Monoclonal War: The Antibody Arsenal and Targets for Expanded Application
Abstract
:1. Introduction
2. Methods
3. The Biological Role of Antibodies in the Normal Human Immune Response
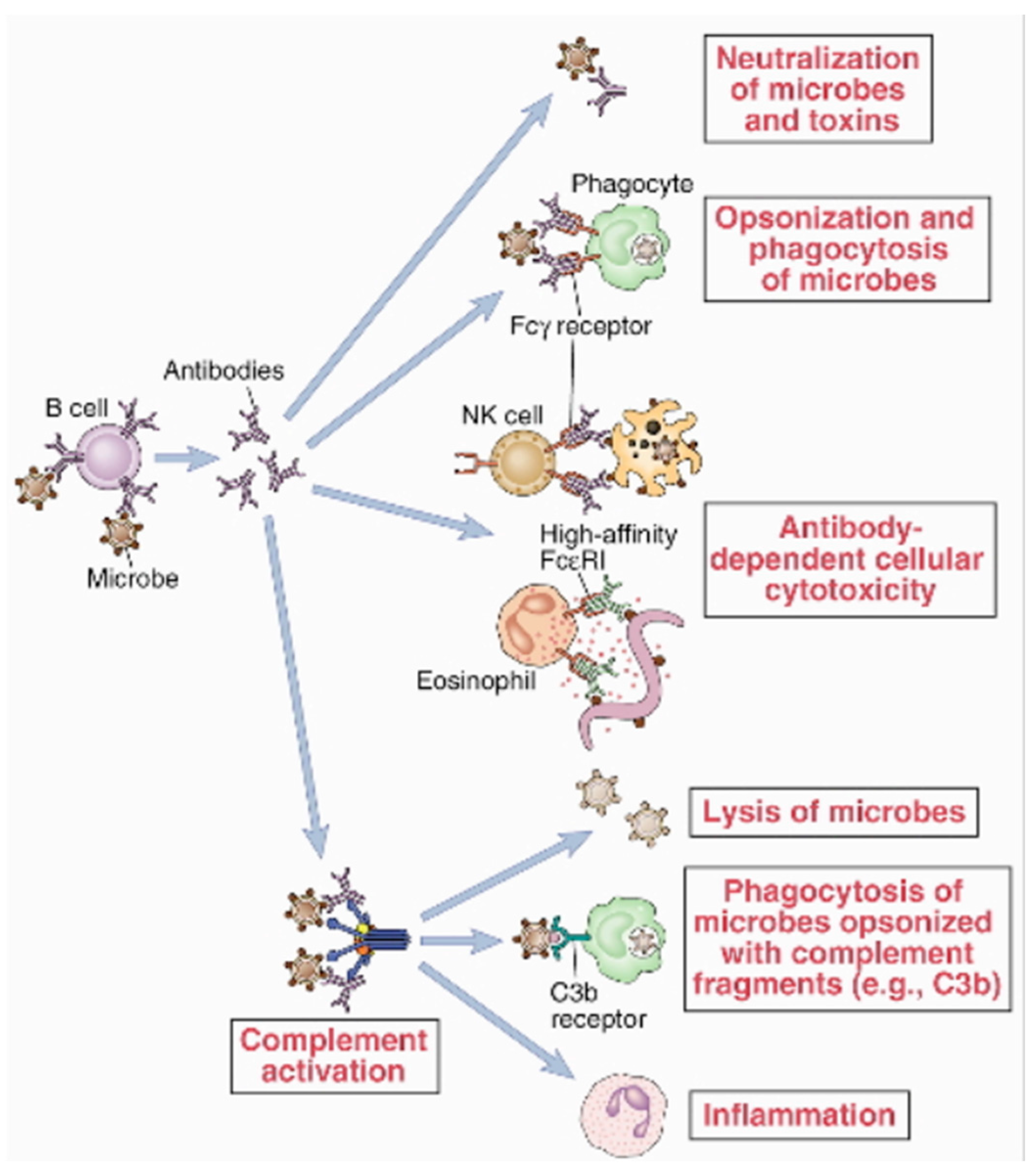
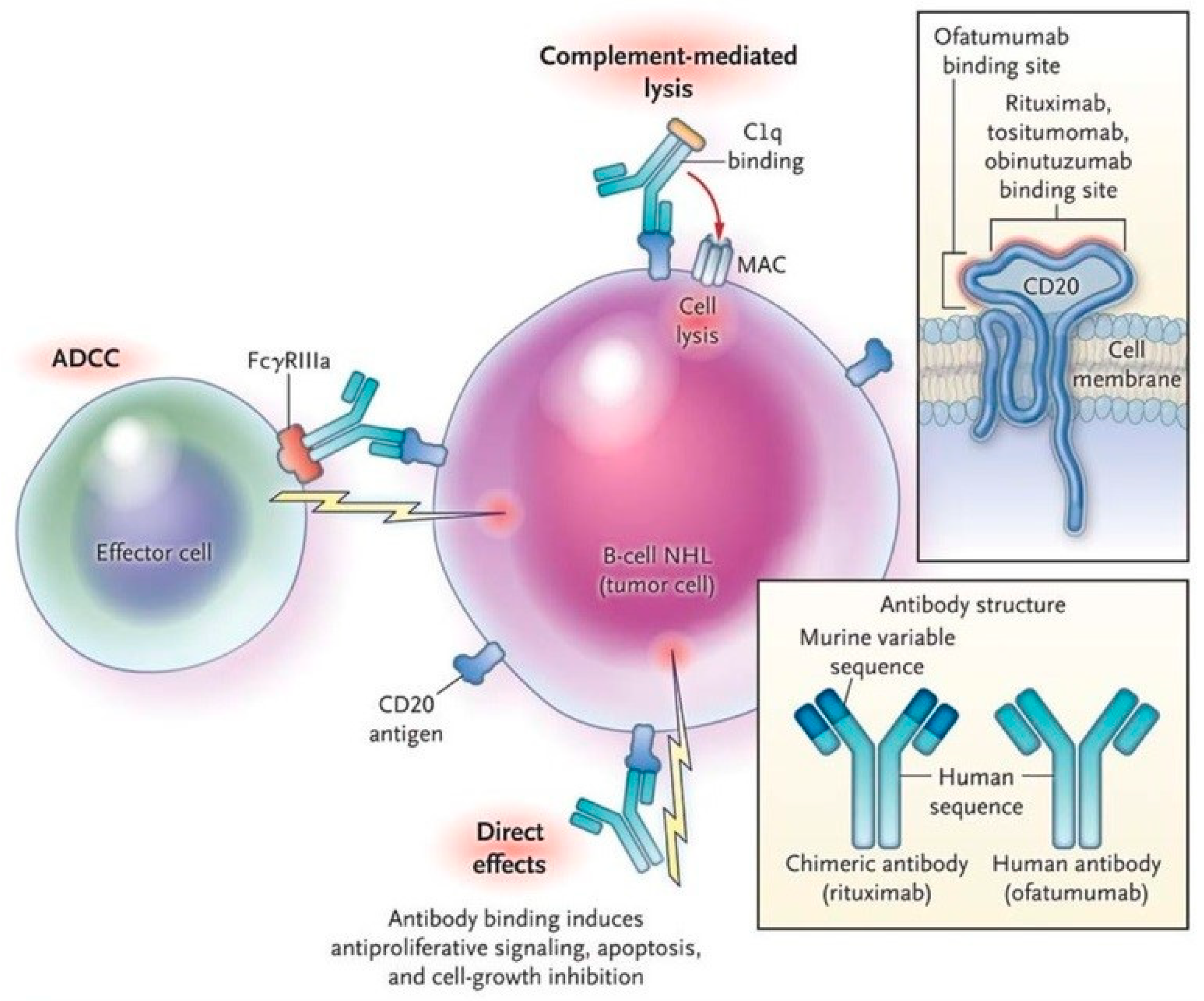
4. Production of mABs and Therapeutic Dynamics
5. Examples of Antibody Treatment Mechanisms Applied in Current Disease
6. Advances in Development and Emerging Treatments
7. Obstacles in Development
8. Autoimmune Neuropathies as an Example of the Challenges in the Current Research
9. Addressing Challenges and Future Directions: mABs as the Therapeutic Swiss-Army Knife
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takemasa, E.; Liu, S.; Hasegawa, H. Production of Neutralizing Antibody. Methods Mol. Biol. 2018, 1868, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Minnee, J.; Landau, N.R. Vectored immunoprophylaxis and treatment of SARS-CoV-2 infection in a preclinical model. Proc. Natl. Acad. Sci. USA 2023, 120, e2303509120. [Google Scholar] [CrossRef] [PubMed]
- Sanatkar, S.A.; Heidari, A.; Arya, S.; Ghasemi, M.; Rezaei, N. The potential role of immunotherapy in Wilms' tumor: Opportunities and challenges. Curr. Pharm. Des. 2023, 29, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Goulet, D.R.; Atkins, W.M. Considerations for the Design of Antibody-Based Therapeutics. J. Pharm. Sci. 2019, 109, 74–103. [Google Scholar] [CrossRef] [PubMed]
- Zivojnovic, M. Somatic Hypermutation of Immunoglobulin Genes: Correlation with the Cell Cycle and Contribution of Mutagenic Repair Pathways. Ph.D. Thesis, Université René Descartes-Paris V, Paris, France, 2013. [Google Scholar]
- Parola, C.; Neumeier, D.; Reddy, S.T. Integrating high-throughput screening and sequencing for monoclonal antibody discovery and engineering. Immunology 2017, 153, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Maixnerova, D.; Tesar, V. Emerging role of monoclonal antibodies in the treatment of IgA nephropathy. Expert Opin. Biol. Ther. 2023, 23, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Balocco, R.; Koch, S.D.S.G.; Thorpe, R.; Weisser, K.; Malan, S. New INN nomenclature for monoclonal antibodies. Lancet 2022, 399, 24. [Google Scholar] [CrossRef]
- Manso, T.; Kushwaha, A.; Abdollahi, N.; Duroux, P.; Giudicelli, V.; Kossida, S. Mechanisms of action of monoclonal antibodies in oncology integrated in IMGT/mAb-DB. Front. Immunol. 2023, 14, 1129323. [Google Scholar] [CrossRef]
