CDX-585, a Bispecific Antibody with Dual Targeting of ILT4 and PD-1 Checkpoint Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Development and Characterization of Bispecific Antibodies
2.2. Affinity Determination Using Bio-Layer Interferometry
2.3. ELISA Assays
2.4. Flow Cytometry
2.5. Cell-Based PD-1 Signaling Assay
2.6. Immune Cell Activation Assays
2.7. Human T Cell Stimulation
2.8. Mixed Lymphocyte Reaction
2.9. Mouse Xenograft Tumor Model
2.10. Non-Good Laboratory Practice (GLP) Pilot Non-Human Primate Study
2.11. Statistical Analysis
3. Results
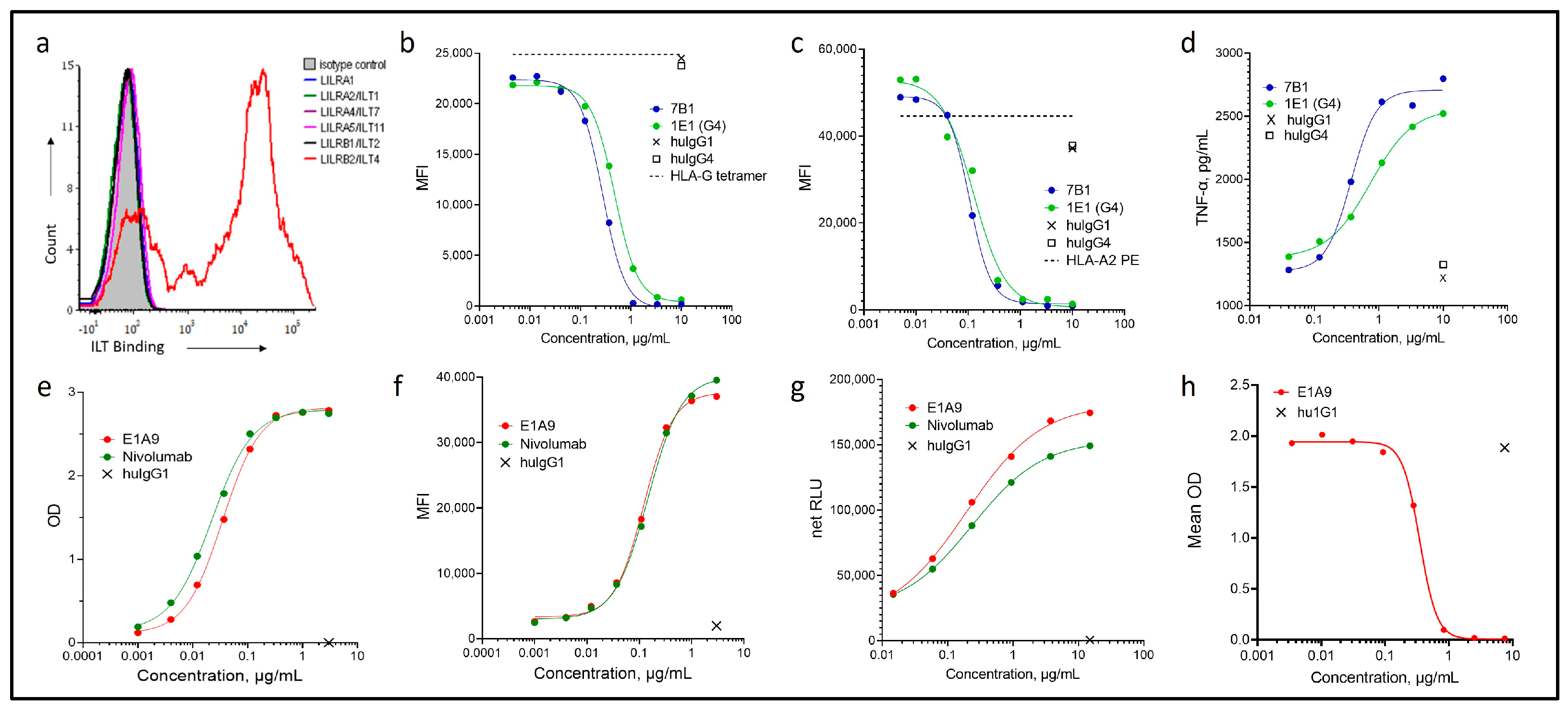
3.1. Characterization of Novel ILT4 and PD-1 Antibodies
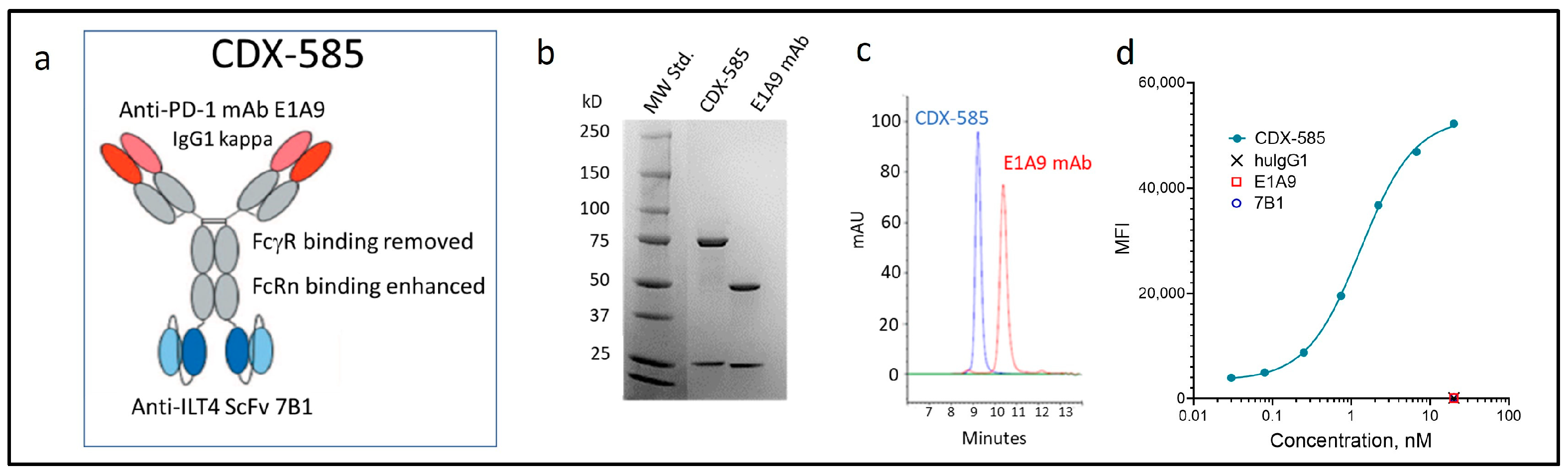
3.2. Development of the CDX-585 bsAb
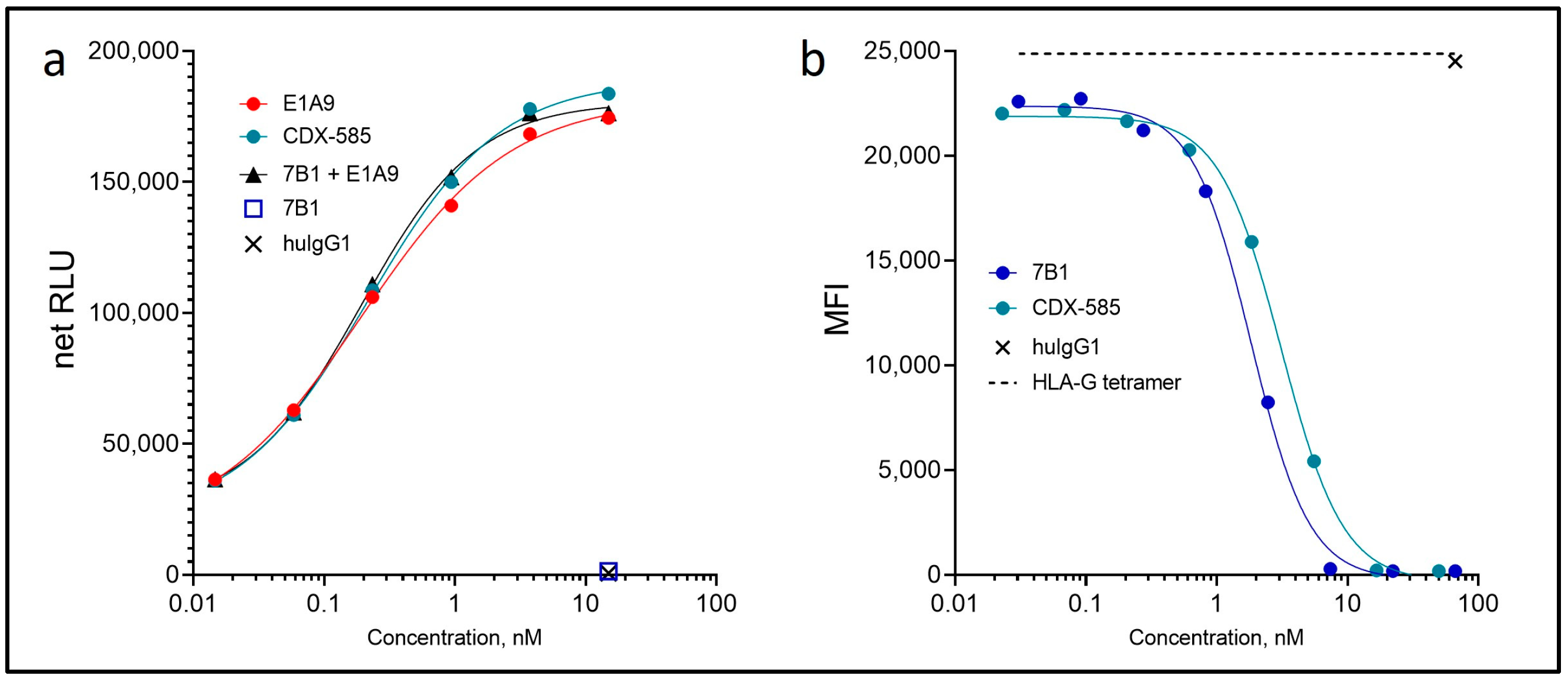
3.3. CDX-585 Potently Inhibits PD-1 Signaling and HLA-G Ligand Binding
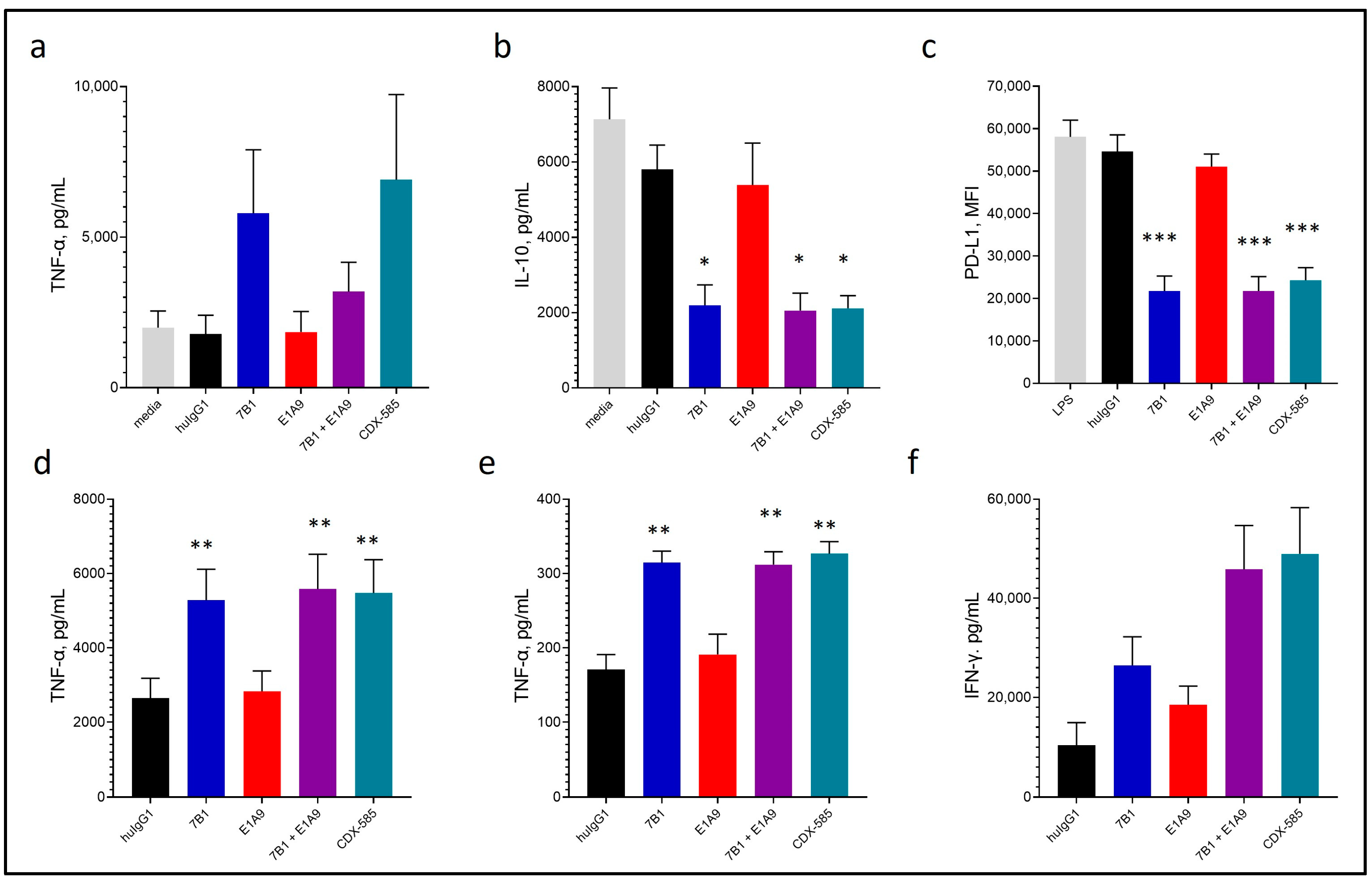
3.4. CDX-585 Drives M1 Macrophage Polarization
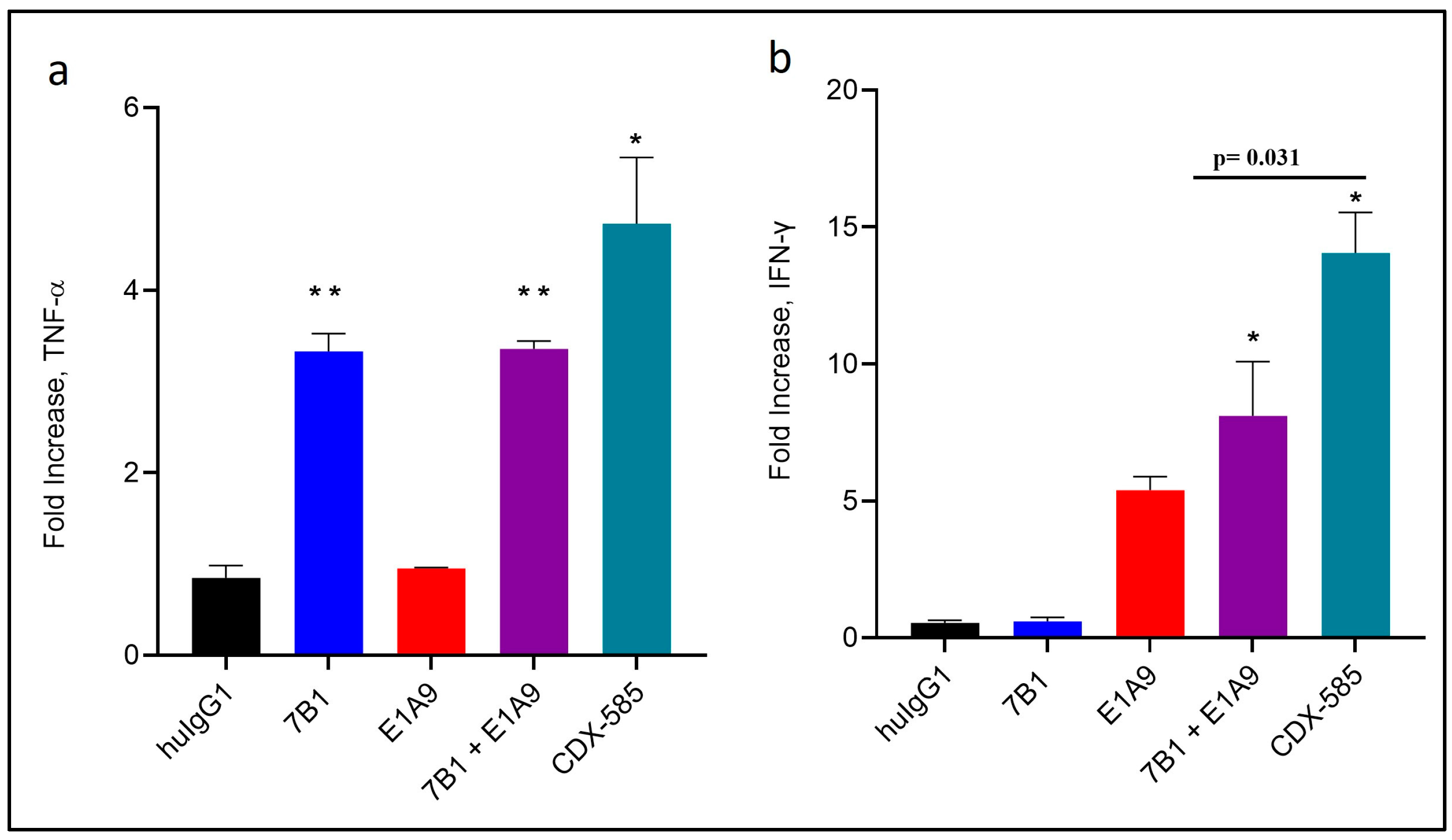
3.5. CDX-585 Enhances Both Myeloid and T Cell Activation
3.6. CDX-585 Enhances T Cell Activation in Mixed Lymphocyte Reactions
3.7. CDX-585 Potentiates CD40-Mediated Immune Cell Activation
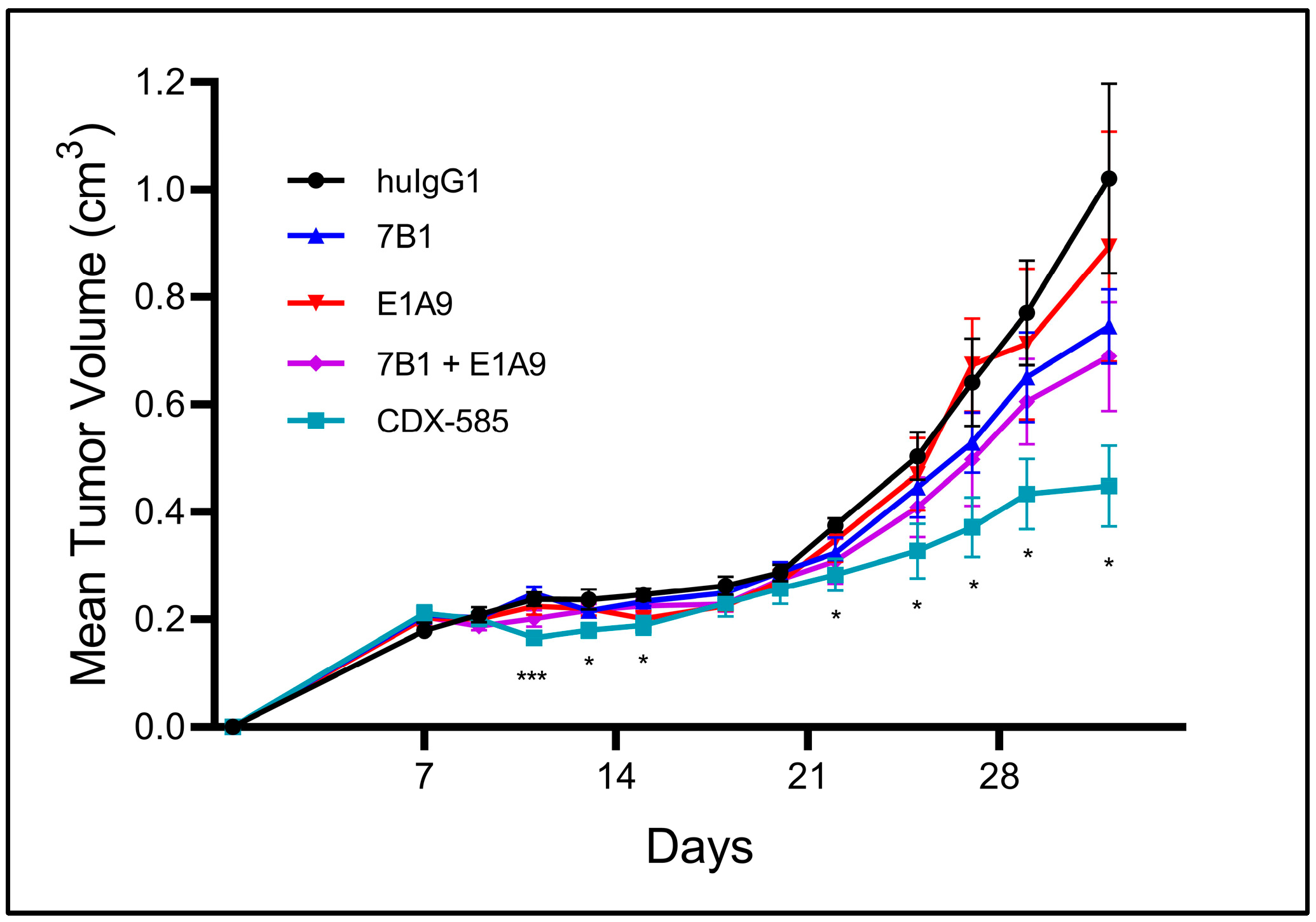
3.8. CDX-585 Enhances Antitumor Activity in a SKMEL-5 Mouse Model
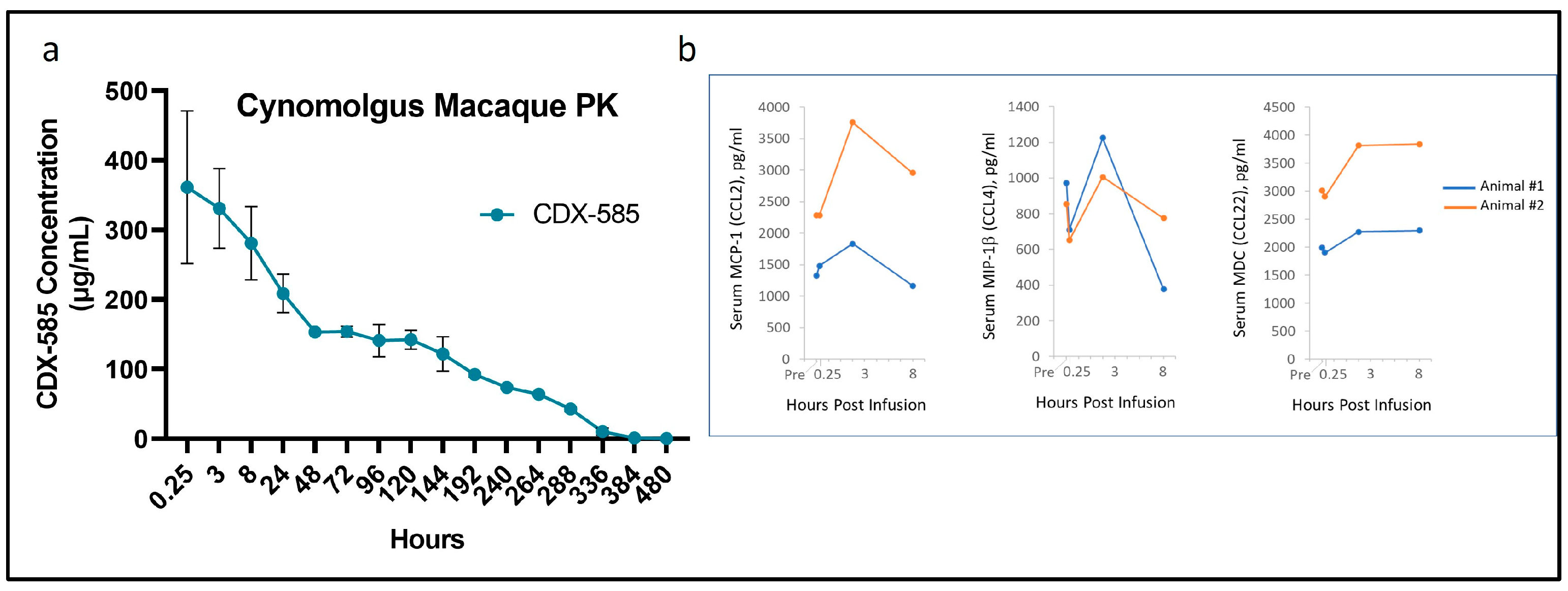
3.9. Non-GLP Pilot Study in Non-Human Primates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solinas, C.; Gombos, A.; Latifyan, S.; Piccart-Gebhart, M.; Kok, M.; Buisseret, L. Targeting Immune Checkpoints in Breast Cancer: An Update of Early Results. ESMO Open 2017, 2, e000255. [Google Scholar] [CrossRef]
