Inflammatory Profile of Th9 Cells and Their Protective Potential in Helminth Infections
Abstract
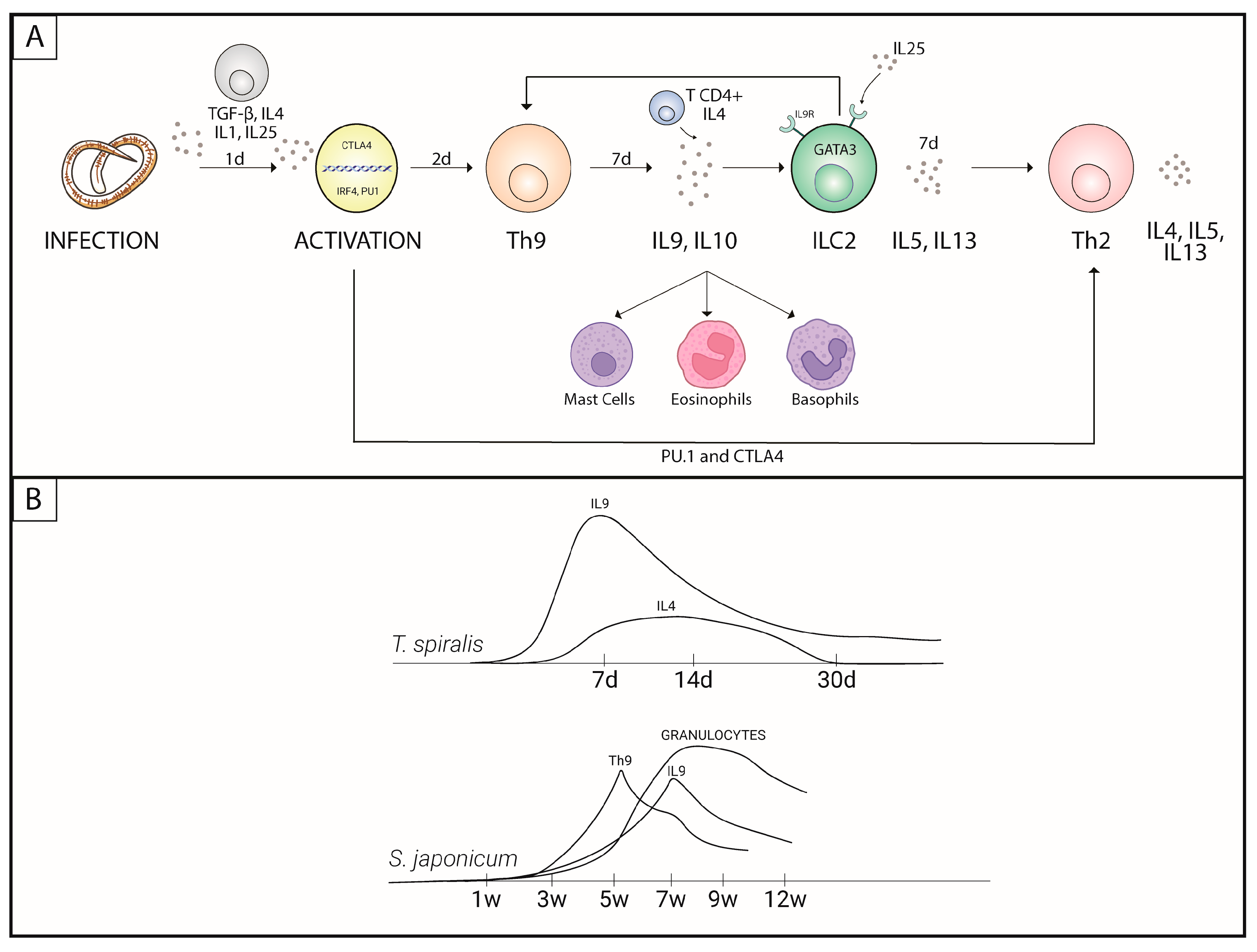
1. Introduction
2. The Participation of Th9 in Inflammation
3. Protective Potential of Th9 in Helminth Infections
3.1. Nematodes
3.2. Cestodes
3.3. Trematodes
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Licona-Limón, P.; Arias-Rojas, A.; Olguín-Martínez, E. IL-9 and Th9 in parasite immunity. Semin. Immunopathol. 2017, 39, 29–38. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/health-topics/schistosomiasis#tab=tab_1 (accessed on 25 April 2023).