- Alfaleh, M.A.; Alsaab, H.O.; Mahmoud, A.B.; Alkayyal, A.A.; Jones, M.L.; Mahler, S.M.; Hashem, A.M. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front. Immunol. 2020, 11, 1986. [Google Scholar] [CrossRef]
- Carter, P.J.; Lazar, G.A. Next generation antibody drugs: Pursuit of the ‘high-hanging fruit’. Nat. Rev. Drug Discov. 2017, 17, 197–223. [Google Scholar] [CrossRef]
- Fischman, S.; Ofran, Y. Computational design of antibodies. Curr. Opin. Struct. Biol. 2018, 51, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quintero, M.L.; Ljungars, A.; Waibl, F.; Greiff, V.; Andersen, J.T.; Gjølberg, T.T.; Jenkins, T.P.; Voldborg, B.G.; Grav, L.M.; Kumar, S.; et al. Assessing developability early in the discovery process for novel biologics. mAbs 2023, 15, 2171248. [Google Scholar] [CrossRef]
- Maloney, D.G. Anti-CD20 antibody therapy for B-cell lymphomas. N. Engl. J. Med. 2012, 366, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Tsao, L.-C.; Force, J.; Hartman, Z.C. Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res. 2021, 81, 4641–4651. [Google Scholar] [CrossRef]
- Royce, M.; Osgood, C.L.; Amatya, A.K.; Fiero, M.H.; Chang, C.G.; Ricks, T.K.; Shetty, K.A.; Kraft, J.; Qiu, J.; Song, P.; et al. FDA Approval Summary: Margetuximab plus Chemotherapy for Advanced or Metastatic HER2-Positive Breast Cancer. Clin. Cancer Res. 2021, 28, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Zinn, S.; Vazquez-Lombardi, R.; Zimmermann, C.; Sapra, P.; Jermutus, L.; Christ, D. Advances in antibody-based therapy in oncology. Nat. Cancer 2023, 4, 165–180. [Google Scholar] [CrossRef]
- Petric, Z.; Goncalves, J.; Paixao, P. Under the Umbrella of Clinical Pharmacology: Inflammatory Bowel Disease, Infliximab and Adalimumab, and a Bridge to an Era of Biosimilars. Pharmaceutics 2022, 14, 1766. [Google Scholar] [CrossRef]
- Lázár-Molnár, E.; Delgado, J.C. Implications of Monoclonal Antibody Therapeutics Use for Clinical Laboratory Testing. Clin. Chem. 2019, 65, 393–405. [Google Scholar] [CrossRef]
- Vaisman-Mentesh, A.; Gutierrez-Gonzalez, M.; DeKosky, B.J.; Wine, Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation following Treatment with Monoclonal Antibodies. Front. Immunol. 2020, 11, 1951. [Google Scholar] [CrossRef]
- Cardoso Alves, L.; Corazza, N.; Micheau, O.; Krebs, P. The multifaceted role of TRAIL signaling in cancer and immunity. FEBS J. 2021, 288, 5530–5554. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wang, L.; Luo, D.; Soni, V.; Rosenn, E.H.; Wang, Z. Mycobacterium smegmatis, a Promising Vaccine Vector for Preventing TB and Other Diseases: Vaccinomics Insights and Applications. Vaccines 2023, 11, 1302. [Google Scholar] [CrossRef] [PubMed]
