction in IFN-γ production [
48,
49].
Further, the virus has the ability to control the expression of viral antigens on the surface of the infected cells, either by inhibiting antigen presentation machinery or by masking antigenic epitopes with viral proteins. This makes infected cells less susceptible to detection and elimination by immune cells, allowing the Mpox virus to develop a long-term infection of the host [
41].
Mpox virus infections have an effect on the complement system as well. This interference is mediated by the production of viral proteins known as virokines [
50], which mimic complement regulators and prevent complement activation. The VV complement control protein (VCP), one of these virokines, has been found to be crucial in inhibiting the complement system during poxvirus infections [
51]. Additionally, virulent Mpox encodes a secretory protein similar to the protein that controls the complement system in the case of a vaccina virus infection. This protein is called Mpox Inhibitor of Complement Enzymes (MOPICE). MOPICE reacts with c3b and c4b leading to its cleavage either directly or via plasma serine protease and so c3 convertase formation is prevented [
52].
The Mpox virus has also acquired methods to escape host cell apoptosis, allowing infected cells to survive longer and facilitating viral reproduction and propagation. The virus encodes proteins that prevent apoptosis, either by disrupting the activation of proapoptotic signaling pathways or directly inhibiting the mechanism of apoptotic cell death. This extends the lifespan of infected cells, allowing the Mpox virus to reproduce rapidly and spread to neighboring cells without being cleared by the immune system [
53].
Regarding cell-mediated immunity, the Mpox virus can suppress T-cell-receptor-mediated T cell activation by employing alternative antigen presentation pathways [
54]. The Mpox virus disrupts the major histocompatibility complex (MHC) class I antigen presentation by either decreasing MHC class I expression on infected cells or suppressing viral antigen processing and presentation. This makes it more difficult for CTLs to identify infected cells, which permits the Mpox virus to escape immune monitoring and develop persistent infection. In addition, the Mpox virus can prevent co-stimulatory molecules from being expressed on antigen-presenting cells, which hinders the T cell activation and proliferation in response to viral antigens [
37,
55].
Regarding the adaptive immune response, the Mpox virus has developed strategies to avoid detection by the host’s adaptive immune cells. The virus can undergo antigenic change, resulting in a wide range of viral variants with changed antigenic profiles that avoid detection by neutralizing antibodies and T cells. Because of this antigenic variety, the Mpox virus is able to evade immune monitoring and persist in host populations [
37].
As a whole, we can say that Mpox is a very resistant virus that can modulate the immune response to keep its spread and division.
5. Neuroinvasive Proprieties of Mpox
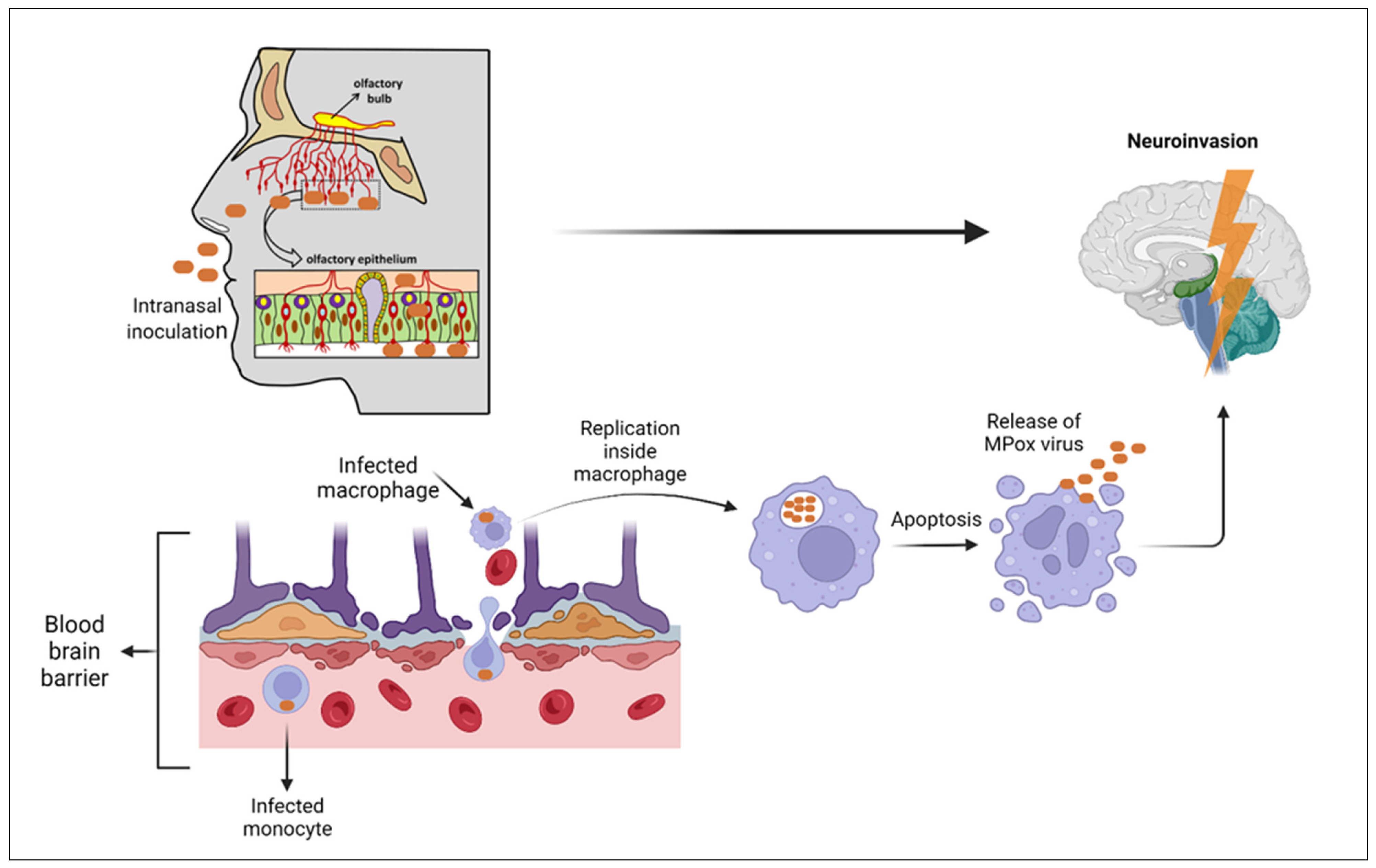
No human studies show precise information about that, but several animal studies show some relevant data. Several studies show the ability of Mpox to cross the blood–brain barrier [
4,
56]. During the 2003 outbreak of Mpox in the Midwest region of the United states, the virus was identified in the brain tissue of four rodents out of fifty-two using polymerase chain reaction and electrochemiluminescence (ECL) assays [
57]. During this outbreak, a 6-year-old girl developed the symptoms of encephalitis (new onset seizures, headache, and rigidity) which required her transfer to the emergency department and tracheal intubation. On further workup, the cerebrospinal fluid (CSF) analysis by PCR and ELISA showed negative results for Mpox antigens and positive for Mpox IgM antibodies, respectively, which suggests Mpox neuroinvasion [
4]. During the current outbreak, two cases of acute disseminated encephalomyelitis (ADEM) have been reported in the district of Colombia, but the PCR nucleic acid analysis for CSF was negative and ELISA IgM for Mpox virus was not performed, making the underlying pathology of these cases unclear, which may be due to Mpox neuroinvasion or the para-infectious autoimmune response to Mpox [
58]. In another case of a 35-year-old female in the UK diagnosed with encephalitis, her CSF analysis showed a PCR positive for Mpox virus antigens and ELISA IgM positive for Mpox [
59]. Animal studies suggest that one of the routes of invasion to the brain is through the olfactory epithelium after the increasing replication within the nasal mucosa, especially that which covers the nasal septum [
60]. Another animal study using intracellular staining identifies the pox virus antigens within cerebral tissues [
61]. Another route of viral transmission to the brain is the blood route, through the infected macrophages and neutrophils which show the highest concentration of Mpox Zaire 79 in primates; the former undergoes apoptosis releasing the virus particles with the latter infecting the brain tissue [
61] (
Figure 4).
6. Neurological Manifestations
The symptoms vary from nonspecific less serious symptoms such as headache, myalgias, fatigue, and pain to more serious symptoms such as seizures, blindness, photophobia, delirium, disturbed conscious level (DCL) up to coma, encephalitis, and transverse myelitis [
4,
14,
15,
17,
56,
59,
62,
63] (
Table 1). According to a recently published systematic review and meta-analysis, the most prevalent clinical manifestations are rash (93%, 95%; CI: 80–100%), fever (72%, 95%; CI: 30–99%), pruritus (65%, 95%; CI: 47–81%), and lymphadenopathy (62%, 95%; CI:47–76%). Other manifestations included fatigue (60%, 95%; CI: 32–85%), sore throat (57%, 95%; CI: 36–77%), headache (50%, 95%; CI: 25–75%), cough (47%, 95%; CI: 38–57%), myalgias (45%, 95%; CI: 16–76%), photophobia (32%, 95%; CI: 3–71%), arthralgia (26%, 95%; CI: 1–65%), difficult breathing (25%, 95%; CI: 3–58%), conjunctivitis (19%, 95%; CI: 9–32%), nausea/vomiting (19%, 95%; CI: 9–30%), diarrhea (4%, 95%; CI: 2–7%) [
64]. In most studies, headache is found in more than half of the cases [
15,
18,
65,
66]. According to a prospective observational study of 216 patients in the Democratic Republic of Congo, less than 10% of patients suffer from severe neurological symptoms such as confusion, disorientation, and stupor [
18].
