Abstract
The nasal cavity is a primary checkpoint for the invasion of respiratory pathogens. Numerous pathogens, including SARS-CoV-2, S. pneumoniae, S. aureus, etc., can adhere/colonize nasal lining to trigger an infection. Secretory IgA (sIgA) serves as the first line of immune defense against foreign pathogens. sIgA facilitates clearance of pathogenic microbes by intercepting their access to epithelial receptors and mucus entrapment through immune exclusion. Elevated levels of neutralizing IgA at the mucosal surfaces are associated with a high level of protection following intranasal immunizations. This review summarizes recent advances in intranasal vaccination technology and challenges in maintaining nominal IgA levels at the mucosal surface. Overall, the review emphasizes the significance of IgA-mediated nasal immunity, which holds a tremendous potential to mount protection against respiratory pathogens.
1. Introduction
The nasal cavity plays a protective role in trapping airborne particles and pathogen-laden droplets owing to the intricate anatomy and adhesive property of mucus. The captured particles and respiratory droplets are eliminated through mucociliary clearance [1,2]. However, high expression of angiotensin-converting enzyme 2 (ACE2) and serine protease TMPRSS2 facilitate the interaction with spike protein of SARS-CoV-2, making the nose a primary entry point for the virus [3,4,5,6]. Along similar lines, bacteria such as S. pneumoniae have an affinity to human mucin [7,8], while S. aureus relies on the surface protein (SasG) of epithelial cells to colonize the nasal cavity [9,10].
Immunoglobulin A (IgA) plays a pivotal role in the forefront defense to intercept the binding or colonization of respiratory pathogens to the airway epithelium while conserving the commensal flora [11,12,13]. The protective role of IgA is reported against numerous respiratory pathogens including SARS-CoV-2, influenza, Streptococcus pneumoniae, etc. The IgA is secreted by IgA-producing B lymphocytes in lymphoid organs, such as MALT (mucosal-associated lymphoid tissue) [14]. Cytokines such as IL-4, IL-5, IL-6, IL-10, and transforming growth factor-β are critical in the production and maintenance of IgA at the mucosal surface [15]. A nominal IgA level (61 to 365 mg/dL) is required to maintain homeostasis on the mucosal surface. Different factors such as age, autoimmune diseases, immunodeficiency, drug usage, etc., alter the level and function of IgA at the mucosal surfaces [16]. Moreover, IgA deficiencies can increase the risk of allergies and respiratory infections [17,18,19,20].
Growing evidence highlights the significance of intranasal immunization in producing elevated levels of neutralizing IgA that is associated with efficient protection against COVID-19 and influenza infections [21]. A higher level of antigen-specific IgA was observed with intranasal vaccination compared to the parenteral route [22,23,24,25]. This review highlights the current understanding of the protective role of IgA in upper airways and advances in intranasal vaccine technologies targeting sterilizing immunity in local mucosa.
2. Cross-Reactivity of IgA against Respiratory Pathogens
A major challenge associated with the respiratory tract is the susceptibility to pathogen entry despite the epithelial and mucosal barrier. In this context, IgA plays a crucial role in modulating mucosal immunity and conserving homeostasis. Contrary to other immunoglobulins, IgA mediates the clearance of toxins and pathogens from the mucosal tissue by immune exclusion, receptor blockade, and steric hindrance [15,26]. Secretion of IgA is orchestrated in mucosa-associated lymphoid tissue (MALT) through the crosstalk between innate and adaptive immune cells, mainly macrophages, dendritic cells (DCs), and B and T lymphocytes. MALT is essentially the primary site for IgA class switching and production of IgA-secreting B cell population. Pathogen entry to the nasal lining is detected by DCs residing underneath the nasal epithelium, leading to subsequent activation and migration to MALT for antigen presentation [27]. Upon specific immunomodulatory cues, IgA class switching occurs along with affinity maturation leading to increased transportation of antigen-specific IgA-producing B and T cells to the effector site to mount an immune response against pathogens [28].