- Rini, B.I.; Battle, D.; Figlin, R.A.; George, D.J.; Hammers, H.; Hutson, T.; Jonasch, E.; Joseph, R.W.; McDermott, D.F.; Motzer, R.J.; et al. The Society for Immunotherapy of Cancer Consensus Statement on Immunotherapy for the Treatment of Advanced Renal Cell Carcinoma (RCC). J. Immunother. Cancer 2019, 7, 354. [Google Scholar] [CrossRef]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The Future of Immune Checkpoint Therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune Checkpoint Inhibitors in Melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Cimadamore, A.; Blanca, A.; Massari, F.; Vau, N.; Scarpelli, M.; Cheng, L.; Montironi, R. Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer. Cancers 2021, 13, 131. [Google Scholar] [CrossRef]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune Checkpoint Inhibitors for Lung Cancer Treatment: A Review. J. Clin. Med. 2020, 9, 1362. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of Resistance to Immune Checkpoint Inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Bonavida, B.; Chouaib, S. Resistance to Anticancer Immunity in Cancer Patients: Potential Strategies to Reverse Resistance. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 457–467. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Fleming, V.; Hu, X.; Weber, R.; Nagibin, V.; Groth, C.; Altevogt, P.; Utikal, J.; Umansky, V. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front. Immunol. 2018, 9, 398. [Google Scholar] [CrossRef]
- Ai, L.; Mu, S.; Wang, Y.; Wang, H.; Cai, L.; Li, W.; Hu, Y. Prognostic Role of Myeloid-Derived Suppressor Cells in Cancers: A Systematic Review and Meta-Analysis. BMC Cancer 2018, 18, 1220. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J. Identification of CD4+CD25+CD127− Regulatory T Cells and CD14+HLA−DR−/Low Myeloid-Derived Suppressor Cells and Their Roles in the Prognosis of Breast Cancer. Biomed. Rep. 2016, 5, 208–212. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, P.; Du, J.; Chen, G.; Wan, X.; Li, Z.; Xu, B. Relationship of preoperative and postoperative myeloid-derived suppressor cells percentage with the prognosis in rectal cancer patients. Zhonghua Wei Chang Wai Ke Za Zhi Chin. J. Gastrointest. Surg. 2015, 18, 1139–1143. [Google Scholar]