- Pan American Health Organization (PAHO). Available online: https://www.paho.org/en/topics/soil-transmitted-helminthiasis (accessed on 25 April 2023).
- Haase, P.; Voehringer, D. Regulation of the humoral type 2 immune response against allergens and helminths. Eur. J. Immunol. 2021, 51, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, H.; Renauld, J.C.; Van Snick, J.; Grencis, R.K. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect. Immun. 1998, 66, 3832–3840. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.F.; Lind, E.F.; Gondek, D.C.; Bennett, K.A.; Gleeson, M.W.; Pino-Lagos, K.; Scott, Z.A.; Coyle, A.J.; Reed, J.L.; Van Snick, J.; et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 2006, 442, 997–1002. [Google Scholar] [CrossRef]
- Li, L.; Xie, H.; Wang, M.; Qu, J.; Cha, H.; Yang, Q.; Feng, Y.; Qi, Y.; Qiu, H.; Dong, N.; et al. Characteristics of IL-9 induced by Schistosoma japonicum infection in C57BL/6 mouse liver. Sci. Rep. 2017, 7, 2343. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Gery, I. The unique features of Th9 cells and their products. Crit. Rev. Immunol. 2012, 32, 1–10. [Google Scholar] [CrossRef]
- Veldhoen, M.; Uyttenhove, C.; van Snick, J.; Helmby, H.; Westendorf, A.; Buer, J.; Martin, B.; Wilhelm, C.; Stockinger, B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008, 9, 1341–1346. [Google Scholar] [CrossRef]
- Bilate, A.M.B. Inflamação, citosinas, proteínas da fase aguda e implicações terapêuticas. Reumatol. Clín. 2007, 8, 47–51. [Google Scholar]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Imunologia Celular e Molecular, 6th ed.; Editora Elsevier: Rio de Janeiro, Brasil, 2008. [Google Scholar]
- Balbino, C.A.; Pereira, L.M.; Curi, R. Mecanismos envolvidos na cicatrização: Uma revisão. Rev. Bras. Farm. 2005, 41, 27–51. [Google Scholar] [CrossRef]
- Chamusca, F.V.; Reis, S.R.A.; Lemaire, D.; Medrado, A.P. Mediadores do efeito sistêmico do processo inflamatório e terapias fotobiomoduladoras: Uma revisão de literatura. Rev. Ciên. Méd. Biol. 2012, 11, 70–78. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, J.; Chen, H.; Nie, H.; Miller, H.; Gong, Q.; Liu, C. T Lymphocyte-Mediated Liver Immunopathology of Schistosomiasis. Front. Immunol. 2020, 11, 61. [Google Scholar] [CrossRef]
- Dardalhon, V.; Awasthi, A.; Kwon, H.; Galileos, G.; Gao, W.; Sobel, R.A.; Mitsdoerffer, M.; Strom, T.B.; Elyaman, W.; Ho, I.C.; et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 2008, 9, 1347–1355. [Google Scholar] [CrossRef]
- Chang, H.C.; Sehra, S.; Goswami, R.; Yao, W.; Yu, Q.; Stritesky, G.L.; Jabeen, R.; McKinley, C.; Ahyi, A.N.; Han, L.; et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat. Immunol. 2010, 11, 527–534. [Google Scholar] [CrossRef]
- Staudt, V.; Bothur, E.; Klein, M.; Lingnau, K.; Reuter, S.; Grebe, N.; Gerlitzki, B.; Hoffmann, M.; Ulges, A.; Taube, C.; et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 2010, 33, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Niedbala, W.; Besnard, A.G.; Nascimento, D.C.; Donate, P.B.; Sonego, F.; Yip, E.; Guabiraba, R.; Chang, H.D.; Fukada, S.Y.; Salmond, R.J.; et al. Nitric oxide enhances Th9 cell differentiation and airway inflammation. Nat. Commun. 2014, 5, 4575. [Google Scholar] [CrossRef]