According to several studies, age is inversely proportional to the severity of the neurological manifestations. Most of the children presented required management in the intensive care unit (ICU) due to encephalitis-associated complications with a high fatality rate [
4,
15,
63,
67,
68]. A 6-year-old girl with suspected Mpox encephalitis showed slow waves activity on electroencephalogram (EEG) and diffuse cortical, thalamic, and brain stem edema, and left thalamic and right parietal hyper-intensity on brain magnetic resonance imaging (MRI), with the CSF examination showing polymorph nuclear pleocytosis, with positive ELISA IgM for Mpox with normal glucose and protein levels [
4]. Another 3-year-old girl, diagnosed with encephalitis on the first day of hospital admission, died on the second day with no CSF analysis performed to confirm Mpox infection [
63]. A recent case of a 35-year-old female diagnosed with Mpox encephalitis showed MRI changes similar to that of the 6-year-old girl [
59].
Management of these cases differs according to institutions, but generally, they include supportive and symptomatic treatment (non-steroidal anti-inflammatory drugs, antibiotics, antiepileptic, and sedatives for seizures), antiviral treatment (such as acyclovir), hospitalization of severe cases, and with some cases requiring invasive procedures such as intubation [
4,
14,
15,
17,
69,
70]. The spread of Mpox to the CNS may be devastating and carry poor outcomes, so we recommend the early diagnosis, isolation, and management of these cases to prevent such complications.
Table 1.
Compilation of reports on the neurological manifestations of Mpox.
Table 1.
Compilation of reports on the neurological manifestations of Mpox.
| Serial Number | Study ID | Neurological Manifestations |
|---|
| Headache | Myalgia/
Flaccid Paralysis | Seizure | Encephalitis | Pain/
Fatigue | Dizziness | Photophobia/
Visual Deficit | Confusion/Delirium/
Encephalopathy | Altered Mental
Status/
Coma |
|---|
| 1 | Adler et al., 2022 [14] | 1 | - | - | - | 1 | - | - | - | - |
| 2 | Akar et al., 2020 [71] | 78 | - | - | - | - | - | - | - | - |
| 3 | Anderson et al., 2003 [72] | 1 | 1 | - | - | 1 | - | - | - | - |
| 4 | Boumandouki P et al., 2007 [73] | - | 2 | - | - | - | - | - | - | - |
| 5 | Cole et al., 2022 [59] | 1 | 1 | - | 1 | 1 | 1 | - | 1 | - |
| 6 | Croft et al., 2007 [74] | 13 | - | - | - | - | - | - | - | - |
| 7 | Eseigbe et al., 2021 [69] | 2 | - | - | - | - | - | - | - | - |
| 8 | Hughes et al., 2021 [69] | 99 | 90 | - | - | 115 | - | - | - | - |
| 9 | Huhn et al., 2005 [15] | 23 | 19 | 1 | 1 | - | - | - | 2 | - |
| 10 | Ježek et al., 1987 [63] | - | - | - | 1 | - | - | - | - | 1 |
| 11 | Kalthan et al., 2016 [68] | 2 | - | - | - | - | - | - | - | - |
| 12 | Learned et al., 2005 [70] | 1 | - | - | - | 2 | - | - | - | - |
| 13 | Ogoina et al., 2019 [17] | 12 | 5 | - | - | 5 | - | 3 | - | - |
| 14 | Ogoina et al., 2020 [75] | 19 | 25 | 1 | 3 | - | - | 9 | - | - |
| 15 | Ogunleye et al., 2019 [76] | 61 | 42 | - | - | - | - | 27 | - | - |
| 16 | Pittman et al., 2022 [18] | 49 | 15 | - | - | 11 | 3 | 5 | 4 | - |
| 17 | Reed et al., 2004 [77] | 11 | 1 | - | - | - | - | - | - | - |
| 18 | Reynolds et al., 2006 [66] | 32 | 36 | - | - | - | - | - | - | - |
| 19 | Reynolds et al., 2013 [78] | 1 | - | - | - | 1 | - | - | - | - |
| 20 | Sejvar et al., 2004 [4] | 2 | - | 1 | 1 | - | - | - | 1 | 1 |
7. Psychiatric Manifestations in Mpox Patients
The typical manifestations of Mpox are fever and skin lesions, but in some patients, it can also present with uncommon clinical conditions such as psychological disorders [
79].
The association between Mpox and mental disorders is still unclear. However, Ogoina et al. stated that 25% of Mpox hospitalized patients were complicated by depression and anxiety [
75], and another study stated that the infected individuals also developed low mood and emotional liability manifestations [
14], so both necessitated treatment [
14,
75]. Other psychological manifestations mentioned in the literature are emotional lability and acute alcohol withdrawal [
80] (
Table 2).
It is believed that the preexistence of mental disorders makes infected individuals more vulnerable to developing psychiatric manifestations [
81]. Furthermore, scars and deformities caused by skin lesions may exacerbate mental health issues associated with Mpox infection [
75].
While the presence of neuropsychiatric symptoms alongside Mpox infection may suggest an association, it is crucial to highlight that this association does not automatically imply causation. Further in-depth research, including longitudinal studies, clinical trials, and mechanistic investigations, is essential to establish whether Mpox virus infection indeed causes neuropsychiatric symptoms or if the association is coincidental.
8. Psychosocial Aspects Associated with Mpox Infection
Infectious diseases have played an important part in shaping human history; they are responsible for more fatalities than any other human disease in the past outbreaks. So, these outbreaks have engraved an automatic response in our subconscious of a fear of infection. This is what happens in Mpox outbreaks. Furthermore, a lack of readiness on the side of medical institutions and inaccurate information copied by the media may aggravate the state of fear and abnormal psychological responses [
82]. Previous reports noted that Mpox symptoms and management strategies are connected to psychosocial aspects such as fear, panic, worry, anger, boredom, weariness, social isolation, financial loss, and stigmatization [
83].
During the outbreak, patients and their family exhibited a variety of opinions and behaviors. The majority said they were afraid and anxious about being stigmatized and subjected to discrimination by family members, community members, and hospital staff. Some patients and their family members refused isolation, feeling that they may face greater stigma and discrimination if others in their community knew they had been admitted to the isolation ward [
17]. Sadly, any of these elements might raise the risk of suicidal thoughts and actual suicide [
84]. Public health programs that raise awareness of an illness can help allay public worries. More widespread access to health information and improved health education can help identify cases early, lessen stigma associated with disease, and reduce people’s fear of being sick [
82]. So, healthcare providers should show interest in counseling the patients and provide them with accurate information about the disease to deal with their fear of Mpox disease, maintain communication with the patients virtually, manage the associated pain, and prevent and treat the dermal lesions to prevent the disfigurement.
9. Differential Diagnosis of Mpox Infection
Mpox belongs to the orthopox virus family as does the smallpox virus. The latter presents with a clinical picture similar to that of the former, most commonly, fever with rigors, malaise, abdominal pain, vomiting, headache, and the characteristic papulovesicular rash [
85], even though early lymphadenopathy in Mpox is characteristically unique [
29].
One of the most common causes of death due to smallpox infection is encephalitis and ADEM according to the cases reported [
85,
86]. Although the clinical picture of smallpox infection is very similar to Mpox infection, it cannot be considered as a differential diagnosis because it has been eradicated since 1980 according to the World Health Assembly [
87]. Also, encephalitis is a documented side effect of the smallpox vaccine [discussed in detail below]. Despite its rarity, it carries a high mortality rate [
88] (
Table 2).
Varicella-zoster virus is another important differential diagnosis that can be presented, especially in the primary infection in children, by encephalitis; myelitis; cranial nerve palsies, particularly involving the abdicant and facial nerves (the latter involved in Ramsay Hunt syndrome); radiculitis with paralysis of a limb; meningitis; and the characteristic but rare cerebellar ataxia [
89]. The Ramsay Hunt syndrome is specific to HZV and consists of a triad of facial nerve palsy, otalgia, and a vesicular rash which may be associated with hearing loss, tinnitus, and hyperacusis due to affection of the vestibulocochlear nerve [
90]. Another specific common manifestation is postherpetic neuralgia, which commonly occurs in old age after shingles “Zoster” with a characteristic radicular distribution [
91]. Almost all the cases recover without sequelae [
89]. The characteristic croupy rash (which appears in different stages of papules, vesicles, pustules, and crustation) of the varicella can differentiate it from Mpox [
92,
93] (
Table 3).
The oral and genital lesions of HSV type 1 and 2 are quiet similar to those of Mpox. The oral lesions are caused by HSV-1, while the genital lesions can be caused by both HSV type 1 and 2. HSV, especially type 1, can cause encephalitis in the acute or subacute onset presented by fever, DCL, personality changes, seizures, and focal neurological deficits. Other findings may include Guillain-Barré syndrome (GBS), benign recurrent meningitis, mania, and facial nerve palsy (Bell’s palsy) [
94]. The Klüver-Busy syndrome is a highly specific but rare neuropsychiatric disorder characterized by an abnormal urge to put objects in the oral cavity (hyperorality) and inappropriate sexual activity (hypersexuality) [
95]. HSV encephalitis can be differentiated from Mpox encephalitis by the characteristic MRI findings, which are inferomedial temporal lobe changes and spared basal ganglia [
94]. Also, the CSF analysis commonly shows lymphocytic pleocytosis, in contrast to Mpox encephalitis, which is reported to show polymorph nuclear pleocytosis [
4,
94] (
Table 3).