- Adah, D.; Hussain, M.; Qin, L.; Qin, L.; Zhang, J.; Chen, X. Implications of MDSCs-Targeting in Lung Cancer Chemo-Immunotherapeutics. Pharmacol. Res. 2016, 110, 25–34. [Google Scholar] [CrossRef]
- Monu, N.R.; Frey, A.B. Myeloid-Derived Suppressor Cells and Anti-Tumor T Cells: A Complex Relationship. Immunol. Investig. 2012, 41, 595–613. [Google Scholar] [CrossRef]
- Komohara, Y.; Jinushi, M.; Takeya, M. Clinical Significance of Macrophage Heterogeneity in Human Malignant Tumors. Cancer Sci. 2014, 105, 1–8. [Google Scholar] [CrossRef]
- Ruffell, B.; Coussens, L.M. Macrophages and Therapeutic Resistance in Cancer. Cancer Cell 2015, 27, 462–472. [Google Scholar] [CrossRef]
- Wagtmann, N.; Rojo, S.; Eichler, E.; Mohrenweiser, H.; Long, E.O. A New Human Gene Complex Encoding the Killer Cell Inhibitory Receptors and Related Monocyte/Macrophage Receptors. Curr. Biol. 1997, 7, 615–618. [Google Scholar] [CrossRef]
- Colonna, M.; Navarro, F.; Bellón, T.; Llano, M.; García, P.; Samaridis, J.; Angman, L.; Cella, M.; López-Botet, M. A Common Inhibitory Receptor for Major Histocompatibility Complex Class I Molecules on Human Lymphoid and Myelomonocytic Cells. J. Exp. Med. 1997, 186, 1809–1818. [Google Scholar] [CrossRef]
- Allan, D.S.; Colonna, M.; Lanier, L.L.; Churakova, T.D.; Abrams, J.S.; Ellis, S.A.; McMichael, A.J.; Braud, V.M. Tetrameric Complexes of Human Histocompatibility Leukocyte Antigen (HLA)-G Bind to Peripheral Blood Myelomonocytic Cells. J. Exp. Med. 1999, 189, 1149–1156. [Google Scholar] [CrossRef]
- Lewis Marffy, A.L.; McCarthy, A.J. Leukocyte Immunoglobulin-Like Receptors (LILRs) on Human Neutrophils: Modulators of Infection and Immunity. Front. Immunol. 2020, 11, 857. [Google Scholar] [CrossRef]