- Goswami, R.; Jabeen, R.; Yagi, R.; Pham, D.; Zhu, J.; Goenka, S.; Kaplan, M.H. STAT6-dependent regulation of Th9 development. J. Immunol. 2012, 188, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Wiener, Z.; Falus, A.; Toth, S. IL-9 increases the expression of several cytokines in activated mast cells, while the IL-9-induced IL-9 production is inhibited in mast cells of histamine-free transgenic mice. Cytokine 2004, 26, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.T.; Ye, J.J.; Alonso, M.N.; Landrigan, A.; Cheung, R.K.; Engleman, E.; Utz, P.J. Regulation of human Th9 differentiation by type I interferons and IL-21. Immunol. Cell. Biol. 2010, 88, 624–631. [Google Scholar] [CrossRef]
- Meylan, F.; Siegel, R.M. TNF superfamily cytokines in the promotion of Th9 differentiation and immunopathology. Semin. Immunopathol. 2017, 39, 21–28. [Google Scholar] [CrossRef]
- Liu, J.; Harberts, E.; Tammaro, A.; Girardi, N.; Filler, R.B.; Fishelevich, R.; Temann, A.; Licona-Limón, P.; Girardi, M.; Flavell, R.A.; et al. IL-9 regulates allergen-specific Th1 responses in allergic contact dermatitis. J. Investig. Dermatol. 2014, 134, 1903–1911. [Google Scholar] [CrossRef]
- Gu, Z.W.; Wang, Y.X.; Cao, Z.W. Neutralization of interleukin-9 ameliorates symptoms of allergic rhinitis by reducing Th2, Th9, and Th17 responses and increasing the Treg response in a murine model. Oncotarget 2017, 28, 14314–14324. [Google Scholar] [CrossRef]
- Faulkner, H.; Humphreys, N.; Renauld, J.C.; Van Snick, J.; Grencis, R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur. J. Immunol. 1997, 27, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Temann, U.A.; Geba, G.P.; Rankin, J.A.; Flavell, R.A. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J. Exp. Med. 1998, 188, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Temann, U.A.; Ray, P.; Flavell, R.A. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. J. Clin. Investig. 2002, 109, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Else, K.J.; Finkelman, F.D.; Maliszewski, C.R.; Grencis, R.K. Cytokine-mediated regulation of chronic intestinal helminth infection. J. Exp. Med. 1994, 179, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Samblas, M.; García-Rodríguez, J.J.; Trelis, M.; Bernal, D.; Lopez-Jaramillo, F.J.; Santoyo-Gonzalez, F.; Vilchez, S.; Espino, A.M.; Bolás-Fernández, F.; Osuna, A. Self-adjuvanting C18 lipid vinil sulfone-PP2A vaccine: Study of the induced immunomodulation against. Open Biol. 2017, 7, 170031. [Google Scholar] [CrossRef]
- Pennock, J.L.; Grencis, R.K. The mast cell and gut nematodes: Damage and defence. Chem. Immunol. Allergy 2006, 90, 128–140. [Google Scholar] [CrossRef]
