Synergistic Effect of Co-Administered SARS-CoV-2 Vaccines Improves Immune Responses in BALB/c Mice: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. SARS-CoV-2 Vaccines Used in This Study
2.2. Animal Model and Immunization Protocol
2.3. Sample Collection
2.4. ELISA for SARS-CoV-2 Spike-Specific IgG
2.5. Total RNA Extraction and cDNA Synthesis
2.6. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
2.7. Histopathological Analysis
2.8. Statistical Analysis
3. Results
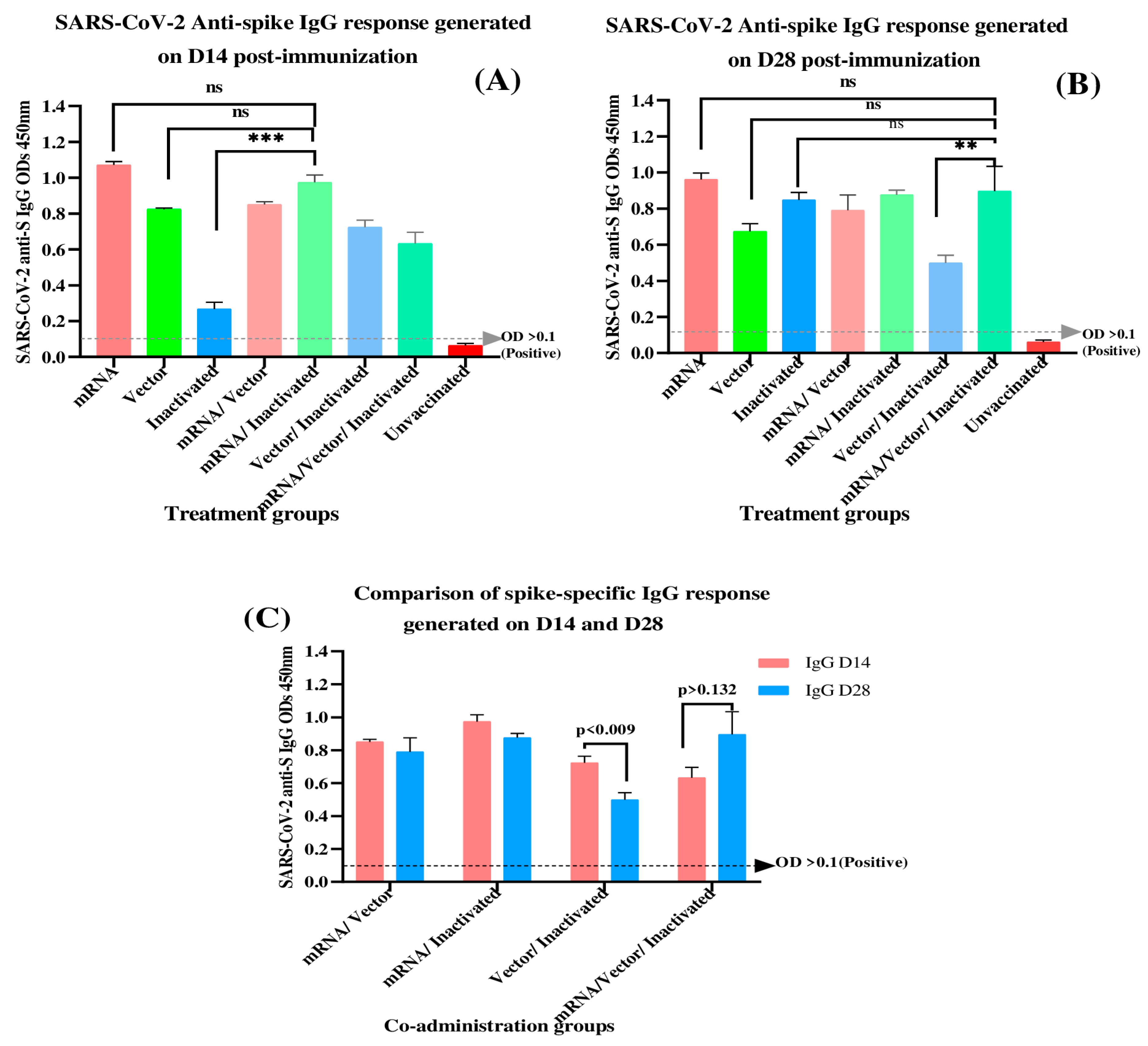
3.1. Determination of SARS-CoV-2 Spike-Specific IgG Antibodies at 14- and 28-Days Post-Immunization
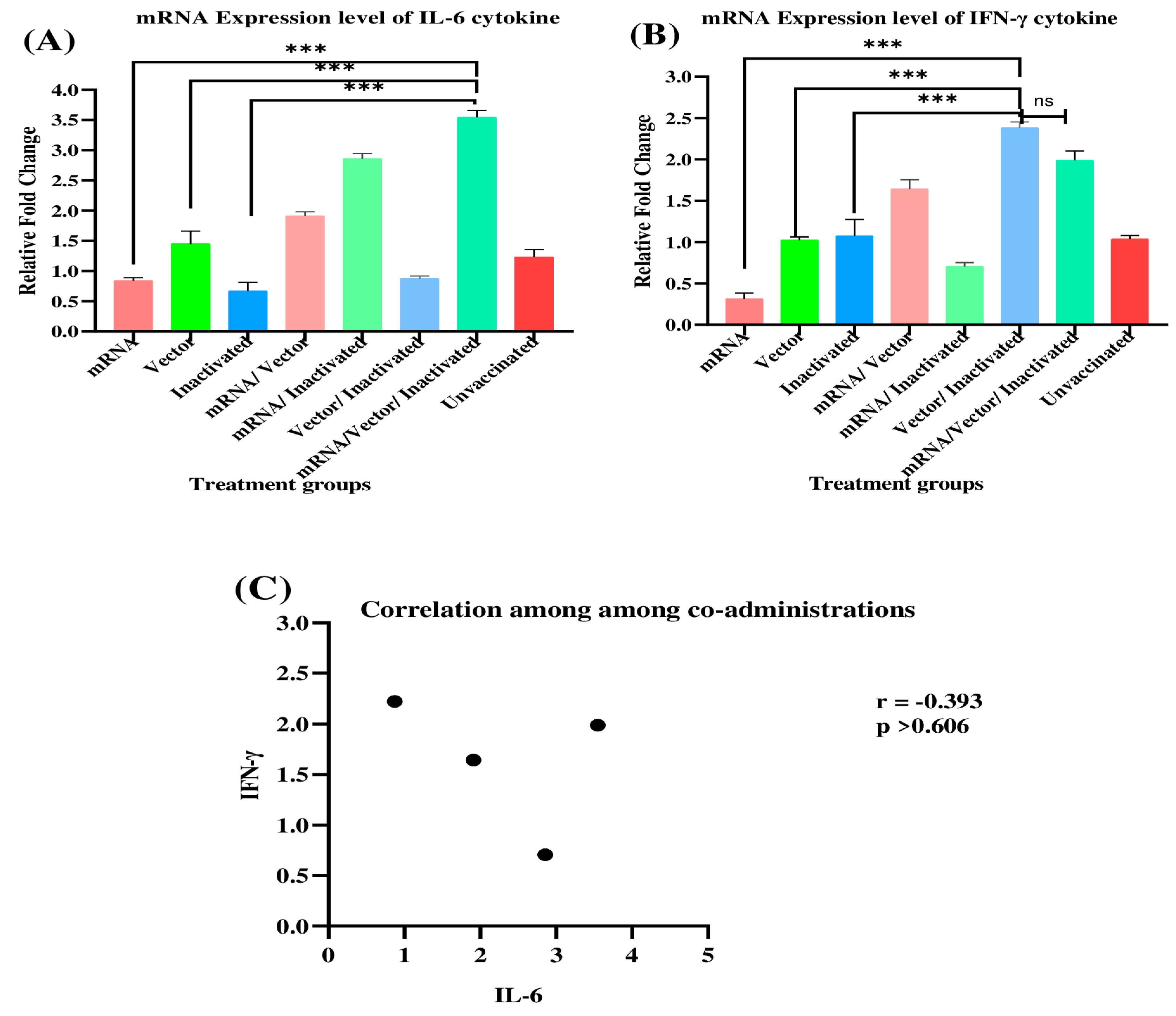
3.2. Evaluation of IL-6 and IFN-γ mRNA Expression Profiles in Immunized BALB/c Mice
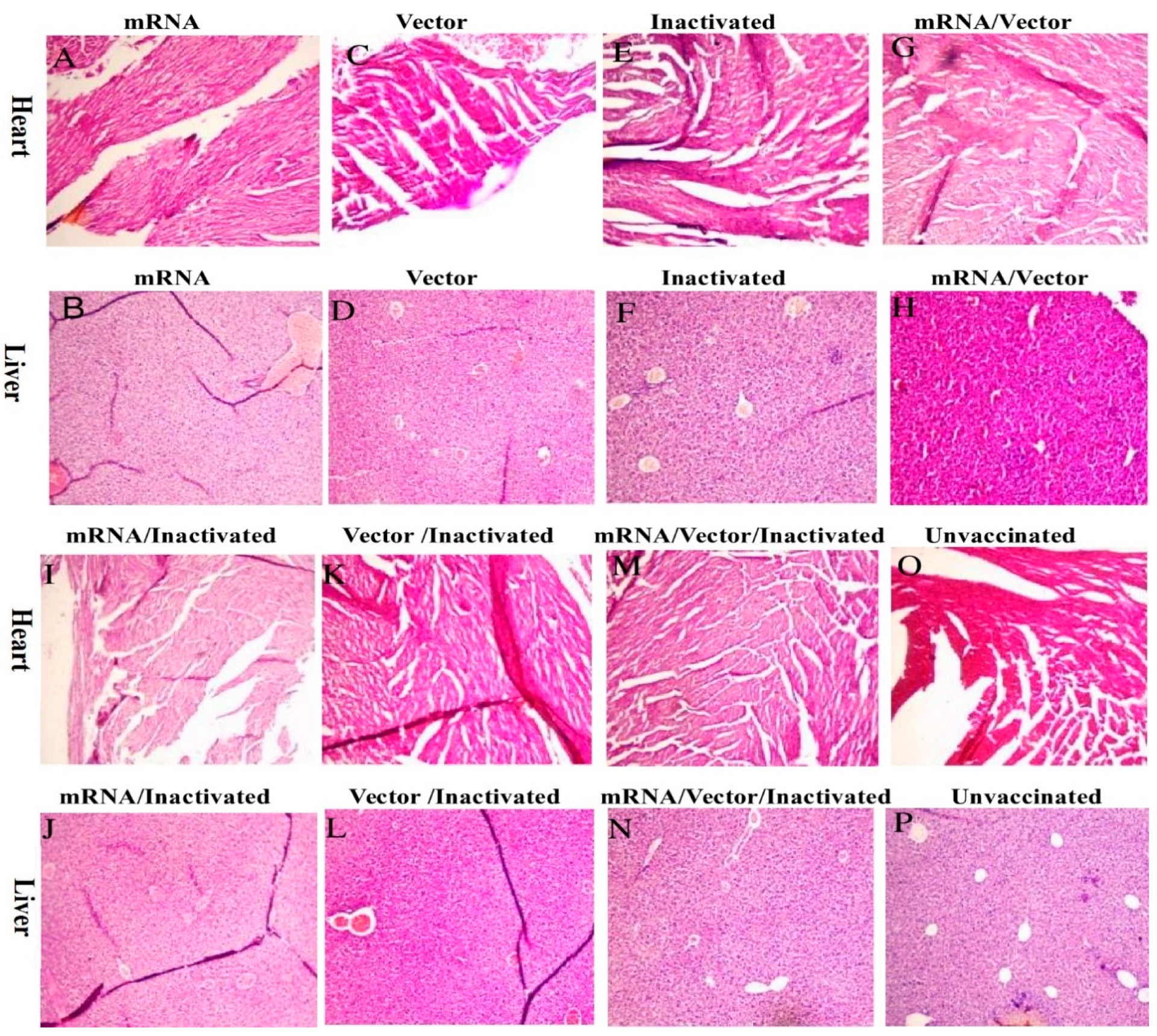
3.3. Safety Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Chang, X.; He, Y.; Tan, K.J.K. The Determinants of COVID-19 Morbidity and Mortality across Countries. Sci. Rep. 2022, 12, 5888. [Google Scholar] [CrossRef] [PubMed]
- Richards, F.; Kodjamanova, P.; Chen, X.; Li, N.; Atanasov, P.; Bennetts, L.; Patterson, B.J.; Yektashenas, B.; Mesa-Frias, M.; Tronczynski, K.; et al. Economic Burden of COVID-19: A Systematic Review. Clin. Outcomes Res. 2022, 14, 293–307. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronavirus (COVID-19) Dashboard. Geneva: World Health Organization. 2023. Available online: https://covid19.who.int/ (accessed on 11 November 2023).
- Chams, N.; Chams, S.; Badran, R.; Shams, A.; Araji, A.; Raad, M.; Mukhopadhyay, S.; Stroberg, E.; Duval, E.J.; Barton, L.M.; et al. COVID-19: A Multidisciplinary Review. Front. Public Health 2020, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, V.; Di Lucia, P.; Ravà, M.; Marotta, D.; Bono, E.; Grassi, S.; Donnici, L.; Cannalire, R.; Stefanelli, I.; Ferraro, A.; et al. Nirmatrelvir Treatment of SARS-CoV-2-infected Mice Blunts Antiviral Adaptive Immune Responses. EMBO Mol. Med. 2023, 15, e17580. [Google Scholar] [CrossRef]