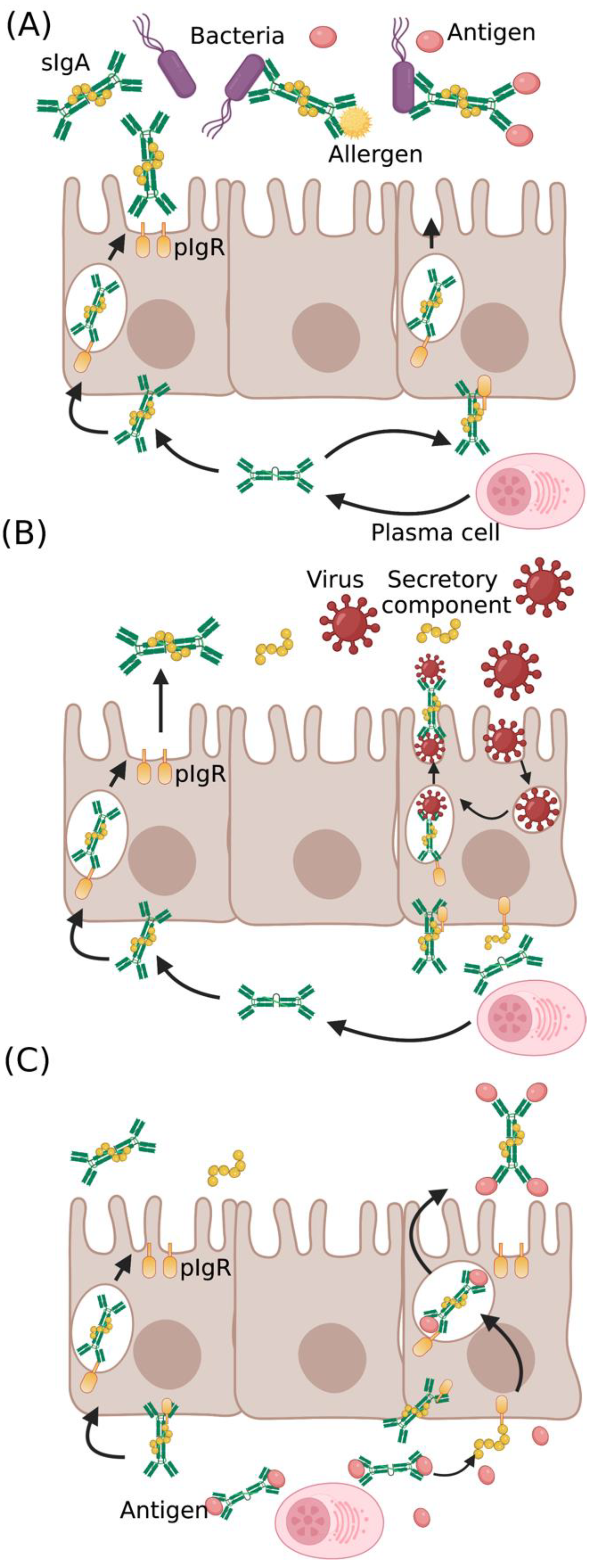
IgA occurs in monomeric and dimeric isoforms. The dimeric IgA comprises two IgA covalently linked with 15 kDa polypeptide, known as the J chain, and a secretory component (SC) [29]. IgA produced in lamina propria undergoes transcytosis through the epithelial layer with the aid of polymeric Ig receptor (pIgR) and is secreted into mucosa as sIgA. It is important to note that pIgR is essential for antibody stabilization and facilitates efficient binding of sIgA with pathogenic proteins. sIgA can recognize a diverse variety of epitopes of pathogens or toxins and impede their affinity or entry towards epithelium by a phenomenon called “immune exclusion” (Figure 1A) [30,31]. The IgA–pIgR complex also interferes with virus proliferation in infected cells and eliminates the virus. The intracellular virus neutralization is demonstrated with adenovirus, which causes respiratory infections [32]. Pathogens that breach the mucosal barrier are neutralized by polymeric IgA in the lamina propria and are cleared into the luminal surface (Figure 1B). sIgA also binds to antigenic domains of bacteria and viruses to induce an agglutination [11]. These processes have been shown to disrupt the microbial membranes, affect their motility, and cause detrimental alteration of their gene expression, thus interfering with their virulence (Figure 1C) [33,34].