10. Mpox Vaccination
The history of the smallpox vaccine began in 1796, when Edward Jenner observed that milkmaids who had caught cowpox were immune to smallpox, inspiring him to test cowpox as a way to prevent smallpox. To test this idea, he inoculated a young boy with cowpox, successfully creating immunity to smallpox and developing the world’s first vaccine. By the 19th century, cowpox was replaced by horsepox in mass vaccinations due to supply challenges. However, in the 20th century, a virus called vaccinia became the standard in smallpox eradication efforts. When it comes to neurological side effects, cowpox and horsepox are thought to be milder, as they naturally occur less often in humans and typically cause minimal symptoms. Vaccinia, however, can lead to rare but serious neurological complications, including encephalitis, particularly in those with compromised immune systems [
96,
97,
98,
99]. There is no vaccine for Mpox itself, even though the smallpox vaccine (vaccinia strain) gives cross-protection against Mpox [
100], with protection against disease up to 85% according to an old study [
101]. A study in Africa showed that people vaccinated against smallpox were less likely to have Mpox and, if infected, had milder symptoms. [
102]. Nowadays, there are three licensed types of vaccines for the smallpox virus, ACAM2000, JYNNEOSTM (also known as IMVAMUNE, IMVANEX, MVA-BN), and Aventis Pasteur Smallpox Vaccine (APSV), that may be used in a smallpox outbreak under the proper regulatory actions (i.e., Investigational New Drug application [IND] or Emergency Use Authorization [EUA]) [
103,
104,
105].
APSV was historically used to provide protection against smallpox, and by extension, it was also believed to offer some cross-protection against Mpox, which is caused by a related orthopox virus. APSV contains the live vaccinia virus and works by stimulating the immune system to produce antibodies against vaccinia, offering immunity against smallpox and, to a lesser extent, other orthopox viruses such as Mpox. However, due to safety concerns, particularly the risk of serious side effects in immunocompromised patients such as neurological complications, APSV has largely been replaced by newer, safer vaccines like ACAM2000 and JYNNEOS [
106,
107].
ACAM2000 is a modified live vaccinia virus vaccine. ACAM2000 works by stimulating an immune response through its viral replication in the body, which can result in a stronger immune activation. While it is safer than APSV, it still carries risks of severe side effects like myocarditis, pericarditis, and skin infections. ACAM2000 has a very bad record when used in healthy young adults, resulting in permanent disability, hospitalization or prolongation of hospitalization, life-threatening illness, or death [
108,
109]. It was used as a replacement for APSV but is still not recommended for individuals with certain health conditions (such as immunocompromised individuals and pregnant women). ACAM2000 is given as a single dose by a multiple-puncture technique with the maximum antibody titer after 28 days [
107,
110].
JYNNEOS is the safest of the three. It is a non-replicating, attenuated vaccine that induces immunity by stimulating the production of neutralizing antibodies without the risk of causing active infection. Subsequently, JYNNEOS is preferred for high-risk populations, including pregnant women and people with weakened immune systems, because it does not pose the same risks of severe complications. For children too, JYNNEOS is the preferred choice. This is because it is a non-replicating, attenuated vaccine, which makes it safer for young children compared to other vaccines. JYNNEOS is administered by subcutaneous injection as a 2-dose series delivered 28 days apart, and vaccine protection is not conferred until 2 weeks after receipt of the second dose [
107,
110].
10.1. Pre-Exposure Prophylaxis
Pre-exposure prophylaxis is indicated in the groups highly susceptible to infection as healthcare workers and laboratory personnel [
110,
111]; also, it is recommended for immunocompromised patients, children, and pregnant women (these groups carry a great risk for severe disease and fatal complications) with the living attenuated vaccine (JYNNEOS) [
112]. It is also advisable in immunosuppressed neurological patients such as those with multiple sclerosis or neuromyelitis optica spectrum, who may suffer from severe manifestations if infected. Moreover, it must be taken into consideration to administer JYNNEOS during the quiescent periods of the disease to avoid exacerbation or relapse of activity [
113].
10.2. Post-Exposure Prophylaxis
Post-exposure prophylaxis can be achieved within four days of exposure to prevent disease or within 4–14 days of exposure to attenuate the disease. It is highly recommended for high-risk exposure contacts according to the Interim Community, such as direct contact with infected skin and mucous membrane, sexual activity with infected cases, or exposure to aerosol from the patients without adequate wearing a mask (N95 equivalent or higher respirators) [
114]. JYNNEOS is the preferred choice for individuals who need protection after exposure to monkeypox, as it offers protection without the risks of severe side effects like myocarditis or encephalitis, which are more common with live virus vaccines like ACAM2000. However, ACAM2000 may still be used in certain high-risk populations under specific circumstances, but it is not the first-line choice for post-exposure prophylaxis due to these concerns [
106,
107].
11. Neurological Complications of Vaccination
As with any other vaccine, the smallpox vaccine has many complications. However, their risk is quiet higher than that of other vaccines such as those for measles or varicella [
115]. In our review, we emphasize the neurological complications of the vaccine.
Variable neurological complications have been reported with the smallpox vaccine, especially with the first generation of the vaccine (Dryvax) which was used in the past to eradicate the disease. It has been replaced by ACAM2000 in 2010 after its approval by the FDA, as it has less neurological complications [
115]. These complications can be categorized as transient, such as headache, limb paresthesia, mononeuropathy, cranial nerve palsy such as Bell’s palsy, flaccid or spastic paraparesis, GBS, seizures, and relapses of multiple sclerosis and myasthenia gravis [
116,
117,
118,
119] and permanent complications such as permanent brain damage, movement disorders, epilepsy, neurosis, psychosis, and deafness [
88,
117,
118]. The most devastating complication is PVEM (post-vaccinal encephalomyelitis), which has a high mortality rate ranging from 30 to 50% after 2–3 weeks of the onset of manifestations and occurs in all age groups, even those below two years old [
116]. PVEM is characterized by an abrupt onset after 10–12 days of vaccination with a range from 2 to 30 days of fever, headache, dizziness, DCL, vomiting, leg and back pain, and seizures. A few days later, the neurological manifestations worsen to stupor, limb paresis, cranial nerve palsies, aphasia, seizures, and extrapyramidal symptoms such as chorea, athetosis, and tremors [
116,
120]. Most of the patients in those cases recovered without sequelae; however, some of the patients in those cases have permanent residuals such as seizures, intellectual disabilities, cranial nerve palsies, and psychiatric symptoms [
121]. In children below two years of age, PVE (post-vaccinal encephalopathy) is a condition similar to PVEM but milder [
120]. These complications have been reported more in primary vaccination than revaccination [
119,
120]. According to Sejvar et al., who follow up the complications of the Dryvax vaccine “the first generation of the vaccine”, there were three cases of PVE, all of which occurred after primary vaccination, and “Headache” is the most common neurological manifestation (44% of 216 reports of 665,000 case) with only 39 serious complications which include 4 cases of encephalitis and/or myelitis (the diagnosis is excluded in one of them), 3 cases of GBS, 13 cases of suspected meningitis (only 2 of the 13 cases confirmed by investigations), 11 cases of Bell’s palsy, and 8 cases of seizures [
119].
LC16m8 is an attenuated smallpox vaccine derived from the Lister strain of vaccinia. It has been licensed and safely used in Japan since 1970, but it has not been approved by the FDA yet. Animal studies showed that it has much lower neurotoxicity than ACAM2000 [
122].
In conclusion, there are diverse neurological complications associated with the smallpox vaccine, though severe complications are less common with ACAM2000. However, ACAM2000 may still be used in certain high-risk populations under specific circumstances, but it is not the first-line choice. JYNNEOS stands out as the safest and most effective option, especially for vulnerable individuals.
12. Diagnosis
The diagnosis of any disease depends mainly on the clinical picture of the disease, with or without other lab and radiological methods. The same goes for Mpox diagnosis, which mainly depends on clinical symptoms and signs along with the history of animal contact. But the definitive diagnosis is through skin lesion PCR (Polymerase Chain Reaction). In contrast, PCR from blood sample is not definitive [
13].
Most cases were among young homosexual men. Also, contact with the lesion, blood, body fluids, and contaminated objects of the infected people or animals are considered other routes of infection [
123].
Typically, we use real-time PCR to detect the genetic materials of the orthopox virus and identify Mpox by testing the genome sequence. Different types of samples can be used, but skin samples are the most common. Other possible samples include rectal swabs, feces, urine, nasopharyngeal swabs, and semen [
124]. Previous information suggests the role of bodily fluids in the spread of the infection [
124]. Real-time PCR needs about two days to examine and detect the genetic materials of the virus. DNA sequencing is another technique that needs at least four days. PCR is a very sensitive and specific technique by which we can differentiate Mpox from smallpox disease [
125]. Real-time PCR is an available, easy, and accurate diagnostic tool [
126,
127]. Multiplex assay is one of the most updated techniques of PCR. GeneXpert platform is a device used for quick diagnosis in endemic areas using multiplex assay [
128].
Serology is not the best option as it may give a misleading result of a false positive and may show cross-reaction with other orthopox species of viruses. Also, people who took smallpox vaccine may give a false positive. But serology is very useful in screening cases. Radioimmunoassay adsorption is the test used for the serological screening searching for specific antibodies to Mpox or previous vaccination [
129]. Finally, we can say that PCR is a very precious and essential tool for diagnosis, but we a need much faster method to overcome these outbreaks [
130].
CSF is an important and unique type of fluid in the body. Various changes may occur in CSF due to neurological diseases or infection. Low protein, normal glucose, and high cell count are the changes recorded in CSF due to Mpox infection [
131]. Test searching for IgM in CSF can be used for Mpox diagnosis [
131]. CSF examination shows some pathological and neurological changes associated with this infection. So, CSF examination may be given priority in some cases with special neurological complaints [
132].
13. Multimodalities Treatment of Mpox Disease and Its Associated Neuropsychiatric Manifestations
To date, there has been no definitive treatment for Mpox. However, the preclinical trials on Mpox have shown promising results for some drugs. The strategy of management should be multimodal, incorporating both pharmacological and non-pharmaceutical approaches and should be adjusted to relieve the symptoms, and manage the related complications. Furthermore, the patients should be closely monitored along their clinical course to detect the onset of long-term post-viral disorders as soon as possible.