- Panza, F.; Fiorino, F.; Pastore, G.; Fiaschi, L.; Tumbarello, M.; Medaglini, D.; Ciabattini, A.; Montagnani, F.; Fabbiani, M. Does Nirmatrelvir/Ritonavir Influence the Immune Response against SARS-CoV-2, Independently from Rebound? Microorganisms 2023, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Pant, A.B. Efforts at COVID-19 Vaccine Development: Challenges and Successes. Vaccines 2020, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H. Covid-19 Vaccines—Immunity, Variants, Boosters. N. Engl. J. Med. 2022, 387, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Dolan, C.; Barouch, D.H. COVID-19 Vaccines: Adenoviral Vectors. Annu. Rev. Med. 2022, 73, 41–54. [Google Scholar] [CrossRef]
- Gebre, M.S.; Rauch, S.; Roth, N.; Gergen, J.; Yu, J.; Liu, X.; Cole, A.C.; Mueller, S.O.; Petsch, B.; Barouch, D.H. MRNA Vaccines Induce Rapid Antibody Responses in Mice. NPJ Vaccines 2022, 7, 88. [Google Scholar] [CrossRef]
- Kanokudom, S.; Assawakosri, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Yorsaeng, R.; Srimuan, D.; Thatsanatorn, T.; et al. Safety and Immunogenicity of the Third Booster Dose with Inactivated, Viral Vector, and MRNA COVID-19 Vaccines in Fully Immunized Healthy Adults with Inactivated Vaccine. Vaccines 2022, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Van Dromme, I.; Spiessens, B.; et al. Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S. N. Engl. J. Med. 2022, 386, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and Safety of COVID-19 Vaccines. Cochrane Database Syst. Rev. 2022, 2023. [Google Scholar] [CrossRef]
- Piechotta, V.; Harder, T. Waning of COVID-19 Vaccine Effectiveness: Individual and Public Health Risk. Lancet 2022, 399, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Belayachi, J.; Obtel, M.; Mhayi, A.; Razine, R.; Abouqal, R. Long Term Effectiveness of Inactivated Vaccine BBIBP-CorV (Vero Cells) against COVID-19 Associated Severe and Critical Hospitalization in Morocco. PLoS ONE 2022, 17, e0278546. [Google Scholar] [CrossRef] [PubMed]