- Tedla, N.; Gibson, K.; McNeil, H.P.; Cosman, D.; Borges, L.; Arm, J.P. The Co-Expression of Activating and Inhibitory Leukocyte Immunoglobulin-like Receptors in Rheumatoid Synovium. Am. J. Pathol. 2002, 160, 425–431. [Google Scholar] [CrossRef]
- Colonna, M.; Samaridis, J.; Cella, M.; Angman, L.; Allen, R.L.; O’Callaghan, C.A.; Dunbar, R.; Ogg, G.S.; Cerundolo, V.; Rolink, A. Human Myelomonocytic Cells Express an Inhibitory Receptor for Classical and Nonclassical MHC Class I Molecules. J. Immunol. 1998, 160, 3096–3100. [Google Scholar] [CrossRef]
- Sloane, D.E.; Tedla, N.; Awoniyi, M.; MacGlashan, D.W.; Borges, L.; Austen, K.F.; Arm, J.P. Leukocyte Immunoglobulin-like Receptors: Novel Innate Receptors for Human Basophil Activation and Inhibition. Blood 2004, 104, 2832–2839. [Google Scholar] [CrossRef]
- Baudhuin, J.; Migraine, J.; Faivre, V.; Loumagne, L.; Lukaszewicz, A.-C.; Payen, D.; Favier, B. Exocytosis Acts as a Modulator of the ILT4-Mediated Inhibition of Neutrophil Functions. Proc. Natl. Acad. Sci. USA 2013, 110, 17957–17962. [Google Scholar] [CrossRef]
- Köstlin, N.; Ostermeir, A.-L.; Spring, B.; Schwarz, J.; Marmé, A.; Walter, C.B.; Poets, C.F.; Gille, C. HLA-G Promotes Myeloid-Derived Suppressor Cell Accumulation and Suppressive Activity during Human Pregnancy through Engagement of the Receptor ILT4. Eur. J. Immunol. 2017, 47, 374–384. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, X.; Li, J.; Yu, S.; Wang, L.; Jiang, G.; Yang, D.; Wei, Z.; Zhang, N.; Liu, J.; et al. Immunoglobulin-like Transcript 4 Promotes Tumor Progression and Metastasis and up-Regulates VEGF-C Expression via ERK Signaling Pathway in Non-Small Cell Lung Cancer. Oncotarget 2015, 6, 13550–13563. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Gao, W.; Li, L.; Cui, X.; Yang, H.; Lin, W.; Dang, Q.; Zhang, N.; Sun, Y. Inhibitory Receptor Immunoglobulin-like Transcript 4 Was Highly Expressed in Primary Ductal and Lobular Breast Cancer and Significantly Correlated with IL-10. Diagn. Pathol. 2014, 9, 85. [Google Scholar] [CrossRef]