Figure 1.
Protective role of IgA in pathogen neutralization and antigen clearance: IgA at the mucosal surface provides (A) cross-protection against diverse pathogens and antigens, (B) intracellular virus neutralization in infected epithelial cells, and (C) antigen clearance from lamina propria through the complex formation of antigen with IgA–pIgR complex (sIgA—secretory IgA, pIgR—polymeric Ig receptor).
In addition, pathogens are entrapped in the mucus layer and eliminated by the native mucociliary clearance [35]. Although these processes have been researched for decades, there is more to comprehend and warrants further research. Various experiments have been conducted to study the immunological functions of the mucus layer, SC, and polysaccharide chains in SC. Studies carried out in the absence of SC showed poor adherence and retention of IgA molecules in the epithelia or mucus layer, significantly lowering neutralization efficiency [36]. The deletion of carbohydrate moieties of SC in the sIgA complex hindered its anchoring to mucin and led to the failure of its protective role [31]. Hence, it is evident that the mucus layer, along with SC glycosylation, is essential to augment and preserve the functions of sIgA [37,38]. Murine models of lung infection with shigella flexneri revealed that the binding of sIgA with mucus is necessary for providing first-line defense against the invasion of bacteria [39]. In another instance, intranasal administration of neutralizing IgA followed by respiratory syncytial virus (RSV) challenge resulted in a significant reduction in lung viral titer and, subsequently, mitigated pneumonia in the murine model [40]. Similar findings on IgA protection were reported in animals infected with influenza and reovirus [41,42,43]. Interestingly, intravenous administration of antigen-specific IgA, specifically in the polymeric isoform, protected the mice from influenza infection owing to the nasal secretion of IgA from serum. However, the monomeric IgA was not effective in preventing infection [35].