- Deming, M.E.; Lyke, K.E. A ‘Mix and Match’ Approach to SARS-CoV-2 Vaccination. Nat. Med. 2021, 27, 1510–1511. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.C.; Cravioto, A.; Rees, H.; Higgins, J.P.T.; Boutron, I.; et al. Considerations in Boosting COVID-19 Vaccine Immune Responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Camargo, C.A.; Fal, A.; Flisiak, R.; Gwenzi, W.; Kelishadi, R.; Leemans, A.; Nieto, J.J.; Ozen, A.; Perc, M.; et al. COVID-19 Vaccine Boosters: The Good, the Bad, and the Ugly. Vaccines 2021, 9, 1299. [Google Scholar] [CrossRef]
- Zhang, J.; He, Q.; An, C.; Mao, Q.; Gao, F.; Bian, L.; Wu, X.; Wang, Q.; Liu, P.; Song, L.; et al. Boosting with Heterologous Vaccines Effectively Improves Protective Immune Responses of the Inactivated SARS-CoV-2 Vaccine. Emerg. Microbes Infect. 2021, 10, 1598–1608. [Google Scholar] [CrossRef]
- Bauwens, J.; De Lusignan, S.; Weldesselassie, Y.G.; Sherlock, J.; Künzli, N.; Bonhoeffer, J. Safety of Routine Childhood Vaccine Coadministration versus Separate Vaccination. BMJ Glob. Health 2022, 7, e008215. [Google Scholar] [CrossRef]
- Wagenhäuser, I.; Reusch, J.; Gabel, A.; Höhn, A.; Lâm, T.T.; Almanzar, G.; Prelog, M.; Krone, L.B.; Frey, A.; Schubert-Unkmeir, A.; et al. Immunogenicity and Safety of Coadministration of COVID-19 and Influenza Vaccination. Eur. Respir. J. 2023, 61, 2201390. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, J.S.; Clemmensen, H.S.; Battey, H.; Dijkman, K.; Lindenstrøm, T.; Laureano, R.S.; Taplitz, R.; Morgan, J.; Aagaard, C.; Rosenkrands, I.; et al. A Mycobacterium Tuberculosis-Specific Subunit Vaccine That Provides Synergistic Immunity upon Co-Administration with Bacillus Calmette-Guérin. Nat. Commun. 