- Nagashima, H.; Mahlakõiv, T.; Shih, H.Y.; Davis, F.P.; Meylan, F.; Huang, Y.; Harrison, O.J.; Yao, C.; Mikami, Y.; Urban, J.F.; et al. Neuropeptide CGRP Limits Group 2 Innate Lymphoid Cell Responses and Constrains Type 2 Inflammation. Immunity 2019, 51, 682–695.e686. [Google Scholar] [CrossRef]
- Inclan-Rico, J.M.; Hernandez, C.M.; Henry, E.K.; Federman, H.G.; Sy, C.B.; Ponessa, J.J.; Lemenze, A.D.; Joseph, N.; Soteropoulos, P.; Beaulieu, A.M.; et al. Trichinella spiralis-induced mastocytosis and erythropoiesis are simultaneously supported by a bipotent mast cell/erythrocyte precursor cell. PLoS Pathog. 2020, 16, e1008579. [Google Scholar] [CrossRef]
- Licona-Limón, P.; Henao-Mejia, J.; Temann, A.U.; Gagliani, N.; Licona-Limón, I.; Ishigame, H.; Hao, L.; Herbert, D.R.; Flavell, R.A. Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity 2013, 39, 744–757. [Google Scholar] [CrossRef]
- Henry, E.K.; Sy, C.B.; Inclan-Rico, J.M.; Espinosa, V.; Ghanny, S.S.; Dwyer, D.F.; Soteropoulos, P.; Rivera, A.; Siracusa, M.C. Carbonic anhydrase enzymes regulate mast cell-mediated inflammation. J. Exp. Med. 2016, 213, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Angkasekwinai, P.; Sodthawon, W.; Jeerawattanawart, S.; Hansakon, A.; Pattanapanyasat, K.; Wang, Y.H. ILC2s activated by IL-25 promote antigen-specific Th2 and Th9 functions that contribute to the control of Trichinella spiralis infection. PLoS ONE 2017, 12, e0184684. [Google Scholar] [CrossRef]
- Turner, J.E.; Morrison, P.J.; Wilhelm, C.; Wilson, M.; Ahlfors, H.; Renauld, J.C.; Panzer, U.; Helmby, H.; Stockinger, B. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J. Exp. Med. 2013, 210, 2951–2965. [Google Scholar] [CrossRef] [PubMed]
- Angkasekwinai, P.; Srimanote, P.; Wang, Y.H.; Pootong, A.; Sakolvaree, Y.; Pattanapanyasat, K.; Chaicumpa, W.; Chaiyaroj, S.; Dong, C. Interleukin-25 (IL-25) promotes efficient protective immunity against Trichinella spiralis infection by enhancing the antigen-specific IL-9 response. Infect. Immun. 2013, 81, 3731–3741. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, R.; Munisankar, S.; Bhootra, Y.; Jagannathan, J.; Dolla, C.; Kumaran, P.; Nutman, T.B.; Babu, S. IL-10- and TGFβ-mediated Th9 Responses in a Human Helminth Infection. PLoS Negl. Trop. Dis. 2016, 10, e0004317. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Munisankar, S.; Dolla, C.; Nutman, T.B.; Babu, S. Cytotoxic T-Lymphocyte-Associated Antigen 4 (CTLA-4)- and Programmed Death 1 (PD-1)-Mediated Regulation of Monofunctional and Dual Functional CD4. Infect. Immun. 2019, 87, e00469-19. [Google Scholar] [CrossRef]
- Anuradha, R.; George, P.J.; Hanna, L.E.; Chandrasekaran, V.; Kumaran, P.; Nutman, T.B.; Babu, S. IL-4-, TGF-β-, and IL-1-dependent expansion of parasite antigen-specific Th9 cells is associated with clinical pathology in human lymphatic filariasis. J. Immunol. 2013, 191, 2466–2473. [Google Scholar] [CrossRef]
- Schmitt, E.; Beuscher, H.U.; Huels, C.; Monteyne, P.; van Brandwijk, R.; van Snick, J.; Ruede, E. IL-1 serves as a secondary signal for IL-9 expression. J. Immunol. 1991, 147, 3848–3854. [Google Scholar] [CrossRef]