Tecovirimat is an antiviral drug that was initially approved by the FDA in 2018 as a smallpox treatment, but recently, it was also approved by the European Medicines Agency for the treatment of Mpox infection [
133,
134]. It acts by the inhibition of the VP37 protein; this protein is necessary to interact with TIP47 and GTPase and in the formation of the essential enveloped virions [
135]. It had been found that tecovirimat reduced the mortality rate in Mpox-infected animals, with a survival rate of 90% [
136,
137,
138,
139]. However, in animals with delayed treatment, the mortality rate was much higher [
139].
Another drug is Brincidofovir, which is converted to the conjugate of cidofovir (CDV) and phosphorylated to give the active metabolite cidofovir diphosphate. Cidofovir diphosphate in turn interferes with viral replication by the inhibition of DNA polymerization [
102]. It also had positive results in clinical studies. However, it has low efficacy, with the highest survival rate being only 57% [
140,
141,
142]. A recent study by Hutson CL et al. suggested that Brincidofovir may be more effective when combined with other drugs such as tecovirimat [
65]. Monoclonal antibodies and Interferon-Beta are also possible therapeutic agents that may be used in the treatment of Mpox [
143,
144].
Treatment should not only be focused on the viral load, but it should also include the management of Mpox general manifestations including pain, skin lesion, and neuropsychiatric complications. As mentioned above, beside the pharmacological treatment, we need a complementary supportive care including hemodynamic balance restoration, rehydration, supplementary oxygen, management of bacterial infections of skin lesions, and ocular complications using lubricants, topical antivirals, and topical antibiotics [
145,
146].
Very little is known about the analgesic strategy for Mpox infection in the present outbreak. A recent study by Pfäfflin et al. found that five out of six hospitalized patients described their pain as an unprecedent pain that was relieved by a combination of local and systemic analgesics [
147].
The World Health Organization (WHO) and The Centers for Disease Control and Prevention (CDC) have provided guidelines to manage and control the present Mpox outbreak. However, these reports have limited information regarding analgesic treatment alternatives. Each patient should have a therapeutic approach based on their somatosensory complaints with the use of atypical analgesic medications for neuropathic pain disorders [
146].
There is no fixed approach for the management of Mpox patients, so each case is individualized according to the associated symptoms and the severity of the disease. Management strategies range from just analgesics for pain and antiviral drugs to intubation and ventilatory support for some serious cases of encephalitis [
148]. Corticosteroids may be considered for an of acute disseminated encephalomyelitis (ADEM)-like presentation, but they should be used cautiously due to their immunosuppressive complications [
69].
All psychiatric complications of the Mpox disease resulted from stigmatization, discrimination, disfigurement, isolation, and physical pain. So, healthcare providers should show interest in counseling the patients and provide them with accurate information about the disease to deal with their fears of the Mpox disease, maintain communication with the patients virtually, manage the associated pain, and prevent and treat the dermal lesions to prevent the disfigurement.
Mpox infection does not only affect the mental health of patients, but it also has an impact on healthcare providers’ mental health, so it is important to put strategies to reduce the burden of this problem from the start. A recent publication by Ahmed et al. recommended some ideas to reduce this problem, for instance, increase physical activity and exercise; receive adequate rest and sleep; avoid of alcohol and smoking; avoid negative thoughts about having the infection; and most importantly, properly understanding the risk factors and avoiding them, such as unsafe sexual practices [
149].
Management of the neurological manifestations associated with the Mpox disease differs according to institutions, but it generally includes the supportive and symptomatic treatment (non-steroidal anti-inflammatory drugs, antibiotics, antiepileptic, and sedatives for seizures), antiviral treatment (such as acyclovir), hospitalization of severe cases, and with some cases requiring invasive procedures such as intubation [
4,
14,
15,
17,
69,
70]. The spread of Mpox to the CNS may be devastating and carry poor outcomes, so we recommend the early diagnosis, isolation, and management of these cases to prevent such complications.
14. Limitations
While exploring the potential associations between Mpox infection and neuropsychiatric manifestations, it is essential to acknowledge several limitations that affect the ability to establish causation. Understanding these limitations is crucial for interpreting the existing literature accurately and guiding future research endeavors. Many studies investigating the neuropsychiatric manifestations of Mpox infection are cross-sectional in nature, primarily focusing on observing the presence of symptoms at a single point in time. This design limits the ability to infer causation, as causality cannot be determined from cross-sectional data alone. The presence of confounding variables, such as pre-existing psychiatric conditions, comorbidities, or other concurrent infections, poses a significant challenge in establishing a direct causal relationship between Mpox infection and neuropsychiatric manifestations. Failure to adequately account for these confounders can impede the accurate assessment of causation. The scarcity of longitudinal studies tracking individuals over an extended period following Mpox infection hinders the ability to establish a temporal relationship between the onset of neuropsychiatric symptoms and the presence of the virus. Longitudinal designs are essential for determining the direction of causality. While an association between Mpox infection and neuropsychiatric symptoms may be observed, the biological mechanisms underlying this relationship remain inadequately understood. Without a comprehensive understanding of the pathophysiological pathways linking the virus to neuropsychiatric manifestations, inferring causation becomes challenging. Ethical considerations regarding the deliberate exposure of individuals to the Mpox virus for the purpose of experimental studies pose a significant barrier to conducting controlled trials that could elucidate a causal relationship. This limitation restricts the ability to establish causation through traditional experimental methods. The absence of comprehensive mechanistic studies exploring how Mpox infection may directly influence the central nervous system and lead to neuropsychiatric symptoms represents a critical limitation. Without detailed mechanistic insights, establishing a causal link remains speculative.
Acknowledging these limitations is imperative for maintaining scientific rigor and ensuring that interpretations of the associations between Mpox infection and the neuropsychiatric manifestations are cautious and evidence-based. Addressing these limitations through rigorous study designs and interdisciplinary collaborations is essential to advance our understanding of this complex relationship.
15. Recommendations
The comprehensive set of recommendations underscores the multifaceted nature of addressing Mpox infection, encompassing public health, vaccination, mental health, stigma reduction, and global collaboration. By integrating these recommendations into public health policies and interventions, it is possible to create a coordinated, evidence-based response to the Mpox outbreak, ensuring the well-being and safety of the affected populations. These recommendations emphasize the urgent need to prevent close contact, especially feco-oral sexual activities, to mitigate the risk of Mpox transmission during the endemic period. We stress the importance of carefully screening and vaccinating men who have sex with men (MSM) to reduce stigma and ensure the targeted protection of this demographic [
150]. We highlight the significance of vaccinating immunosuppressed neurological patients, such as those with multiple sclerosis or neuromyelitis optica spectrum, to mitigate the risk of severe manifestations if infected [
113]. We advocate for investigating the disease and its prevalence to understand the multifaceted factors contributing to Mpox, thereby reducing the stigma associated with the LGBTQ community [
151].
To enhance the response to Mpox outbreaks, it is crucial to strengthen surveillance systems and community engagement through targeted educational campaigns. Additionally, supporting mental health services for affected individuals and fostering global collaboration for equitable vaccine access may further ensure the effective control and mitigation of the disease. Continued research is vital to assess the long-term efficacy and safety of current vaccines and treatments, as well as to explore new therapeutic options for managing severe cases.
16. Conclusions
Mpox is a viral disease that occurs mostly in central and western Africa. Recent research proved that the main cause of the Mpox spread worldwide is the cessation of smallpox vaccination along with direct human transmission. Notably, to date, Mpox mainly affects children and young adults. All these records and observations may expose us to the emerging danger of a widespread resistant disease. The clinical picture of Mpox is very similar to smallpox except for early lymphadenopathy, which is highly supportive of human Mpox. Mpox can lead to many complications in severe cases, including acute respiratory distress, sepsis, bronchopneumonia, GIT complications, and corneal affection with visual loss. The typical manifestations of Mpox are fever and skin lesions, but in some patients, it can also present with uncommon clinical conditions such as neuropsychiatric manifestations. The neurological symptoms vary from nonspecific less serious symptoms such as headache, myalgias, fatigue, and pain to more serious symptoms such as seizures, blindness, photophobia, delirium, disturbed conscious level (DCL) up to coma, encephalitis, and transverse myelitis. Human Mpox should be differentiated from smallpox and other orthopox viruses such as herpes simplex virus (HSV) 1 and 2 and varicella-zoster. All psychiatric complications of the Mpox disease resulted from stigmatization, discrimination, disfigurement, isolation, and physical pain. So, healthcare providers should show interest in counseling the patients and provide them with accurate information about the disease to deal with their fear of Mpox disease, maintain communication with the patients virtually, manage the associated pain, and prevent and treat the dermal lesions to prevent the disfigurement. Management of the neurological cases associated with Mpox disease differs according to institutions, but generally, they include supportive and symptomatic treatment (non-steroidal anti-inflammatory drugs, antibiotics, antiepileptic, and sedatives for seizures), antiviral treatment (such as acyclovir), hospitalization of severe cases, and with some cases requiring invasive procedures such as intubation. The spread of Mpox to the CNS may be devastating and carry poor outcomes, so we recommend the early diagnosis, isolation, and management of these cases to prevent such complications.