2021, 12, 6658. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Zhao, Y.; Lung, D.C.; Ye, Z.; Song, W.; Liu, F.-F.; Cai, J.-P.; Wong, W.-M.; Yip, C.C.-Y.; et al. Intravenous Injection of Coronavirus Disease 2019 (COVID-19) MRNA Vaccine Can Induce Acute Myopericarditis in Mouse Model. Clin. Infect. Dis. 2022, 74, 1933–1950. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Güler, A.; et al. BNT162b Vaccines Protect Rhesus Macaques from SARS-CoV-2. Nature 2021, 592, 283–289. [Google Scholar] [CrossRef]
- Celise, D.A.; Kimotho, J.; Kimani, J.W.; Muriithi, A.K.; Odari, E.O. Increase in the Immune Response in Balb/c Mice after the Co-Administration of a Vector-Based COVID-19 Vaccine with Cytosine Phosphoguanine Oligodeoxynucleotide. Vaccines 2022, 11, 53. [Google Scholar] [CrossRef]
- Mudenda, M.; Kimani, J.; Kinyua, J.; Kimotho, J. Preliminary In Vivo Evidence of Oral Selenium Supplementation as a Potentiating Agent on a Vector-Based COVID-19 Vaccine in BALB/c Mice. Vaccines 2022, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Mao, Q.; An, C.; Zhang, J.; Gao, F.; Bian, L.; Li, C.; Liang, Z.; Xu, M.; Wang, J. Heterologous Prime-Boost: Breaking the Protective Immune Response Bottleneck of COVID-19 Vaccine Candidates. Emerg. Microbes Infect. 2021, 10, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, A.; Mostafa, A.; Hegazy, R.R.; El-Shesheny, R.; El Taweel, A.; Gomaa, M.R.; Shehata, M.; Elbaset, M.A.; Kayed, A.E.; Mahmoud, S.H.; et al. Immunogenicity and Safety of an Inactivated SARS-CoV-2 Vaccine: Preclinical Studies. Vaccines 2021, 9, 214. [Google Scholar] [CrossRef]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef]
- Sakurai, F.; Tachibana, M.; Mizuguchi, H. Adenovirus Vector-Based Vaccine for Infectious Diseases. Drug Metab. Pharmacokinet. 2022, 42, 100432. [Google Scholar] [CrossRef]