3. T-Dependent and T-Independent Mechanisms of IgA Induction
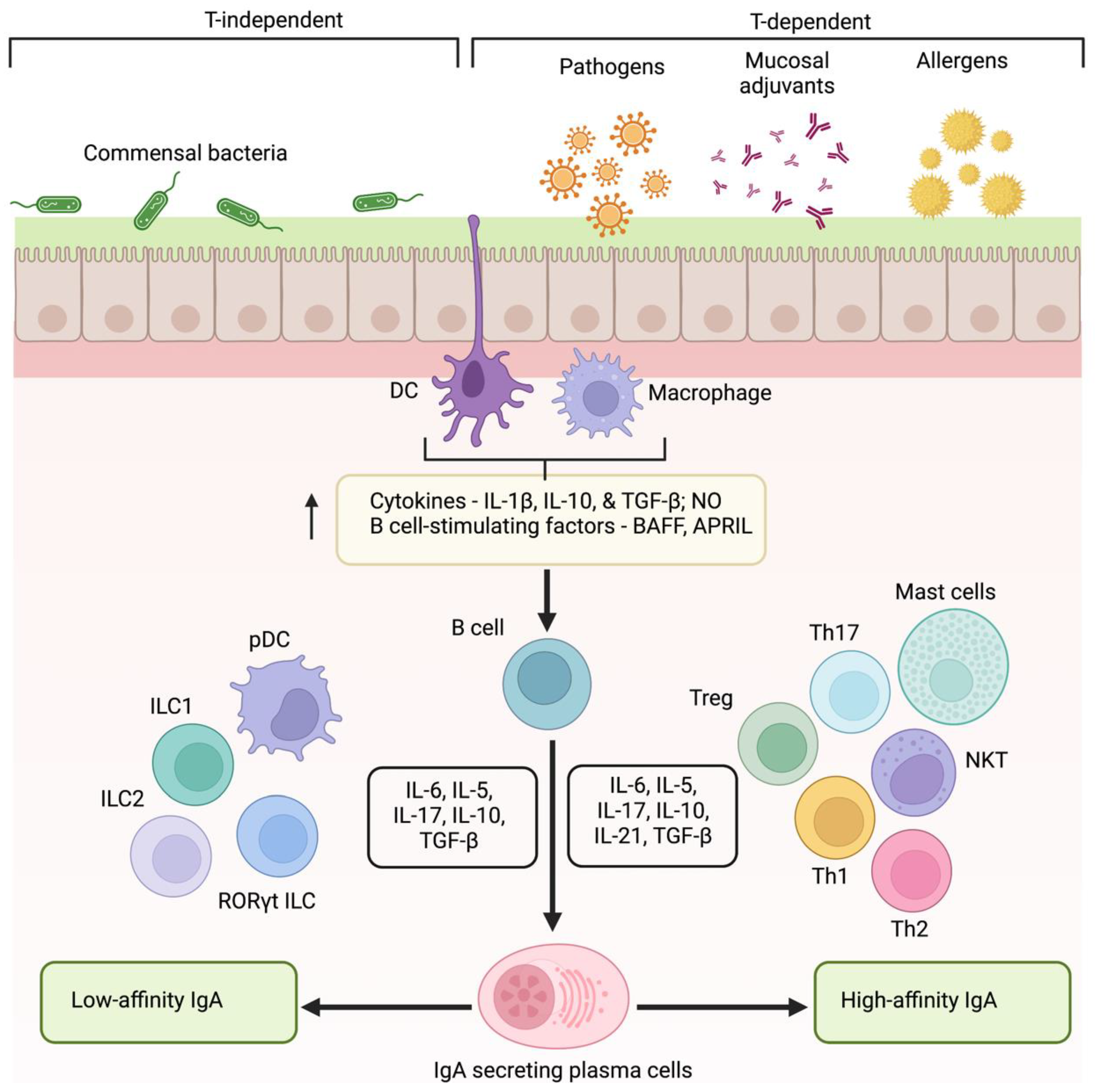
B lymphocytes induce IgA secretion in MALT in response to endogenous antigens from commensal flora, invasion of pathogenic microorganisms, or immunization. Foreign antigens induce T cell-dependent pathways to produce high-affinity IgA antibodies. On the other hand, antigens from commensal flora generate low-affinity antibody molecules through T cell-independent pathways. Dendritic cells (DCs) underlying mucosal epithelia sample luminal antigens through its extended dendrites to capture and present antigen to B cells in MALT (Figure 2) [44]. Consequently, T cells are activated, leading to the IgA class switching recombination (Ig CSR) [28]. Ig CSR leads to IgA production in a T cell-dependent and independent manner. Activation of high-affinity IgA in T cell-dependent pathways demands the interaction between CD 40 of B cell with CD 40 L of T cell [45]. This interaction upregulates the activation of T follicular helper cells (Tfh cells), Th17 cells, and FOxp3 + Treg cells. Subsequently, results in the aggravated release of proinflammatory cytokines such as IL4, IL5, IL6, IL10, IL13, IL17, IL21, and TGF β that trigger the Ig CSR and production of high-affinity IgA molecules [46,47,48]. However, studies have shown IgA secretion in CD40-deficient mice and human cells indicating the role of T cell-independent mechanism. In T-cell independent pathway, the capture of commensal antigen enhances the production of TNF family subtypes, BAFF (B cell activating factor) and APRIL (a proliferation-inducing ligand) that in turn activates ILC1, ILC2, RORYt, and pDCs, and the production of IL5, IL6, IL10, IL17, and TGFβ. This results in the stimulation of Ig CSR and the production of low-affinity IgG and IgA molecules [30].