- Fanger, N.A.; Cosman, D.; Peterson, L.; Braddy, S.C.; Maliszewski, C.R.; Borges, L. The MHC Class I Binding Proteins LIR-1 and LIR-2 Inhibit Fc Receptor-Mediated Signaling in Monocytes. Eur. J. Immunol. 1998, 28, 3423–3434. [Google Scholar] [CrossRef]
- Liang, S.; Ristich, V.; Arase, H.; Dausset, J.; Carosella, E.D.; Horuzsko, A. Modulation of Dendritic Cell Differentiation by HLA-G and ILT4 Requires the IL-6—STAT3 Signaling Pathway. Proc. Natl. Acad. Sci. USA 2008, 105, 8357–8362. [Google Scholar] [CrossRef]
- Chen, H.-M.; van der Touw, W.; Wang, Y.S.; Kang, K.; Mai, S.; Zhang, J.; Alsina-Beauchamp, D.; Duty, J.A.; Mungamuri, S.K.; Zhang, B.; et al. Blocking Immunoinhibitory Receptor LILRB2 Reprograms Tumor-Associated Myeloid Cells and Promotes Antitumor Immunity. J. Clin. Investig. 2018, 128, 5647–5662. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Liu, X.; Lin, W.; Wang, J.; Wang, S.; Si, F.; Huang, L.; Zhao, Y.; Sun, Y.; Peng, G. Tumor-Derived ILT4 Induces T Cell Senescence and Suppresses Tumor Immunity. J. Immunother. Cancer 2021, 9, e001536. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Pan, P.-Y.; Eisenstein, S.; Divino, C.M.; Lowell, C.A.; Takai, T.; Chen, S.-H. Paired Immunoglobin-like Receptor-B Regulates the Suppressive Function and Fate of Myeloid-Derived Suppressor Cells. Immunity 2011, 34, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Siu, L.L.; Wang, D.; Hilton, J.; Geva, R.; Rasco, D.; Perets, R.; Abraham, A.K.; Wilson, D.C.; Markensohn, J.F.; Lunceford, J.; et al. First-in-Class Anti-Immunoglobulin-like Transcript 4 Myeloid-Specific Antibody MK-4830 Abrogates a PD-1 Resistance Mechanism in Patients with Advanced Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 57–70. [Google Scholar] [CrossRef]