- Bergami, F.; Arena, F.; Sammartino, J.C.; Ferrari, A.; Zavaglio, F.; Zelini, P.; Paolucci, S.; Comolli, G.; Percivalle, E.; Lilleri, D.; et al. Differential Kinetics of Effector and Memory Responses Induced by Three Doses of SARS-CoV-2 MRNA Vaccine in a Cohort of Healthcare Workers. Vaccines 2022, 10, 1809. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Ma, D.; Duan, S.; Zhang, J.; Yue, R.; Li, X.; Gao, Y.; Li, X.; Zeng, F.; Xu, X.; et al. Immunological Study of Combined Administration of SARS-CoV-2 DNA Vaccine and Inactivated Vaccine. Vaccines 2022, 10, 929. [Google Scholar] [CrossRef]
- Torresi, J.; Edeling, M.A.; Nolan, T.; Godfrey, D.I. A Complementary Union of SARS-CoV2 Natural and Vaccine Induced Immune Responses. Front. Immunol. 2022, 13, 914167. [Google Scholar] [CrossRef] [PubMed]
- Shenyu, W.; Xiaoqian, D.; Bo, C.; Xuan, D.; Zeng, W.; Hangjie, Z.; Qianhui, Z.; Zhenzhen, L.; Chuanfu, Y.; Juan, Y.; et al. Immunogenicity and Safety of a SARS-CoV-2 Inactivated Vaccine (CoronaVac) Co-Administered with an Inactivated Quadrivalent Influenza Vaccine: A Randomized, Open-Label, Controlled Study in Healthy Adults Aged 18 to 59 Years in China. Vaccine 2022, 40, 5356–5365. [Google Scholar] [CrossRef]
- Lv, J.; Wu, H.; Xu, J.; Liu, J. Immunogenicity and Safety of Heterologous versus Homologous Prime-Boost Schedules with an Adenoviral Vectored and MRNA COVID-19 Vaccine: A Systematic Review. Infect. Dis. Poverty 2022, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-Dose Administration and the Influence of the Timing of the Booster Dose on Immunogenicity and Efficacy of ChAdOx1 NCoV-19 (AZD1222) Vaccine: A Pooled Analysis of Four Randomised Trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of Standard and Extended Dosing Intervals of BNT162b2 MRNA Vaccine. Cell 2021, 184, 5699–5714.e11. [Google Scholar] [CrossRef]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral Vectored Vaccines: Design, Development, Preventive and Therapeutic Applications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef]