Author Contributions
H.F.H., A.A.A., S.M.A., T.T.A., A.A., A.S.A., H.H.S., M.E.A.M., Y.A.M., Y.N.R. and R.S.: literature search, data analysis curation, and visualization; H.F.H., A.A.A., S.M.A., T.T.A., A.A., A.S.A., H.H.S., M.E.A.M., Y.A.M., Y.N.R. and R.S.: writing—original draft preparation; H.F.H., A.A.A., S.M.A., T.T.A., H.H.S., M.E.A.M., Y.A.M., Y.N.R. and R.S.: writing—review and editing; H.F.H., A.A.A., S.M.A., T.T.A., H.H.S., M.E.A.M., Y.A.M., Y.N.R. and R.S.: funding acquisition: H.F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chatelain, R. Monkeypox outbreak—Nine states, May 2022. Weekly 2022, 71, 764–769. [Google Scholar]
- Breman, J.G.; Kalisa-Ruti; Steniowski, M.V.; Zanotto, E.; Gromyko, A.I.; Arita, I. Human monkeypox, 1970–79. Bull. World Health Organ. 1980, 58, 165–182. [Google Scholar]
- Meyer, H.; Perrichot, M.; Stemmler, M.; Emmerich, P.; Schmitz, H.; Varaine, F.; Shungu, R.; Tshioko, F.; Formenty, P. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002, 40, 2919–2921. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J.; Chowdary, Y.; Schomogyi, M.; Stevens, J.; Patel, J.; Karem, K.; Fischer, M.; Kuehnert, M.J.; Zaki, S.R.; Paddock, C.D. Human monkeypox infection: A family cluster in the midwestern United States. J. Infect. Dis. 2004, 190, 1833–1840. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Emerson, G.L.; Carroll, D.S.; Zhao, H.; Li, Y.; Reynolds, M.G.; Karem, K.L.; Olson, V.A.; Lash, R.R.; Davidson, W.B. Phylogenetic and ecologic perspectives of a monkeypox outbreak, southern Sudan, 2005. Emerg. Infect. Dis. 2013, 19, 237. [Google Scholar] [CrossRef]
- Nia, Z.M.; Bragazzi, N.L.; Wu, J.; Kong, J.D. A Twitter dataset for Monkeypox, May 2022. Data Brief 2023, 48, 109118. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Girometti, N.; Byrne, R.; Bracchi, M.; Heskin, J.; McOwan, A.; Tittle, V.; Gedela, K.; Scott, C.; Patel, S.; Gohil, J. Epidemiological characteristics and clinical features of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, United Kingdom. Lancet Infect. Dis. 2022, 10, S1473–S3099. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Kong, J.D.; Mahroum, N.; Tsigalou, C.; Khamisy-Farah, R.; Converti, M.; Wu, J. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: A preliminary pooled data analysis and literature review. J. Med. Virol. 2023, 95, e27931. [Google Scholar] [CrossRef]
- CDC. Clinical Considerations for Mpox in Children and Adolescents in the U.S. Available online: https://www.cdc.gov/mpox/hcp/clinical-care/pediatric.html (accessed on 10 November 2024).
- Weinstein, R.A.; Nalca, A.; Rimoin, A.W.; Bavari, S.; Whitehouse, C.A. Reemergence of monkeypox: Prevalence, diagnostics, and countermeasures. Clin. Infect. Dis. 2005, 41, 1765–1771. [Google Scholar] [CrossRef]
- Adalja, A.; Inglesby, T. A novel international monkeypox outbreak. Ann. Intern. Med. 2022, 175, 1175–1176. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Vaccines and Immunization for Monkeypox: Interim Guidance; World Health Organization: Geneva, Switzerland, 2022. Available online: https://www.who.int/publications/i/item/WHO-MPX-Immunization (accessed on 10 November 2024).
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Mungmunpuntipantip, R.; Wiwanitkit, V. Diarrhea and monkeypox: A consideration. Rev. Esp. Enferm. Dig. 2022, 114, 763–764. [Google Scholar] [CrossRef]
- Ogoina, D.; Izibewule, J.H.; Ogunleye, A.; Ederiane, E.; Anebonam, U.; Neni, A.; Oyeyemi, A.; Etebu, E.N.; Ihekweazu, C. The 2017 human monkeypox outbreak in Nigeria—Report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS ONE 2019, 14, e0214229. [Google Scholar] [CrossRef]
- Pittman, P.R.; Martin, J.W.; Kingebeni, P.M.; Tamfum, J.-J.M.; Wan, Q.; Reynolds, M.G.; Quinn, X.; Norris, S.; Townsend, M.B.; Satheshkumar, P.S. Clinical characterization of human monkeypox infections in the Democratic Republic of the Congo. medRxiv 2022. [Google Scholar] [CrossRef]
- Dimmock, S. Machiavellian machinations. Nurs. Times 1986, 82, 35–36. [Google Scholar]
- Beer, E.M.; Rao, V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007791. [Google Scholar] [CrossRef]
- Hughes, L.J.; Goldstein, J.; Pohl, J.; Hooper, J.W.; Pitts, R.L.; Townsend, M.B.; Bagarozzi, D.; Damon, I.K.; Karem, K.L. A highly specific monoclonal antibody against monkeypox virus detects the heparin binding domain of A27. Virology 2014, 464, 264–273. [Google Scholar] [CrossRef]
- Montanuy, I.; Alejo, A.; Alcami, A. Glycosaminoglycans mediate retention of the poxvirus type I interferon binding protein at the cell surface to locally block interferon antiviral responses. FASEB J. 2011, 25, 1960–1971. [Google Scholar] [CrossRef]
- Hsiao, J.-C.; Chung, C.-S.; Chang, W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 1999, 73, 8750–8761. [Google Scholar] [CrossRef] [PubMed]
- Pickup, D.J. Extracellular Virions: The Advance Guard of Poxvirus Infections. PLoS Pathog. 2015, 11, e1004904. [Google Scholar] [CrossRef] [PubMed]
- Whitbeck, J.C.; Foo, C.H.; Ponce de Leon, M.; Eisenberg, R.J.; Cohen, G.H. Vaccinia virus exhibits cell-type-dependent entry characteristics. Virology 2009, 385, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Kmiec, D.; Kirchhoff, F. Monkeypox: A new threat? Int. J. Mol. Sci. 2022, 23, 7866. [Google Scholar] [CrossRef]
- Sivan, G.; Weisberg, A.S.; Americo, J.L.; Moss, B. Retrograde Transport from Early Endosomes to the trans-Golgi Network Enables Membrane Wrapping and Egress of Vaccinia Virus Virions. J. Virol. 2016, 90, 8891–8905. [Google Scholar] [CrossRef]
- Realegeno, S.; Priyamvada, L.; Kumar, A.; Blackburn, J.B.; Hartloge, C.; Puschnik, A.S.; Sambhara, S.; Olson, V.A.; Carette, J.E.; Lupashin, V.; et al. Conserved Oligomeric Golgi (COG) Complex Proteins Facilitate Orthopoxvirus Entry, Fusion and Spread. Viruses 2020, 12, 707. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Hetta, H.F.; Alexiou, A.; Papadakis, M.; Batiha, G.E.-S. Monkeypox epidemic at the door: Should we remain idly by or prepare strongly? AMB Express 2023, 13, 5. [Google Scholar] [CrossRef]
- Zandi, M.; Shafaati, M.; Hosseini, F. Mechanisms of immune evasion of monkeypox virus. Front. Microbiol. 2023, 14, 1106247. [Google Scholar] [CrossRef]
- Patrono, L.V.; Pléh, K.; Samuni, L.; Ulrich, M.; Röthemeier, C.; Sachse, A.; Muschter, S.; Nitsche, A.; Couacy-Hymann, E.; Boesch, C. Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity. Nat. Microbiol. 2020, 5, 955–965. [Google Scholar] [CrossRef]
- Davido, B.; D’Anglejan, E.; Baudoin, R.; Dahmane, L.; Chaud, A.; Cortier, M.; Vauloup-Fellous, C.; De Truchis, P.; Ghosn, J. Monkeypox outbreak 2022: An unusual case of peritonsillar abscess in a person previously vaccinated against smallpox. J. Travel Med. 2022, 29, taac082. [Google Scholar] [CrossRef]
- Cann, J.; Jahrling, P.; Hensley, L.; Wahl-Jensen, V. Comparative pathology of smallpox and monkeypox in man and macaques. J. Comp. Pathol. 2013, 148, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, Q.-Z.; Zhang, H.; Liu, Z.-X.; Chen, X.-H.; Ye, L.-L.; Luo, Y. The land-scape of immune response to monkeypox virus. EBioMedicine 2023, 87, 104424. [Google Scholar] [CrossRef] [PubMed]
- Tsalik, E.L.; Fiorino, C.; Aqeel, A.; Liu, Y.; Henao, R.; Ko, E.R.; Burke, T.W.; Reller, M.E.; Bodinayake, C.K.; Nagahawatte, A. The host response to viral infections reveals common and virus-specific signatures in the peripheral blood. Front. Immunol. 2021, 12, 741837. [Google Scholar] [CrossRef] [PubMed]
- Parnian, R.; Heydarifard, F.; Mousavi, F.S.; Heydarifard, Z.; Zandi, M. Innate Immune Response to Monkeypox Virus Infection: Mechanisms and Immune Escape. J. Innate Immun. 2024, 16, 413–424. [Google Scholar] [CrossRef]
- Fang, D.; Liu, Y.; Dou, D.; Su, B. The unique immune evasion mechanisms of the mpox virus and their implication for developing new vaccines and immunotherapies. Virol. Sin. 2024, 39, 709–718. [Google Scholar] [CrossRef]
- Mellman, I. Dendritic cells: Master regulators of the immune response. Cancer Immunol. Res. 2013, 1, 145–149. [Google Scholar] [CrossRef]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding roles for CD4+ T cells in immunity to viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef]