- Helmby, H.; Grencis, R.K. Interleukin 1 plays a major role in the development of Th2-mediated immunity. Eur. J. Immunol. 2004, 34, 3674–3681. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Brombacher, F.; Van Snick, J. TGF-β interactions with IL-1 family members trigger IL-4-independent IL-9 production by mouse CD4(+) T cells. Eur. J. Immunol. 2010, 40, 2230–2235. [Google Scholar] [CrossRef]
- Poveda, C.; Fresno, M.; Gironès, N.; Martins-Filho, O.A.; Ramírez, J.D.; Santi-Rocca, J.; Marin-Neto, J.A.; Morillo, C.A.; Rosas, F.; Guhl, F. Cytokine profiling in Chagas disease: Towards understanding the association with infecting Trypanosoma cruzi discrete typing units (a BENEFIT TRIAL sub-study). PLoS ONE 2014, 9, e91154. [Google Scholar] [CrossRef] [PubMed]
- Amorim, E.A.D.S.; de França, Á.; Pereira, V.R.A.; Brelaz-de-Castro, M.C.A. IL-1 family and Cutaneous Leishmaniasis: A poorly understood relationship. Cytokine Growth Factor Rev. 2021, 57, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.A.; Maizels, R.M. Cutting edge: In the absence of TGF-β signaling in T cells, fewer CD103+ regulatory T cells develop, but exuberant IFN-γ production renders mice more susceptible to helminth infection. J. Immunol. 2012, 189, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, R.; Munisankar, S.; Bhootra, Y.; Dolla, C.; Kumaran, P.; Nutman, T.B.; Babu, S. Modulation of CD4+ and CD8+ T cell function and cytokine responses in Strongyloides stercoralis infection by interleukin-27 (IL-27) and IL-37. Infect. Immun. 2017, 85, e00500-17. [Google Scholar] [CrossRef]
- Sibi, J.M.; Mohan, V.; Munisankar, S.; Babu, S.; Aravindhan, V. Augmented Innate and Adaptive Immune Responses Under Conditions of Diabetes-Filariasis Comorbidity. Front. Immunol. 2021, 12, 716515. [Google Scholar] [CrossRef]
- Woolsey, I.D.; Miller, A.L. Echinococcus granulosus sensu lato and Echinococcus multilocularis: A review. Res. Vet. Sci. 2021, 135, 517–522. [Google Scholar] [CrossRef]
- Tuxun, T.; Wang, J.H.; Lin, R.Y.; Shan, J.Y.; Tai, Q.W.; Li, T.; Zhang, J.H.; Zhao, J.M.; Wen, H. Th17/Treg imbalance in patients with liver cystic echinococcosis. Parasite Immunol. 2012, 34, 520–527. [Google Scholar] [CrossRef]
- Tuxun, T.; Apaer, S.; Ma, H.Z.; Zhang, H.; Aierken, A.; Lin, R.Y.; Wen, H. The Potential Role of Th9 Cell Related Cytokine and Transcription Factors in Patients with Hepatic Alveolar Echinococcosis. J. Immunol. Res. 2015, 2015, 895416. [Google Scholar] [CrossRef]
- Pang, N.; Zhang, F.; Li, S.; Zhu, Y.; Zhang, C.; An, M.; Wang, H.; Mamuti, W.; Ding, J.; Fan, H. TGF-β/Smad signaling pathway positively up-regulates the differentiation of Interleukin-9-producing CD4. J. Infect. 2018, 76, 406–416. [Google Scholar] [CrossRef]
- Pang, N.; Zhang, F.; Ma, X.; Zhang, Z.; Zhao, H.; Xin, Y.; Wang, S.; Zhu, Y.; Wen, H.; Ding, J. Th9/IL-9 profile in human echinococcosis: Their involvement in immune response during infection by Echinococcus granulosus. Mediat. Inflamm. 2014, 2014, 781649. [Google Scholar] [CrossRef]