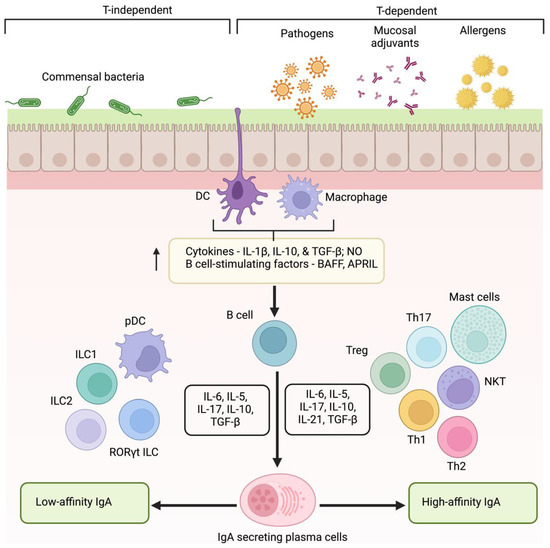
Figure 2.
Mechanism of low- and high-affinity IgA production. Induction of IgA occurs in a T-dependent and independent manner. Mucosal surface exposure to allergens, pathogens, or vaccines triggers epithelial cells and antigen-presenting cells (DCs and macrophages) to elicit the production of cytokines, NO, and BAFF, and APRIL to activate B lymphocytes. Class switching to high-affinity IgA occurs with the aid of Th cells through a T cell-dependent manner. On the contrary, plasmacytoid DCs and ILCs favor the induction of low-affinity IgA. (DCs—dendritic cells, NO—nitric oxide, BAFF—B cell activating factor, APRIL—a proliferation-inducing ligand, ILCs—innate lymphoid cells).
4. IgA Deficiencies and IgA1 Proteases: A Threat to Nasal Vaccines?
Normal serum IgA levels are relevant for homeostasis between proinflammatory and anti-inflammatory factors to control infections, allergies, and autoimmune disorders. Selective IgA deficiency is predominantly marked by low levels of IgA in serum, without altering other immunoglobulins [49]. Patients with IgA deficiency are reported to have a serum IgA less than 7 mg/dL [50]. This condition increases the susceptibility to recurrent respiratory and gastrointestinal infections, autoimmune disorders, and allergies [51]. Factors contributing to the IgA deficient condition include impaired B cell maturation, calcium modulators, and transmembrane activators. On the contrary, common variable immunodeficiency (CVID) weakens the immunological functions of IgA, IgG, and IgM [52]. Therefore, it is important to monitor the levels of IgA antibodies and study the factors that could lead to the deleterious clinical manifestations caused by IgA deficiency. Significantly low levels of IgA production in respiratory and gut epithelium are observed in vitamin A deficiency (VAD) models in response to viruses and vaccines [53,54]. However, the levels and function of non-IgA immunoglobulins remain unaffected with retinol deficiency, leading to an increased IgG-to-IgA ratio. Hence, VAD makes it difficult to confer IgA-mediated protection with respiratory infections and vaccines. Numerous studies have shown that single intranasal administration of vitamin A palmitate or retinyl palmitate (an ester of retinol and palmitate) aids in improved protection from infections in VAD populations. Here, retinol served as an IgA-class switching factor that restored the mucosal IgA levels in the VAD mice [55].
IgA1 proteases are reported to interfere with IgA’s host defense mechanisms by cleaving IgA1 antibodies and hampering their structural integrity and function [56]. These proteolytic autotransporter proteins are produced by various pathogenic bacterial species such as Haemophilus influenzae, Neisseria meningitidis, Neisseria gonorrhoeae, and S. pneumoniae [57]. They specifically recognize and cleave certain proline–threonine and proline–serine peptide bonds in the IgA1 hinge region sequence TPPTPSPSTPPTPSPS in the IgA1 molecule, generating intact Fcα and Fabα fragments. As a result, recognition of bacterial epitopes by the IgA1 is hindered [58].