- Alvarado, D.; Maurer, M.; Gedrich, R.; Seibel, S.B.; Murphy, M.B.; Crew, L.; Goldstein, J.; Crocker, A.; Vitale, L.A.; Morani, P.A.; et al. Anti-KIT Monoclonal Antibody CDX-0159 Induces Profound and Durable Mast Cell Suppression in a Healthy Volunteer Study. Allergy 2022, 77, 2393–2403. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Dall’Acqua, W.F.; Kiener, P.A.; Wu, H. Properties of Human IgG1s Engineered for Enhanced Binding to the Neonatal Fc Receptor (FcRn). J. Biol. Chem. 2006, 281, 23514–23524. [Google Scholar] [CrossRef]
- Lederman, S.; Yellin, M.J.; Krichevsky, A.; Belko, J.; Lee, J.J.; Chess, L. Identification of a Novel Surface Protein on Activated CD4+ T Cells That Induces Contact-Dependent B Cell Differentiation (Help). J. Exp. Med. 1992, 175, 1091–1101. [Google Scholar] [CrossRef]
- Noelle, R.J.; Roy, M.; Shepherd, D.M.; Stamenkovic, I.; Ledbetter, J.A.; Aruffo, A. A 39-KDa Protein on Activated Helper T Cells Binds CD40 and Transduces the Signal for Cognate Activation of B Cells. Proc. Natl. Acad. Sci. USA 1992, 89, 6550–6554. [Google Scholar] [CrossRef]
- Beatty, G.L.; Chiorean, E.G.; Fishman, M.P.; Saboury, B.; Teitelbaum, U.R.; Sun, W.; Huhn, R.D.; Song, W.; Li, D.; Sharp, L.L.; et al. CD40 Agonists Alter Tumor Stroma and Show Efficacy against Pancreatic Carcinoma in Mice and Humans. Science 2011, 331, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Vitale, L.A.; Thomas, L.J.; He, L.-Z.; O’Neill, T.; Widger, J.; Crocker, A.; Sundarapandiyan, K.; Storey, J.R.; Forsberg, E.M.; Weidlick, J.; et al. Development of CDX-1140, an Agonist CD40 Antibody for Cancer Immunotherapy. Cancer Immunol. Immunother. 2019, 68, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Romieu-Mourez, R.; Solis, M.; Nardin, A.; Goubau, D.; Baron-Bodo, V.; Lin, R.; Massie, B.; Salcedo, M.; Hiscott, J. Distinct Roles for IFN Regulatory Factor (IRF)-3 and IRF-7 in the Activation of Antitumor Properties of Human Macrophages. Cancer Res. 2006, 66, 10576–10585. [Google Scholar] [CrossRef]