- Ai, J.; Guo, J.; Zhang, H.; Zhang, Y.; Yang, H.; Lin, K.; Song, J.; Fu, Z.; Fan, M.; Zhang, Q.; et al. Cellular Basis of Enhanced Humoral Immunity to SARS-CoV-2 upon Homologous or Heterologous Booster Vaccination Analyzed by Single-Cell Immune Profiling. Cell Discov. 2022, 8, 114. [Google Scholar] [CrossRef]
- Jiang, M.; Väisänen, E.; Kolehmainen, P.; Huttunen, M.; Ylä-Herttuala, S.; Meri, S.; Österlund, P.; Julkunen, I. COVID-19 Adenovirus Vector Vaccine Induces Higher Interferon and pro-Inflammatory Responses than MRNA Vaccines in Human PBMCs, Macrophages and MoDCs. Vaccine 2023, 41, 3813–3823. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.B.; Im, S.J.; Namkoong, H.; Kim, S.W.; Choi, Y.W.; Kang, M.C.; Lim, H.S.; Jin, H.T.; Yang, S.H.; Cho, M.L.; et al. Crucial Roles of Interleukin-7 in the Development of T Follicular Helper Cells and in the Induction of Humoral Immunity. J. Virol. 2014, 88, 8998–9009. [Google Scholar] [CrossRef] [PubMed]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Yu, J. Fighting Fire with Fire: Immunogenicity of Viral Vectored Vaccines against COVID-19. Viruses. 2022, 14, 380. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 Vaccine BNT162b1 Elicits Human Antibody and TH1 T Cell Responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Vrdoljak, K.; Brlek, P.; Pavelić, E.; Molnar, V.; Matišić, V.; Erceg Ivkošić, I.; Parčina, M. Adaptive Immune Responses and Immunity to SARS-CoV-2. Front. Immunol. 2022, 13, 848582. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 Infection: The Role of Cytokines in COVID-19 Disease. Cytokine Growth Fac. Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Qudus, M.S.; Tian, M.; Sirajuddin, S.; Liu, S.; Afaq, U.; Wali, M.; Liu, J.; Pan, P.; Luo, Z.; Zhang, Q.; et al. The Roles of Critical Pro-inflammatory Cytokines in the Drive of Cytokine Storm during SARS-CoV-2 Infection. J. Med. Virol. 2023, 95, e28751. [Google Scholar] [CrossRef]