Since Fcα is required for the agglutination process and opsonophagocytic activity, the IgA1 protease-mediated cleavage establishes the way for bacterial survival and colonization. Few studies have shown that the Fabα fragment is known to retain its surface-antigen binding capacity termed “fabulation” even after the cleavage [59]. Several studies revealed that circulating antibodies in serum and nasal secretions can neutralize these proteolytic enzymes [15]. These antibodies regulate the proteases secreted by the commensal flora. Hence, nasal immunity depends on the balance between the level of these neutralizing antibodies and protease enzymes. This is the underlying reason why children with a history of atopic disease encounter recurring immunological dysfunctions that is attributed to the cleavage of IgA molecules by the IgA1 proteases in the absence of protease-neutralizing antibodies [60].
5. Recent Advances in IgA Inducing Nasal Vaccines
Vaccines are aimed to elicit a long-lasting immune response against pathogens. Adjuvants are immunostimulants to trigger adequate innate and adaptive immunity. These components alter the kinetics, longevity, and robustness of the host immune response [61]. The addition of adjuvants in vaccines is beneficial in decreasing the dose of antigens and frequency of vaccine administration. They have proven to bolster immune activation in immunocompromised, elderly, and neonates [62,63,64].
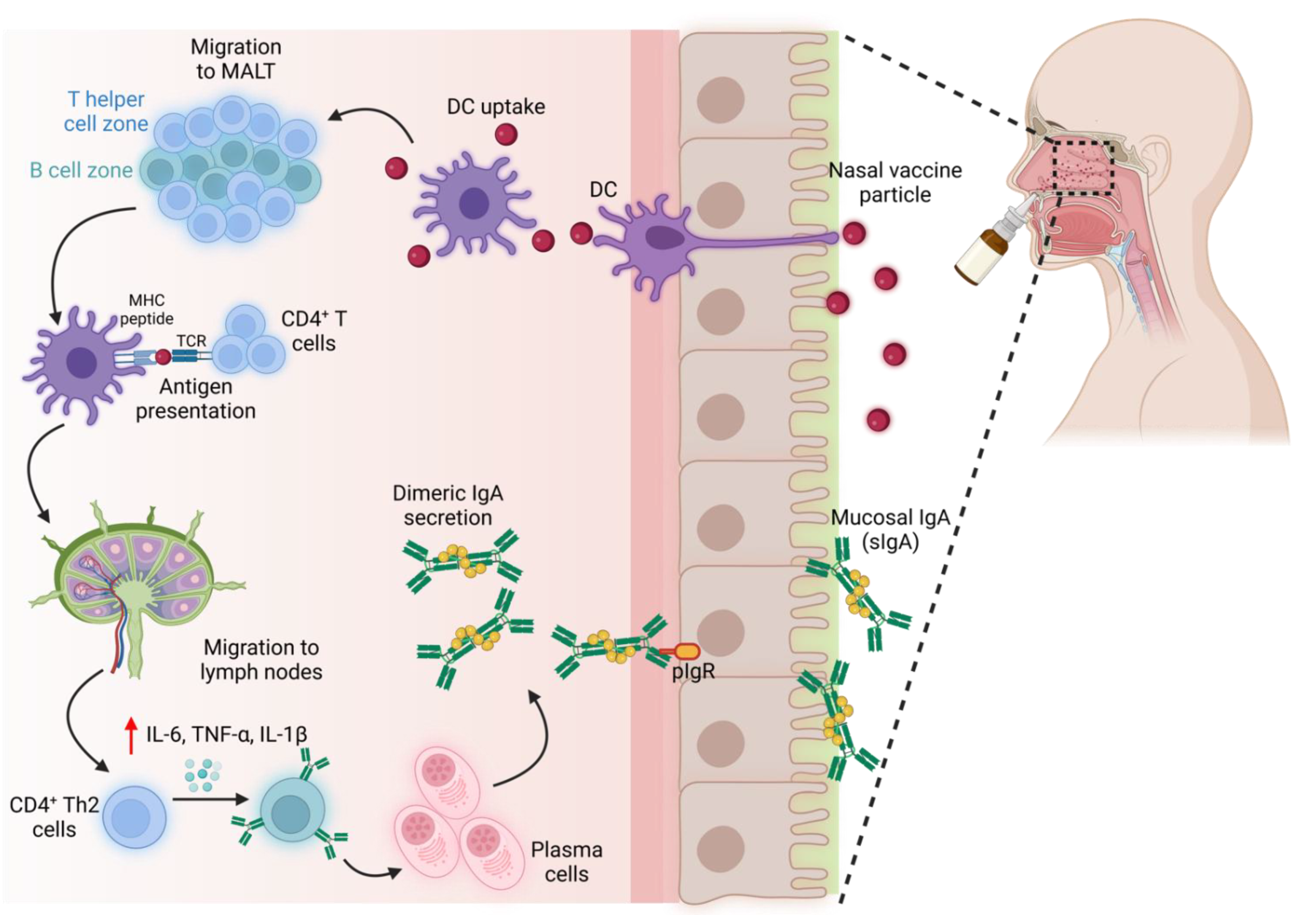
Adjuvants enhance the presentation of antigens and facilitate the maturation of antigen-presenting cells such as macrophages, dendritic cells, etc. Growing evidence indicates a robust humoral response with intranasal vaccines compared to their parenteral route [62]. Intranasal administration of spike proteins elicited local and systemic mucosal IgA levels more than the parenteral route (Figure 3). Virus-like particles, liposomes, nanogels, etc., are being explored as immunostimulants and delivery platforms to