- Pysz, I.; Jackson, P.J.M.; Thurston, D.E. Introduction to antibody–drug conjugates (adcs). In Cytotoxic Payloads for Antibody–Drug Conjugates; Thurston, D.E., Jackson, P.J.M., Eds.; The Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Motley, M.P.; Banerjee, K.; Fries, B.C. Monoclonal antibody-based therapies for bacterial infections. Curr. Opin. Infect. Dis. 2019, 32, 210–216. [Google Scholar] [CrossRef]
- Beretta, M.; Mouquet, H. Advances in human monoclonal antibody therapy for HBV infection. Curr. Opin. Virol. 2022, 53, 101205. [Google Scholar] [CrossRef]
- Shirazi, F.G.; Mohammadi, H.; Amiri, M.M.; Singethan, K.; Xia, Y.; Bayat, A.A.; Bahadori, M.; Rabbani, H.; Jeddi-Tehrani, M.; Protzer, U.; et al. Monoclonal antibodies to various epitopes of hepatitis B surface antigen inhibit hepatitis B virus infection. J. Gastroenterol. Hepatol. 2013, 29, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Politch, J.A.; Cone, R.A.; Zeitlin, L.; Lai, S.K.; Santangelo, P.J.; Moench, T.R.; Whaley, K.J. Engineering monoclonal antibody-based contraception and multipurpose prevention technologies. Biol. Reprod. 2020, 103, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Takemori, T.; Sugimoto-Ishige, A.; Nishitsuji, H.; Futamura, Y.; Harada, M.; Kimura-Someya, T.; Matsumoto, T.; Honma, T.; Tanaka, M.; Yaguchi, M.; et al. Establishment of a Monoclonal Antibody against Human NTCP That Blocks Hepatitis B Virus Infection. J. Virol. 2022, 96, e0168621. [Google Scholar] [CrossRef] [PubMed]
- Nader-Marta, G.; Molinelli, C.; Debien, V.; Martins-Branco, D.; Aftimos, P.; de Azambuja, E.; Awada, A. Antibody–drug conjugates: The evolving field of targeted chemotherapy for breast cancer treatment. Ther. Adv. Med. Oncol. 2023, 15, 17588359231183679. [Google Scholar] [CrossRef]
- Paci, A.; Desnoyer, A.; Delahousse, J.; Blondel, L.; Maritaz, C.; Chaput, N.; Mir, O.; Broutin, S. Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: Part 1, monoclonal antibodies, antibody-drug conjugates and bispecific T-cell engagers. Eur. J. Cancer 2020, 128, 107–118. [Google Scholar] [CrossRef]
- Walsh, S.J.; Bargh, J.D.; Dannheim, F.M.; Hanby, A.R.; Seki, H.; Counsell, A.J.; Ou, X.; Fowler, E.; Ashman, N.; Takada, Y.; et al. Site-selective modification strategies in antibody–drug conjugates. Chem. Soc. Rev. 2020, 50, 1305–1353. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhu, Y.; Zhang, J.; Chen, B. Monoclonal antibodies: New chance in the management of B-cell acute lymphoblastic leukemia. Hematology 2022, 27, 642–652. [Google Scholar] [CrossRef] [PubMed]