- Wang, A.; Pan, D.; Lee, Y.H.; Martinez, G.J.; Feng, X.H.; Dong, C. Cutting edge: Smad2 and Smad4 regulate TGF-β-mediated Il9 gene expression via EZH2 displacement. J. Immunol. 2013, 191, 4908–4912. [Google Scholar] [CrossRef] [PubMed]
- Memon, M.A.; Naqvi, M.A.; Xin, H.; Meng, L.; Hasan, M.W.; Haseeb, M.; Lakho, S.A.; Aimulajiang, K.; Bu, Y.; Xu, L.; et al. Immunomodulatory dynamics of excretory and secretory products on Th9 immune response during Haemonchus contortus infection in goat. PLoS Negl. Trop. Dis. 2020, 14, e0008218. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.H.; Wang, H.; Cheng, J.; Xu, H. Th2 cells as an intermediate for the differentiation of naïve T cells into Th9 cells, associated with the Smad3/Smad4 and IRF4 pathway. Exp. Ther. Med. 2020, 19, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Jin, G.; Fang, J.; Lu, Y. IL-4 together with IL-1β induces antitumor Th9 cell differentiation in the absence of TGF-β signaling. Nat. Commun. 2019, 10, 1376. [Google Scholar] [CrossRef]
- Zhan, T.; Zhang, T.; Wang, Y.; Wang, X.; Lin, C.; Ma, H.; Duan, Z.; Li, C.; Xu, J.; Xia, C. Dynamics of Th9 cells and their potential role in immunopathogenesis of murine schistosomiasis. Parasit. Vectors 2017, 10, 305. [Google Scholar] [CrossRef]
- Costain, A.H.; MacDonald, A.S.; Smits, H.H. Schistosome Egg Migration: Mechanisms, Pathogenesis and Host Immune Responses. Front. Immunol. 2018, 20, 3042. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Ma, H.; Jiang, S.; Zhong, Z.; Wang, X.; Li, C.; Yu, D.; Liu, L.; Xu, J.; Xia, C. Interleukin-9 blockage reduces early hepatic granuloma formation and fibrosis during Schistosoma japonicum infection in mice. Immunology 2019, 158, 296–303. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Christine Oliveira, Y.L.; de Oliveira, Y.L.M.; Cirilo, T.M.; Fujiwara, R.T.; Bueno, L.L.; Dolabella, S.S. Inflammatory Profile of Th9 Cells and Their Protective Potential in Helminth Infections. Immuno 2023, 3, 228-236. https://doi.org/10.3390/immuno3020015
Di Christine Oliveira YL, de Oliveira YLM, Cirilo TM, Fujiwara RT, Bueno LL, Dolabella SS. Inflammatory Profile of Th9 Cells and Their Protective Potential in Helminth Infections. Immuno. 2023; 3(2):228-236. https://doi.org/10.3390/immuno3020015
Chicago/Turabian StyleDi Christine Oliveira, Yvanna Louise, Yrna Lorena Matos de Oliveira, Tatyane Martins Cirilo, Ricardo Toshio Fujiwara, Lilian Lacerda Bueno, and Silvio Santana Dolabella. 2023. "Inflammatory Profile of Th9 Cells and Their Protective Potential in Helminth Infections" Immuno 3, no. 2: 228-236. https://doi.org/10.3390/immuno3020015
APA StyleDi Christine Oliveira, Y. L., de Oliveira, Y. L. M., Cirilo, T. M., Fujiwara, R. T., Bueno, L. L., & Dolabella, S. S. (2023). Inflammatory Profile of Th9 Cells and Their Protective Potential in Helminth Infections. Immuno, 3(2), 228-236. https://doi.org/10.3390/immuno3020015