| SARS-CoV-2 Vaccines | Platforms | Doses Administered in Mice Studies (per Mouse) |
|---|---|---|
| COMIRNATY COVID-19 (Pfizer-BioNTech) vaccine. Lot Number: GN6343 | mRNA (nucleoside modified) vaccine | 5 µg [25,26] |
| Janssen (Ad26.COV2.S (recombinant)) vaccine. Lot number: ACB6959 | Adenovirus26-vectored vaccine | 4 × 109 VP [27,28] |
| SARS-CoV-2 vaccine (Vero Cell), Inactivated (Sinopharm) Product Code: 2021071947 | Inactivated vaccine | 0.8 µg [21,29] |
| Treatment Groups | Treatment and Immunization Protocol |
|---|---|
| mRNA | 5 µg (50 μL) of Pfizer-BioNTech vaccine on D0 and D14 |
| Vector | 4 × 109 VP (40 μL) of Janssen vaccine on D0 |
| Inactivated | 0.8 µg (100 μL) of Sinopharm vaccine on D0 and D14 |
| mRNA/Vector | Coadministration of 5 µg (50 μL) Pfizer-BioNTech and 4 × 109 VP (40 μL) Janssen on D0 |
| mRNA/Inactivated | Coadministration of 5 µg (50 μL) Pfizer-BioNTech and 0.8 µg (100 μL) Sinopharm on D0 |
| Vector /Inactivated | Coadministration of 4 × 109 VP (40 μL) Janssen and 0.8 µg (100 μL) Sinopharm on D0 |
| mRNA/Vector/Inactivated | Coadministration of 5 µg of Pfizer-BioNTech (50 µL), 4 × 109 VP (40 µL) of Janssen and 0.8 µg (100 µL) of Sinopharm on D0 |
| Unvaccinated | 50 μL of 1× phosphate-buffered saline (PBS) on D0 |
| Gene of Interest | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Amplicon Size (bp) | Tm (°C) | %GC | NCBI Accession |
|---|---|---|---|---|---|---|
| IL-6 | CCCACCAGGA ACGAAAGTCA | ACTGGCTGGA AGTCTCTTGC | 70 | 59.89 59.96 | 55.00 55.00 | https://www.ncbi.nlm.nih.gov/nucleotide/930945755# (accessed on 5 September 2023) |
| IFN-γ | GGATGCATTCA TGAGTATTGC | CCTTTTCCGC TTCCTGAGG | 127 | 55.42 58.14 | 42.86 57.89 | https://www.ncbi.nlm.nih.gov/nucleotide/926657655# (accessed on 5 September 2023) |