- High, P.; Carmon, K.S. G protein-coupled receptor-targeting antibody-drug conjugates: Current status and future directions. Cancer Lett. 2023, 564, 216191. [Google Scholar] [CrossRef] [PubMed]
- Carbonetti, S.; Oliver, B.G.; Vigdorovich, V.; Dambrauskas, N.; Sack, B.; Bergl, E.; Kappe, S.H.; Sather, D.N. A method for the isolation and characterization of functional murine monoclonal antibodies by single B cell cloning. J. Immunol. Methods 2017, 448, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Pecetta, S.; Finco, O.; Seubert, A. Quantum leap of monoclonal antibody (mAb) discovery and development in the COVID-19 era. Semin. Immunol. 2020, 50, 101427. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, A.M.; Jager, C.F.D.H.; Kardol-Hoefnagel, T.; Katsburg, M.M.; Knulst, A.C.; Otten, H.G. Comparison of Two Strategies to Generate Antigen-Specific Human Monoclonal Antibodies: Which Method to Choose for Which Purpose? Front. Immunol. 2021, 12, 660037. [Google Scholar] [CrossRef] [PubMed]
- Pedrioli, A.; Oxenius, A. Single B cell technologies for monoclonal antibody discovery. Trends Immunol. 2021, 42, 1143–1158. [Google Scholar] [CrossRef]
- Awwad, S.; Angkawinitwong, U. Overview of Antibody Drug Delivery. Pharmaceutics 2018, 10, 83. [Google Scholar] [CrossRef]
- Neo, S.Y.; Xu, S.; Chong, J.; Lam, K.-P.; Wu, J. Harnessing novel strategies and cell types to overcome immune tolerance during adoptive cell therapy in cancer. J. Immunother. Cancer 2023, 11, e006434. [Google Scholar] [CrossRef]
- Gauthier, M.; Laroye, C.; Bensoussan, D.; Boura, C.; Decot, V. Natural Killer cells and monoclonal antibodies: Two partners for successful antibody dependent cytotoxicity against tumor cells. Crit. Rev. Oncol. 2021, 160, 103261. [Google Scholar] [CrossRef]
- Vallat, J.; Mathis, S. Pathology explains various mechanisms of auto-immune inflammatory peripheral neuropathies. Brain Pathol. 2023, e13184. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/bpa.13184 (accessed on 3 August 2023). [CrossRef] [PubMed]
- Collet, R.; Caballero-Ávila, M.; Querol, L. Clinical and pathophysiological implications of autoantibodies in autoimmune neuropathies. Rev. Neurol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Briani, C.; Visentin, A. Therapeutic Monoclonal Antibody Therapies in Chronic Autoimmune Demyelinating Neuropathies. Neurotherapeutics 2022, 19, 874–884. [Google Scholar] [CrossRef]