- Pollard, J.W. Macrophages Define the Invasive Microenvironment in Breast Cancer. J. Leukoc. Biol. 2008, 84, 623–630. [Google Scholar] [CrossRef]
- Hu, X.; Chakravarty, S.D.; Ivashkiv, L.B. Regulation of IFN and TLR Signaling During Macrophage Activation by Opposing Feedforward and Feedback Inhibition Mechanisms. Immunol. Rev. 2008, 226, 41–56. [Google Scholar] [CrossRef]
- Burshtyn, D.N.; Morcos, C. The Expanding Spectrum of Ligands for Leukocyte Ig-like Receptors. J. Immunol. 2016, 196, 947–955. [Google Scholar] [CrossRef]
- Shiroishi, M.; Tsumoto, K.; Amano, K.; Shirakihara, Y.; Colonna, M.; Braud, V.M.; Allan, D.S.J.; Makadzange, A.; Rowland-Jones, S.; Willcox, B.; et al. Human Inhibitory Receptors Ig-like Transcript 2 (ILT2) and ILT4 Compete with CD8 for MHC Class I Binding and Bind Preferentially to HLA-G. Proc. Natl. Acad. Sci. USA 2003, 100, 8856–8861. [Google Scholar] [CrossRef]
- Reprogramming of IL-10 Activity and Signaling by IFN-γ|The Journal of Immunology. Available online: https://www.jimmunol.org/content/171/10/5034 (accessed on 5 May 2022).
- Hu, X.; Paik, P.K.; Chen, J.; Yarilina, A.; Kockeritz, L.; Lu, T.T.; Woodgett, J.R.; Ivashkiv, L.B. IFN-γ Suppresses IL-10 Production and Synergizes with TLR2 by Regulating GSK3 and CREB/AP-1 Proteins. Immunity 2006, 24, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-K.; Letterio, J.J.; Gorham, J.D. TGF-Β1 Inhibition of IFN-γ-Induced Signaling and Th1 Gene Expression in CD4+ T Cells Is Smad3 Independent but MAP Kinase Dependent. Mol. Immunol. 2007, 44, 3283–3290. [Google Scholar] [CrossRef]
- Cutting Edge: The Th1 Response Inhibits the Generation of Peripheral Regulatory T Cells|The Journal of Immunology. Available online: https://www.jimmunol.org/content/184/1/30 (accessed on 5 May 2022).