- Chaudhri, G.; Tahiliani, V.; Eldi, P.; Karupiah, G. Vaccine-induced protection against orthopoxvirus infection is mediated through the combined functions of CD4 T cell-dependent antibody and CD8 T cell responses. J. Virol. 2015, 89, 1889–1899. [Google Scholar] [CrossRef]
- Branda, F.; Romano, C.; Ciccozzi, M.; Giovanetti, M.; Scarpa, F.; Ciccozzi, A.; Maruotti, A. Mpox: An Overview of Pathogenesis, Diagnosis, and Public Health Implications. J. Clin. Med. 2024, 13, 2234. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Rezaei, N. Poxviruses and the immune system: Implications for monkeypox virus. Int. Immunopharmacol. 2022, 113, 109364. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, C.; Chuai, X.; Chiu, S. Monkeypox virus: A re-emergent threat to humans. Virol. Sin. 2022, 37, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.R.; Isaacs, S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008, 225, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, B.; Alonso-Lobo, J.M.; Montanuy, I.; Fischer, C.; Sauer, S.; Sigal, L.; Sevilla, N.; Alcamí, A. A virus-encoded type I interferon decoy receptor enables evasion of host immunity through cell-surface binding. Nat. Commun. 2018, 9, 5440. [Google Scholar] [CrossRef]
- Felix, J.; Savvides, S.N. Mechanisms of immunomodulation by mammalian and viral decoy receptors: Insights from structures. Nat. Rev. Immunol. 2017, 17, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Marco Mdel, M.; Alejo, A.; Hudson, P.; Damon, I.K.; Alcami, A. The highly virulent variola and monkeypox viruses express secreted inhibitors of type I interferon. FASEB J. 2010, 24, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Arndt, W.D.; White, S.D.; Johnson, B.P.; Huynh, T.; Liao, J.; Harrington, H.; Cotsmire, S.; Kibler, K.V.; Langland, J.; Jacobs, B.L. Monkeypox virus induces the synthesis of less dsRNA than vaccinia virus, and is more resistant to the anti-poxvirus drug, IBT, than vaccinia virus. Virology 2016, 497, 125–135. [Google Scholar] [CrossRef]
- Arndt, W.D.; Cotsmire, S.; Trainor, K.; Harrington, H.; Hauns, K.; Kibler, K.V.; Huynh, T.P.; Jacobs, B.L. Evasion of the innate immune type I interferon system by monkeypox virus. J. Virol. 2015, 89, 10489–10499. [Google Scholar] [CrossRef]
- Smith, S.A.; Kotwal, G.J. Virokines: Novel immunomodulatory agents. Expert Opin. Biol. Ther. 2001, 1, 343–357. [Google Scholar] [CrossRef]
- Johnston, J.B.; McFadden, G. Poxvirus immunomodulatory strategies: Current perspectives. J. Virol. 2003, 77, 6093–6100. [Google Scholar] [CrossRef]
- Hudson, P.N.; Self, J.; Weiss, S.; Braden, Z.; Xiao, Y.; Girgis, N.M.; Emerson, G.; Hughes, C.; Sammons, S.A.; Isaacs, S.N. Elucidating the role of the complement control protein in monkeypox pathogenicity. PLoS ONE 2012, 7, e35086. [Google Scholar] [CrossRef]
- Lucena-Neto, F.D.; Falcão, L.F.M.; Vieira-Junior, A.S.; Moraes, E.C.S.; David, J.P.F.; Silva, C.C.; Sousa, J.R.; Duarte, M.I.S.; Vasconcelos, P.F.C.; Quaresma, J.A.S. Monkeypox Virus Immune Evasion and Eye Manifestation: Beyond Eyelid Implications. Viruses 2023, 15, 2301. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, E.; Dasgupta, A.; Pinilla, C.; Norori, P.; Früh, K.; Slifka, M.K. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc. Natl. Acad. Sci. USA 2008, 105, 14567–14572. [Google Scholar] [CrossRef] [PubMed]
- Qudus, M.S.; Cui, X.; Tian, M.; Afaq, U.; Sajid, M.; Qureshi, S.; Liu, S.; Ma, J.; Wang, G.; Faraz, M. Corrigendum: The prospective outcome of the Monkeypox outbreak in 2022 and characterization of monkeypox disease immunobiology. Front. Cell. Infect. Microbiol. 2023, 13, 1284014. [Google Scholar] [CrossRef] [PubMed]
- Sepehrinezhad, A.; Ashayeri Ahmadabad, R.; Sahab-Negah, S. Monkeypox virus from neurological complications to neuroinvasive properties: Current status and future perspectives. J. Neurol. 2022, 270, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kulesh, D.A.; Loveless, B.M.; Norwood, D.; Garrison, J.; Whitehouse, C.A.; Hartmann, C.; Mucker, E.; Miller, D.; Wasieloski, L.P., Jr.; Huggins, J. Monkeypox virus detection in rodents using real-time 3′-minor groove binder TaqMan® assays on the Roche LightCycler. Lab. Investig. 2004, 84, 1200–1208. [Google Scholar] [CrossRef]
- Pastula, D.M.; Copeland, M.J.; Hannan, M.C.; Rapaka, S.; Kitani, T.; Kleiner, E.; Showler, A.; Yuen, C.; Ferriman, E.M.; House, J. Two cases of monkeypox-associated encephalomyelitis—Colorado and the District of Columbia, July–August 2022. Morb. Mortal Wkly. Rep. 2022, 71, 1212–1215. [Google Scholar] [CrossRef]
- Cole, J.; Choudry, S.; Kular, S.; Payne, T.; Akili, S.; Callaby, H.; Gordon, N.C.; Ankcorn, M.; Martin, A.; Hobson, E. Monkeypox encephalitis with transverse myelitis in a female patient. Lancet Infect. Dis. 2022, 23, e115–e120. [Google Scholar] [CrossRef]
- Sergeev, A.; Kabanov, A.; Bulychev, L.; Sergeev, A.; Pyankov, O.; Bodnev, S.; Galahova, D.; Zamedyanskaya, A.; Titova, K.; Glotova, T. Using the ground squirrel (Marmota bobak) as an animal model to assess monkeypox drug efficacy. Transbound. Emerg. Dis. 2017, 64, 226–236. [Google Scholar] [CrossRef]
- Song, H.; Janosko, K.; Johnson, R.F.; Qin, J.; Josleyn, N.; Jett, C.; Byrum, R.; Claire, M.S.; Dyall, J.; Blaney, J.E. Poxvirus antigen staining of immune cells as a biomarker to predict disease outcome in monkeypox and cowpox virus infection in non-human primates. PLoS ONE 2013, 8, e60533. [Google Scholar] [CrossRef]
- Shafaati, M.; Zandi, M. Monkeypox virus neurological manifestations in comparison to other orthopoxviruses. Travel Med. Infect. Dis. 2022, 49, 102414. [Google Scholar] [CrossRef]
- Ježek, Z.; Szczeniowski, M.; Paluku, K.; Mutombo, M. Human monkeypox: Clinical features of 282 patients. J. Infect. Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Benites-Zapata, V.A.; Ulloque-Badaracco, J.R.; Alarcon-Braga, E.A.; Hernandez-Bustamante, E.A.; Mosquera-Rojas, M.D.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Clinical features, hospitalisation and deaths associated with monkeypox: A systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Kondas, A.V.; Mauldin, M.R.; Doty, J.B.; Grossi, I.M.; Morgan, C.N.; Ostergaard, S.D.; Hughes, C.M.; Nakazawa, Y.; Kling, C. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. MSphere 2021, 6, e00927-20. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Yorita, K.L.; Kuehnert, M.J.; Davidson, W.B.; Huhn, G.D.; Holman, R.C.; Damon, I.K. Clinical manifestations of human monkeypox influenced by route of infection. J. Infect. Dis. 2006, 194, 773–780. [Google Scholar] [CrossRef]
- Ligon, B.L. Monkeypox: A review of the history and emergence in the Western hemisphere. Semin. Pediatr. Infect. Dis. 2004, 15, 280–287. [Google Scholar] [CrossRef]
- Kalthan, E.; Dondo-Fongbia, J.; Yambele, S.; Dieu-Creer, L.; Zepio, R.; Pamatika, C. Epidémie de 12 cas de maladie à virus monkeypox dans le district de Bangassou en République Centrafricaine en décembre 2015. Bull. Soc. Pathol. Exot. 2016, 109, 358–363. [Google Scholar] [CrossRef]
- Eseigbe, E.; Akude, C.; Osagie, I.; Eseigbe, P. Human Monkey Pox Virus Infection in Plateau State, North Central Nigeria: A Report of Two Cases. West Afr. J. Med. 2021, 38, 1242–1246. [Google Scholar] [CrossRef]
- Learned, L.A.; Reynolds, M.G.; Wassa, D.W.; Li, Y.; Olson, V.A.; Karem, K.; Stempora, L.L.; Braden, Z.H.; Kline, R.; Likos, A. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am. J. Trop. Med. Hyg. 2005, 73, 428–434. [Google Scholar] [CrossRef]
- Akar, S.; Adesola, Y.-O.; Burga, J.; Oluwafemi, B.; Akinrogbe, J.; Ihekweazu, C. Descriptive epidemiology of monkeypox in Nigeria, September 2017–June 2019. Int. J. Infect. Dis. 2020, 101, 219–220. [Google Scholar] [CrossRef]
- Anderson, M.G.; Frenkel, L.D.; Homann, S.; Guffey, J. A case of severe monkeypox virus disease in an American child: Emerging infections and changing professional values. Pediatr. Infect. Dis. J. 2003, 22, 1093–1096. [Google Scholar] [CrossRef]
- Boumandouki, P.; Bileckot, R.; Ibara, J.; Satounkazi, C.; Libama, E.; Moudzeo, H.; Bolanda, J.; Ngokaba, C. Simian smallpox (or monkey smallpox): Study of 8 cases observed at Impfondo Hospital in Republic of Congo. Bull. Soc. Pathol. Exot. 2007, 100, 17–21. [Google Scholar] [PubMed]