| HPRT1 | TGAAGTACTCATTG ATAGTCAAGGGCA | CTGGTGAAAA GGACCTCTCG | 109 | 61.94 57.91 | 40.74 55.00 | https://www.ncbi.nlm.nih.gov/nucleotide/96975137# (accessed on 5 September 2023) |
| Treatment Groups | Day 14 | Day 28 | ||||
|---|---|---|---|---|---|---|
| Mean | SD | CV (%) | Mean | SD | CV (%) | |
| mRNA | 1.070 | 0.039 | 3.65 | 0.962 | 0.068 | 7.16 |
| Vector | 0.824 | 0.013 | 1.68 | 0.674 | 0.086 | 12.89 |
| Inactivated | 0.268 | 0.074 | 27.7 | 0.849 | 0.081 | 9.57 |
| mRNA/Vector | 0.851 | 0.031 | 3.66 | 0.790 | 0.171 | 21.68 |
| mRNA/Inactivated | 0.975 | 0.081 | 8.32 | 0.877 | 0.051 | 5.84 |
| Vector/Inactivated | 0.724 | 0.080 | 11.1 | 0.499 | 0.087 | 17.61 |
| mRNA/Vector/Inactivated | 0.633 | 0.125 | 19.8 | 0.897 | 0.276 | 30.77 |
| Unvaccinated | 0.063 | 0.023 | 37.4 | 0.060 | 0.021 | 35.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonas, N.; Kimani, J.; Kimotho, J.; Munyao, M.M.; Nzou, S.M. Synergistic Effect of Co-Administered SARS-CoV-2 Vaccines Improves Immune Responses in BALB/c Mice: A Preliminary Study. Immuno 2024, 4, 172-185. https://doi.org/10.3390/immuno4020012
Jonas N, Kimani J, Kimotho J, Munyao MM, Nzou SM. Synergistic Effect of Co-Administered SARS-CoV-2 Vaccines Improves Immune Responses in BALB/c Mice: A Preliminary Study. Immuno. 2024; 4(2):172-185. https://doi.org/10.3390/immuno4020012
Chicago/Turabian StyleJonas, Nshimirimana, Josephine Kimani, James Kimotho, Matthew Mutinda Munyao, and Samson Muuo Nzou. 2024. "Synergistic Effect of Co-Administered SARS-CoV-2 Vaccines Improves Immune Responses in BALB/c Mice: A Preliminary Study" Immuno 4, no. 2: 172-185. https://doi.org/10.3390/immuno4020012
APA StyleJonas, N., Kimani, J., Kimotho, J., Munyao, M. M., & Nzou, S. M. (2024). Synergistic Effect of Co-Administered SARS-CoV-2 Vaccines Improves Immune Responses in BALB/c Mice: A Preliminary Study. Immuno, 4(2), 172-185. https://doi.org/10.3390/immuno4020012