- B Cells Expressing IFN-γ Suppress Treg-cell Differentiation and Promote Autoimmune Experimental Arthritis—Olalekan—2015—European Journal of Immunology—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/eji.201445036 (accessed on 5 May 2022).
- Detjen, K.M.; Farwig, K.; Welzel, M.; Wiedenmann, B.; Rosewicz, S. Interferon γ Inhibits Growth of Human Pancreatic Carcinoma Cells via Caspase-1 Dependent Induction of Apoptosis. Gut 2001, 49, 251–262. [Google Scholar] [CrossRef]
- Li, Y.; Carpenito, C.; Wang, G.; Surguladze, D.; Forest, A.; Malabunga, M.; Murphy, M.; Zhang, Y.; Sonyi, A.; Chin, D.; et al. Discovery and Preclinical Characterization of the Antagonist Anti-PD-L1 Monoclonal Antibody LY3300054. J. Immunother. Cancer 2018, 6, 31. [Google Scholar] [CrossRef]
- Stewart, R.; Morrow, M.; Hammond, S.A.; Mulgrew, K.; Marcus, D.; Poon, E.; Watkins, A.; Mullins, S.; Chodorge, M.; Andrews, J.; et al. Identification and Characterization of MEDI4736, an Antagonistic Anti–PD-L1 Monoclonal Antibody. Cancer Immunol. Res. 2015, 3, 1052–1062. [Google Scholar] [CrossRef]
- Wang, C.; Thudium, K.B.; Han, M.; Wang, X.-T.; Huang, H.; Feingersh, D.; Garcia, C.; Wu, Y.; Kuhne, M.; Srinivasan, M.; et al. In Vitro Characterization of the Anti-PD-1 Antibody Nivolumab, BMS-936558, and In Vivo Toxicology in Non-Human Primates. Cancer Immunol. Res. 2014, 2, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Vitale, L.A.; He, L.-Z.; Thomas, L.J.; Wasiuk, A.; O’Neill, T.; Widger, J.; Crocker, A.; Mills-Chen, L.; Forsberg, E.; Weidlick, J.; et al. Development of CDX-527: A Bispecific Antibody Combining PD-1 Blockade and CD27 Costimulation for Cancer Immunotherapy. Cancer Immunol. Immunother. 2020, 69, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive Strategies That Are Mediated by Tumor Cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L. Combinatorial Immunotherapy with Checkpoint Blockers Solves the Problem of Metastatic Melanoma-An Exclamation Sign with a Question Mark. Oncoimmunology 2015, 4, e1058037. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Semmling, V.; Franken, L.; Wagner, H.; Kurts, C. Chemokines: A New Dendritic Cell Signal for T Cell Activation. Front. Immunol. 2011, 2, 31. [Google Scholar] [CrossRef]
- Johnson, D.B.; Estrada, M.V.; Salgado, R.; Sanchez, V.; Doxie, D.B.; Opalenik, S.R.; Vilgelm, A.E.; Feld, E.; Johnson, A.S.; Greenplate, A.R.; et al. Melanoma-Specific MHC-II Expression Represents a Tumour-Autonomous Phenotype and Predicts Response to Anti-PD-1/PD-L1 Therapy. Nat. Commun. 2016, 7, 10582. [Google Scholar] [CrossRef]
- Sanlorenzo, M.; Vujic, I.; Floris, A.; Novelli, M.; Gammaitoni, L.; Giraudo, L.; Macagno, M.; Leuci, V.; Rotolo, R.; Donini, C.; et al. BRAF and MEK Inhibitors Increase PD-1-Positive Melanoma Cells Leading to a Potential Lymphocyte-Independent Synergism with Anti–PD-1 Antibody. Clin. Cancer Res. 2018, 24, 3377–3385. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, M.B.; Vitale, L.; Xia, S.; Peng, Z.; O’Neill, T.; Lillquist, J.; Wasiuk, A.; Weidlick, J.; Widger, J.; Mills-Chen, L.; et al. CDX-585, a Bispecific Antibody with Dual Targeting of ILT4 and PD-1 Checkpoint Pathways. Immuno 2023, 3, 273-288. https://doi.org/10.3390/immuno3030018
Murphy MB, Vitale L, Xia S, Peng Z, O’Neill T, Lillquist J, Wasiuk A, Weidlick J, Widger J, Mills-Chen L, et al. CDX-585, a Bispecific Antibody with Dual Targeting of ILT4 and PD-1 Checkpoint Pathways. Immuno. 2023; 3(3):273-288. https://doi.org/10.3390/immuno3030018
Chicago/Turabian StyleMurphy, Michael B., Laura Vitale, Shukai Xia, Zeyu Peng, Thomas O’Neill, Jay Lillquist, Anna Wasiuk, Jeff Weidlick, Jenifer Widger, Laura Mills-Chen, and et al. 2023. "CDX-585, a Bispecific Antibody with Dual Targeting of ILT4 and PD-1 Checkpoint Pathways" Immuno 3, no. 3: 273-288. https://doi.org/10.3390/immuno3030018
APA StyleMurphy, M. B., Vitale, L., Xia, S., Peng, Z., O’Neill, T., Lillquist, J., Wasiuk, A., Weidlick, J., Widger, J., Mills-Chen, L., Crocker, A., Patterson, C., Boyer, J., Baronas, A. R., Chen, M., Davis, H. M., Ma, M., Goldstein, J., Thomas, L. J., ... Keler, T. (2023). CDX-585, a Bispecific Antibody with Dual Targeting of ILT4 and PD-1 Checkpoint Pathways. Immuno, 3(3), 273-288. https://doi.org/10.3390/immuno3030018