- Croft, D.R.; Sotir, M.J.; Williams, C.J.; Kazmierczak, J.J.; Wegner, M.V.; Rausch, D.; Graham, M.B.; Foldy, S.L.; Wolters, M.; Damon, I.K. Occupational risks during a monkeypox outbreak, Wisconsin, 2003. Emerg. Infect. Dis. 2007, 13, 1150. [Google Scholar] [CrossRef]
- Ogoina, D.; Iroezindu, M.; James, H.I.; Oladokun, R.; Yinka-Ogunleye, A.; Wakama, P.; Otike-Odibi, B.; Usman, L.M.; Obazee, E.; Aruna, O. Clinical course and outcome of human monkeypox in Nigeria. Clin. Infect. Dis. 2020, 71, e210–e214. [Google Scholar] [CrossRef] [PubMed]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J. Outbreak of human monkeypox in Nigeria in 2017–18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.G.; Emerson, G.L.; Pukuta, E.; Karhemere, S.; Muyembe, J.J.; Bikindou, A.; McCollum, A.M.; Moses, C.; Wilkins, K.; Zhao, H. Detection of human monkeypox in the Republic of the Congo following intensive community education. Am. J. Trop. Med. Hyg. 2013, 88, 982. [Google Scholar] [CrossRef]
- Wiwanitkit, S.; Wiwanitkit, V. Atypical zoonotic pox: Acute merging illness that can be easily forgotten. J. Acute Dis. 2018, 7, 88–89. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Datta, P.K.; Maitra, S. Monkeypox and its pandemic potential: What the anaesthetist should know. Br. J. Anaesth. 2022, 129, e49–e52. [Google Scholar] [CrossRef]
- Mittal, R.; Pathak, M.; Jain, A. Neuropsychiatric Manifestations of Mpox (monkeypox) Virus Amidst a Global Outbreak. Prim. Care Companion CNS Disord. 2022, 24, 44747. [Google Scholar] [CrossRef]
- Pappas, G.; Kiriaze, I.J.; Giannakis, P.; Falagas, M.E. Psychosocial consequences of infectious diseases. Clin. Microbiol. Infect. 2009, 15, 743–747. [Google Scholar] [CrossRef]
- Aroyewun, T.F.; Olaleye, S.O.; Adebisi, Y.A.; Yusuf, M. Mental health implications of monkeypox: An urgent need for action. Ann. Med. Surg. 2022, 82, 104771. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Mohammed, A.; Yinka-Ogunleye, A.; Ihekweazu, C. A case of suicide during the 2017 monkeypox outbreak in Nigeria. IJID Reg. 2022, 3, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Cleri, D.J.; Villota, F.J.; Porwancher, R.B. Smallpox, bioterrorism, and the neurologist. Arch. Neurol. 2003, 60, 489–494. [Google Scholar] [CrossRef]
- Marsden, J.P. Acute perivascular myelinoclasis “acute disseminated encephalomyelitis”: A report of two further cases associated with exanthemata. Lancet 1934, 224, 871–872. [Google Scholar]
- CDC (Centers for Disease Control and Prevention (CDC)). What Is Smallpox? Centers for Disease Control and Prevention (CDC). 2017. Available online: https://www.cdc.gov/smallpox/about/index.html (accessed on 10 November 2024).
- Lane, J.M.; Ruben, F.L.; Neff, J.M.; Millar, J.D. Complications of smallpox vaccination, 1968: National surveillance in the United States. N. Engl. J. Med. 1969, 281, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Ziebold, C.; von Kries, R.d.; Lang, R.; Weigl, J.; Schmitt, H.J. Severe complications of varicella in previously healthy children in Germany: A 1-year survey. Pediatrics 2001, 108, e79. [Google Scholar] [CrossRef]
- Crouch, A.E.; Hohman, M.H.; Andaloro, C. Ramsay Hunt Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mallick-Searle, T.; Snodgrass, B.; Brant, J.M. Postherpetic neuralgia: Epidemiology, pathophysiology, and pain management pharmacology. J. Multidiscip. Healthc. 2016, 9, 447. [Google Scholar] [CrossRef]
- McEntire, C.R.; Song, K.-W.; McInnis, R.P.; Rhee, J.Y.; Young, M.; Williams, E.; Wibecan, L.L.; Nolan, N.; Nagy, A.M.; Gluckstein, J. Neurologic manifestations of the World Health Organization’s list of pandemic and epidemic diseases. Front. Neurol. 2021, 12, 634827. [Google Scholar] [CrossRef]
- Ayoade, F.; Kumar, S. Varicella-Zoster Virus (Chickenpox). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ajith Kumar, A.K.; Mendez, M.D. Herpes simplex encephalitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Okikiade, A.; Osharode, A.; Aremu, N.; Chibuike, K.U.; Ogunesan, D.; Olubunmi, O. Kluver Bucy Syndrome: An Overview of the Clinical Interplay. Int. Neuropsychiatr. Dis. J. 2022, 18, 1–7. [Google Scholar] [CrossRef]
- Esparza, J.; Nitsche, A.; Damaso, C.R. Beyond the myths: Novel findings for old paradigms in the history of the smallpox vaccine. PLoS Pathog. 2018, 14, e1007082. [Google Scholar] [CrossRef]
- Riedel, S. Edward Jenner and the history of smallpox and vaccination. Proceedings 2005, 18, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Naleway, A.L. Smallpox vaccine: The good, the bad, and the ugly. Clin. Med. Res. 2003, 1, 87–92. [Google Scholar] [CrossRef] [PubMed]
- WHO. History of Smallpox Vaccine—Smallpox Eradication. Available online: https://www.who.int/news-room/spotlight/history-of-vaccination/history-of-smallpox-vaccination?topicsurvey=ht7j2q)&gad_source=1&gclid=Cj0KCQiA88a5BhDPARIsAFj595hwtayrszkOEmL7zzSuST3yYGDEYykquvTit9QwqBbe98cdMd5NzSQaAhY0EALw_wcB (accessed on 20 June 2024).
- Hammarlund, E.; Lewis, M.W.; Carter, S.V.; Amanna, I.; Hansen, S.G.; Strelow, L.I.; Wong, S.W.; Yoshihara, P.; Hanifin, J.M.; Slifka, M.K. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med. 2005, 11, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.; Jezek, Z.; Grab, B.; Dixon, H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988, 17, 643–650. [Google Scholar] [CrossRef]
- Fenner, F.; Henderson, D.; Arita, I.; Jezek, Z.; Ladnyi, I. Smallpox vaccine and vaccination in the intensified smallpox eradication programme. In Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988; pp. 539–592. Available online: https://iris.who.int/handle/10665/39485 (accessed on 20 November 2024).
- Rizk, J.G.; Lippi, G.; Henry, B.M.; Forthal, D.N.; Rizk, Y. Prevention and treatment of monkeypox. Drugs 2022, 82, 957–963. [Google Scholar] [CrossRef]
- See, K.C. Vaccination for monkeypox virus infection in humans: A review of key considerations. Vaccines 2022, 10, 1342. [Google Scholar] [CrossRef]
- CDC (Centers for Disease Control and Prevention (CDC)). Smallpox Vaccines; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/smallpox/index.html#:~:text=Thanks%20to%20the%20success%20of,occurring%20smallpox%20have%20happened%20since (accessed on 10 November 2024).
- CDC. Clinical Guidance for Smallpox Vaccine Use in a Postevent Vaccination Program; CDC: Atlanta, GA, USA, 2015. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6402a1.htm (accessed on 10 November 2024).
- CDC. Vaccine for Mpox Prevention in the United States. Available online: https://www.cdc.gov/mpox/hcp/vaccine-considerations/index.html (accessed on 10 November 2024).
- McNeil, M.M.; Cano, M.; Miller, E.M.; Petersen, B.W.; Engler, R.J.; Bryant-Genevier, M.G. Ischemic cardiac events and other adverse events following ACAM2000(®) smallpox vaccine in the Vaccine Adverse Event Reporting System. Vaccine 2014, 32, 4758–4765. [Google Scholar] [CrossRef]
- Decker, M.D.; Garman, P.M.; Hughes, H.; Yacovone, M.A.; Collins, L.C.; Fegley, C.D.; Lin, G.; DiPietro, G.; Gordon, D.M. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine 2021, 39, 5541–5547. [Google Scholar] [CrossRef]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. Morb. Mortal Wkly. Rep. 2022, 71, 734. [Google Scholar] [CrossRef]
- Vaughan, A.; Aarons, E.; Astbury, J.; Brooks, T.; Chand, M.; Flegg, P.; Hardman, A.; Harper, N.; Jarvis, R.; Mawdsley, S. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg. Infect. Dis. 2020, 26, 782. [Google Scholar] [CrossRef]
- Khalil, A.; Samara, A.; O’Brien, P.; Morris, E.; Draycott, T.; Lees, C.; Ladhani, S. Monkeypox and pregnancy: What do obstetricians need to know? Ultrasound Obstet. Gynecol. 2022, 60, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Galleguillos, L. Monkeypox and Immunosuppressed Neurological Patients: What can we Advise Vaccination in Multiple Sclerosis and Neuromyelitis Optica Patients? J. ISSN 2022, 2766, 2276. [Google Scholar] [CrossRef]
- CDC (Centers for Disease Control and Prevention (CDC)). Mpox Monitoring and Risk Assessment for Persons Exposed in the Community; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/mpox/php/monitoring/index.html (accessed on 10 November 2024).