- Sasongko, P.L.; van Kraaij, M.; So-Osman, C. Using a scenario approach to assess for the current and future demand of immunoglobulins: An interview and literature study from The Netherlands. Transfus. Med. 2022, 32, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, P.; Dalakas, M.C. Evolution of Anti-B Cell Therapeutics in Autoimmune Neurological Diseases. Neurotherapeutics 2022, 19, 691–710. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Eculizumab: A Review in Generalized Myasthenia Gravis. Drugs 2018, 78, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Chaganti, S.; Hannaford, A.; Vucic, S. Rituximab in chronic immune mediated neuropathies: A systematic review. Neuromuscul. Disord. 2022, 32, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Hays, A.P.; Lee, S.S.; Latov, N. Immune reactive C3d on the surface of myelin sheaths in neuropathy. J. Neuroimmunol. 1988, 18, 231–244. [Google Scholar] [CrossRef]
- Sifniotis, V.; Cruz, E.; Eroglu, B.; Kayser, V. Current Advancements in Addressing Key Challenges of Therapeutic Antibody Design, Manufacture, and Formulation. Antibodies 2019, 8, 36. [Google Scholar] [CrossRef]
- McConnell, M.J. Where are we with monoclonal antibodies for multidrug-resistant infections? Drug Discov. Today 2019, 24, 1132–1138. [Google Scholar] [CrossRef]
- Ho, M. Inaugural Editorial: Searching for Magic Bullets. Antib. Ther. 2018, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.; Wurst, J.M.; Liu, T.; Martinez, R.M.; Datta-Mannan, A.; Feng, Y. Antibody Conjugates-Recent Advances and Future Innovations. Antibodies 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Kollár, É.; Balázs, B.; Tari, T.; Siró, I. Development challenges of high concentration monoclonal antibody formulations. Drug Discov. Today Technol. 2020, 37, 31–40. [Google Scholar] [CrossRef]
- Tyagi, P.; Harper, G.; McGeehan, P.; Davis, S.P. Current status and prospect for future advancements of long-acting antibody formulations. Expert Opin. Drug Deliv. 2023, 20, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kankala, R.K.; Yang, Z.; Li, W.; Xie, S.; Li, H.; Chen, A.-Z.; Zou, L. Antibody-based drug delivery systems for cancer therapy: Mechanisms, challenges, and prospects. Theranostics 2022, 12, 3719–3746. [Google Scholar] [CrossRef] [PubMed]