- Kemper, A.R.; Davis, M.M.; Freed, G.L. Expected adverse events in a mass smallpox vaccination campaign. Eff. Clin. Pract. 2002, 5, 84–90. [Google Scholar] [PubMed]
- Nicolopoulos, D.; Matsaniotis, N.; Kattamis, C.; Athanassiades, T. Post-vaccinial complications. Report of 45 cases. Helv. Paediatr. Acta 1969, 24, 378–389. [Google Scholar]
- Spillane, J.D.; Wells, C.E. The neurology of Jennerian vaccination: A clinical account of the neurological complications which occurred during the smallpox epidemic in South Wales in 1962. Brain 1964, 87, 1–44. [Google Scholar] [CrossRef]
- Goswamy, B.M. Neurological complication after smallpox vaccination. J. Assoc. Physicians India 1969, 17, 41–43. [Google Scholar]
- Sejvar, J.J.; Labutta, R.J.; Chapman, L.E.; Grabenstein, J.D.; Iskander, J.; Lane, J.M. Neurologic adverse events associated with smallpox vaccination in the United States, 2002–2004. JAMA 2005, 294, 2744–2750. [Google Scholar] [CrossRef]
- Booss, J.; Davis, L.E. Smallpox and smallpox vaccination: Neurological implications. Neurology 2003, 60, 1241–1245. [Google Scholar] [CrossRef]
- Ford, F.R. Diseases of the nervous system in infancy, childhood and adolescence. Arch. Dis. Child. 1947, 35, 628–629. [Google Scholar] [CrossRef]
- Kenner, J.; Cameron, F.; Empig, C.; Jobes, D.V.; Gurwith, M. LC16m8: An attenuated smallpox vaccine. Vaccine 2006, 24, 7009–7022. [Google Scholar] [CrossRef]
- Hraib, M.; Jouni, S.; Albitar, M.M.; Alaidi, S.; Alshehabi, Z. The outbreak of monkeypox 2022: An overview. Ann. Med. Surg. 2022, 104069, 104069. [Google Scholar] [CrossRef] [PubMed]
- Peiró-Mestres, A.; Fuertes, I.; Camprubí-Ferrer, D.; Marcos, M.Á.; Vilella, A.; Navarro, M.; Rodriguez-Elena, L.; Riera, J.; Català, A.; Martínez, M.J. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Eurosurveillance 2022, 27, 2200503. [Google Scholar] [CrossRef] [PubMed]
- Maksyutov, R.A.; Gavrilova, E.V.; Shchelkunov, S.N. Species-specific differentiation of variola, monkeypox, and varicella-zoster viruses by multiplex real-time PCR assay. J. Virol. Methods 2016, 236, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Olson, V.A.; Laue, T.; Laker, M.T.; Damon, I.K. Detection of monkeypox virus with real-time PCR assays. J. Clin. Virol. 2006, 36, 194–203. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Wilkins, K.; Hughes, C.; Damon, I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods 2010, 169, 223–227. [Google Scholar] [CrossRef]
- Li, D.; Wilkins, K.; McCollum, A.M.; Osadebe, L.; Kabamba, J.; Nguete, B.; Likafi, T.; Balilo, M.P.; Lushima, R.S.; Malekani, J. Evaluation of the GeneXpert for human monkeypox diagnosis. Am. J. Trop Med. Hyg. 2017, 96, 405. [Google Scholar] [CrossRef]
- Jezek, Z.; Nakano, J.; Arita, I.; Mutombo, M.; Szczeniowski, M.; Dunn, C. Serological survey for human monkeypox infections in a selected population in Zaire. J. Trop Med. Hyg. 1987, 90, 31–38. [Google Scholar]
- Nörz, D.; Tang, H.T.; Emmerich, P.; Giersch, K.; Fischer, N.; Addo, M.; Aepfelbacher, M.; Pfefferle, S.; Luetgehetmann, M. Rapid adaptation of established high-throughput molecular testing infrastructure for detection of monkeypoxvirus. Emerg. Infect. Dis. 2022, 28, 1765–1769. [Google Scholar] [CrossRef]
- Jarius, S.; Pache, F.; Körtvelyessy, P.; Jelčić, I.; Stettner, M.; Franciotta, D.; Keller, E.; Neumann, B.; Ringelstein, M.; Senel, M. Cerebrospinal fluid findings in COVID-19: A multicenter study of 150 lumbar punctures in 127 patients. J. Neuroinflamm. 2022, 19, 19. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Wiwanitkit, V. Cerebrospinal fluid examination and advantage in clinical diagnosis for COVID-19 and monkeypox. J. Datta Meghe Inst. Med. Sci. Univ. 2022, 17, 175. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Monkeypox: What You Need to Know; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/multi-media/details/monkeypox--what-you-need-to-know (accessed on 10 November 2024).
- GEN. FDA Approves First Smallpox-Indicated Treatment. SIGA’s TPOXX, 13 July 2018.
- Lanier, R.; Trost, L.; Tippin, T.; Lampert, B.; Robertson, A.; Foster, S.; Rose, M.; Painter, W.; O’Mahony, R.; Almond, M. Development of CMX001 for the treatment of poxvirus infections. Viruses 2010, 2, 2740–2762. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.T.; Grosenbach, D.W.; Brasel, T.L.; Baker, R.O.; Cawthon, A.G.; Reynolds, E.; Bailey, T.; Kuehl, P.J.; Sugita, V.; Agans, K. Effects of treatment delay on efficacy of tecovirimat following lethal aerosol monkeypox virus challenge in cynomolgus macaques. J. Infect. Dis. 2018, 218, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Grosenbach, D.W.; Honeychurch, K.; Rose, E.A.; Chinsangaram, J.; Frimm, A.; Maiti, B.; Lovejoy, C.; Meara, I.; Long, P.; Hruby, D.E. Oral tecovirimat for the treatment of smallpox. N. Engl. J. Med. 2018, 379, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, A.; Prigge, J.T.; Silvera, P.M.; Honeychurch, K.M.; Hruby, D.E.; Grosenbach, D.W. Treatment with the smallpox antiviral tecovirimat (ST-246) alone or in combination with ACAM2000 vaccination is effective as a postsymptomatic therapy for monkeypox virus infection. Antimicrob. Agents Chemother. 2015, 59, 4296–4300. [Google Scholar] [CrossRef]
- Sbrana, E.; Jordan, R.; Hruby, D.E.; Mateo, R.I.; Xiao, S.-Y.; Siirin, M.; Newman, P.C.; Da Rosa, A.P.T.; Tesh, R.B. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 2007, 76, 768–773. [Google Scholar] [CrossRef]
- Foster, S.A.; Parker, S.; Lanier, R. The role of brincidofovir in preparation for a potential smallpox outbreak. Viruses 2017, 9, 320. [Google Scholar] [CrossRef]
- Olson, V.A.; Smith, S.K.; Foster, S.; Li, Y.; Lanier, E.R.; Gates, I.; Trost, L.C.; Damon, I.K. In vitro efficacy of brincidofovir against variola virus. Antimicrob. Agents Chemother. 2014, 58, 5570–5571. [Google Scholar] [CrossRef]
- Rice, A.D.; Adams, M.M.; Wallace, G.; Burrage, A.M.; Lindsey, S.F.; Smith, A.J.; Swetnam, D.; Manning, B.R.; Gray, S.A.; Lampert, B. Efficacy of CMX001 as a post exposure antiviral in New Zealand White rabbits infected with rabbitpox virus, a model for orthopoxvirus infections of humans. Viruses 2011, 3, 47–62. [Google Scholar] [CrossRef]
- Mucker, E.M.; Wollen-Roberts, S.E.; Kimmel, A.; Shamblin, J.; Sampey, D.; Hooper, J.W. Intranasal monkeypox marmoset model: Prophylactic antibody treatment provides benefit against severe monkeypox virus disease. PLoS Negl. Trop. Dis. 2018, 12, e0006581. [Google Scholar] [CrossRef]
- Goodbourn, S.; Didcock, L.; Randall, R. Interferons: Cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 2000, 81, 2341–2364. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chandran, D.; Mohapatra, R.K.; Alagawany, M.; Nahed, A.; Sharma, A.K.; Chakraborty, C.; Dhama, K. Clinical management, antiviral drugs and immunotherapeutics for treating monkeypox. An update on current knowledge and futuristic prospects. Int. J. Surg. 2022, 105, 106847. [Google Scholar] [CrossRef] [PubMed]
- Hans, G.H.; Wildemeersch, D.; Meeus, I. Integrated Analgesic Care in the Current Human Monkeypox Outbreak: Perspectives on an Integrated and Holistic Approach Combining Old Allies with Innovative Technologies. Medicina 2022, 58, 1454. [Google Scholar] [CrossRef] [PubMed]
- Pfäfflin, F.; Wendisch, D.; Scherer, R.; Jürgens, L.; Godzick-Njomgang, G.; Tranter, E.; Tober-Lau, P.; Stegemann, M.S.; Corman, V.M.; Kurth, F. Monkeypox in-patients with severe anal pain. Infection 2022, 51, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Farahat, R.A.; Khan, S.H.; Memish, Z.A. Availability of monkeypox vaccinations for low and middle-income countries: Challenges and recommendations. Travel Med. Infect. Dis. 2022, 50, 102473. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Abdulqadir, S.O.; Hussein, S.H.; Omar, R.M.; Ahmed, N.A.; Essa, R.A.; Dhama, K.; Lorenzo, J.M.; Abdulla, A.Q. The impact of monkeypox outbreak on mental health and counteracting strategies: A call to action. Int. J. Surg. 2022, 106, 106943. [Google Scholar] [CrossRef]
- Manirambona, E.; Shomuyiwa, D.O.; Musa, S.S.; Prisno, D., 3rd. Monkeypox among men who have sex with men in Africa: The need for testing and vaccination beyond stigma. J. Med. Virol. 2022, 95, e28121. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Khamisy-Farah, R.; Tsigalou, C.; Mahroum, N.; Converti, M. Attaching a stigma to the LGBTQI+ community should be avoided during the monkeypox epidemic. J. Med. Virol. 2023, 95, e27913. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).