- Parray, H.A.; Shukla, S.; Perween, R.; Khatri, R.; Shrivastava, T.; Singh, V.; Murugavelu, P.; Ahmed, S.; Samal, S.; Sharma, C.; et al. Inhalation monoclonal antibody therapy: A new way to treat and manage respiratory infections. Appl. Microbiol. Biotechnol. 2021, 105, 6315–6332. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T. Delivery of Intravenously Administered Antibodies Targeting Alzheimer’s Disease-Relevant Tau Species into the Brain Based on Receptor-Mediated Transcytosis. Pharmaceutics 2022, 14, 411. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.S.; Pandey, S.K.; Lasure, V.; Dubey, S.; Singh, R.K. Monoclonal antibodies for the management of central nervous system diseases: Clinical success and future strategies. Expert Opin. Biol. Ther. 2023, 23, 603–618. [Google Scholar] [CrossRef]
- Pardridge, W.M. Kinetics of Blood–Brain Barrier Transport of Monoclonal Antibodies Targeting the Insulin Receptor and the Transferrin Receptor. Pharmaceuticals 2021, 15, 3. [Google Scholar] [CrossRef]
- Li, H.; Saw, P.E.; Song, E. Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics. Cell. Mol. Immunol. 2020, 17, 451–461. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2016, 9, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.; Davidson, M.; Kypreos, E.; Feehan, J.; Muir, J.A.; Nurgali, K.; Apostolopoulos, V. Immunotherapies for the Treatment of Drug Addiction. Vaccines 2022, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Moulahoum, H.; Zihnioglu, F.; Timur, S.; Coskunol, H. Novel technologies in detection, treatment and prevention of substance use disorders. J. Food Drug Anal. 2018, 27, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Baehr, C.A.; Kelcher, A.H.; Khaimraj, A.; Reed, D.E.; Pandit, S.G.; AuCoin, D.; Averick, S.; Pravetoni, M. Monoclonal Antibodies Counteract Opioid-Induced Behavioral and Toxic Effects in Mice and Rats. Experiment 2020, 375, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Agrewala, J.N. Future perspectives of emerging novel drug targets and immunotherapies to control drug addiction. Int. Immunopharmacol. 2023, 119, 110210. [Google Scholar] [CrossRef]
- Xiaoshan, T.; Junjie, Y.; Wenqing, W.; Yunong, Z.; Jiaping, L.; Shanshan, L.; Selva, N.K.; Kui, C. Immunotherapy for treating methamphetamine, heroin and cocaine use disorders. Drug Discov. Today 2020, 25, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.C.; Bremer, P.T.; Hwang, C.S.; Zhou, B.; Ellis, B.; Hixon, M.S.; Janda, K.D. Monoclonal Antibodies for Combating Synthetic Opioid Intoxication. J. Am. Chem. Soc. 2019, 141, 10489–10503. [Google Scholar] [CrossRef]
- Andris, S.; Wendeler, M.; Wang, X.; Hubbuch, J. Multi-step high-throughput conjugation platform for the development of antibody-drug conjugates. J. Biotechnol. 2018, 278, 48–55. [Google Scholar] [CrossRef]
- Kirley, T.L.; Norman, A.B. Reformulation and Thermal Stability of a Therapeutic Anti-Cocaine mAb. J. Pharm. Sci. 2023, 112, 1595–1602. [Google Scholar] [CrossRef]
- Turner, M.E.; Wetzel, H.N.; Zinani, D.B.; Crutchfield, C.A.; Norman, A.B. Effects of a recombinant humanized anti-cocaine monoclonal antibody on the metabolism and distribution of cocaine in vitro and in mice. Pharmacol. Res. Perspect. 2022, 10, e01009. [Google Scholar] [CrossRef]
- A Gorelick, D.; Gardner, E.L.; Xi, Z.-X. Agents in Development for the Management of Cocaine Abuse. Drugs 2004, 64, 1547–1573. [Google Scholar] [CrossRef]
- Rawat, B.S.; Kumar, D.; Soni, V.; Rosenn, E.H. Therapeutic Potentials of Immunometabolomic Modulations Induced by Tuberculosis Vaccination. Vaccines 2022, 10, 2127. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Guevara, M.L.; Persano, F.; Persano, S. Advances in Lipid Nanoparticles for mRNA-Based Cancer Immunotherapy. Front. Chem. 2020, 8, 589959. [Google Scholar] [CrossRef]
- Chauhan, V.M.; Zhang, H.; Dalby, P.A.; Aylott, J.W. Advancements in the co-formulation of biologic therapeutics. J. Control Release 2020, 327, 397–405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenn, E.H.; Benhaim, M.; Siegel, A.; Stein, D.A.; Leonard, J.S.; Katcher, E.; Halperin, D.; Mostel, Z. Monoclonal War: The Antibody Arsenal and Targets for Expanded Application. Immuno 2023, 3, 346-357. https://doi.org/10.3390/immuno3030021
Rosenn EH, Benhaim M, Siegel A, Stein DA, Leonard JS, Katcher E, Halperin D, Mostel Z. Monoclonal War: The Antibody Arsenal and Targets for Expanded Application. Immuno. 2023; 3(3):346-357. https://doi.org/10.3390/immuno3030021
Chicago/Turabian StyleRosenn, Eric H., Mickael Benhaim, Allison Siegel, David A. Stein, Joseph S. Leonard, Erik Katcher, Dania Halperin, and Zachary Mostel. 2023. "Monoclonal War: The Antibody Arsenal and Targets for Expanded Application" Immuno 3, no. 3: 346-357. https://doi.org/10.3390/immuno3030021
APA StyleRosenn, E. H., Benhaim, M., Siegel, A., Stein, D. A., Leonard, J. S., Katcher, E., Halperin, D., & Mostel, Z. (2023). Monoclonal War: The Antibody Arsenal and Targets for Expanded Application. Immuno, 3(3), 346-357. https://doi.org/10.3390/immuno3030021