mitigate respiratory infections [65]. Virus vectors are the most used vaccine delivery platforms. For instance, intranasal COVID-19 vaccine (ChAd-SARS-CoV-2-S) delivered with an adenovirus vector improved the levels of antigen-specific IgA in contrast to intramuscular injection. This approach also facilitated in the dose reduction of antigens [66]. Live/attenuated viral and bacterial vectors are reported to trigger sIgA through recognizing pathogen-associated molecular patterns (PAMPs). It is important to note that these vectors do not elicit an infection. Similarly, intranasal immunization of Ad5-vectored vaccine encoding S protein (Ad5-nCoV) augmented S-specific IgA antibodies from the tracheal and lung washes, following the intramuscular injection [67]. Virus-vectored vaccines may elicit undesirable antivector antibodies, and the presence of those serum antibodies can impede the seroconversion of neutralizing antibodies against the target pathogen. Administration of Ad5-S-nb2 (encoding S1 protein of SARS-CoV-2) via intranasal route reduced the serum antibodies against Ad5 vectors compared to intramuscular injections. Therefore, the choice of intranasal immunization or other delivery vehicles can be adopted to eliminate the undesired influences of virus-vectored vaccines [65].
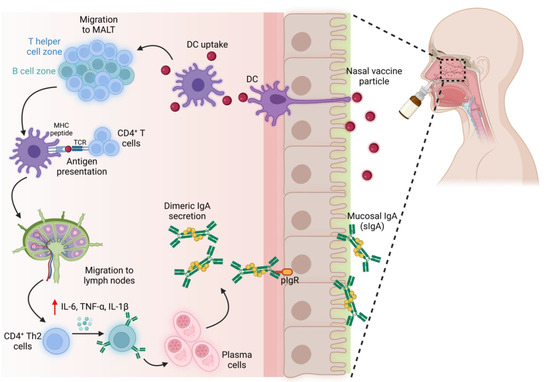
Figure 3.
Induction of neutralizing IgA with intranasal vaccines. An immune response is elicited in MALT, leading to the secretion of IgA on the mucosal surface. (MALT—mucosa-associated lymphoid tissue).
Protein subunit vaccines are less immunogenic and necessitate the incorporation of mucosal adjuvants. The rapid enzymatic degradation of protein subunits also urges the need for a delivery platform. Recently, a group of researchers engineered a nanoemulsion-based mucosal adjuvant that encapsulated RNA agonist, RIG-I, in tandem with recombinant SARS-CoV-2 protein vaccine. This agonist activates TLRs and NLRP3 to enhance immunogenicity and elicited superior Th-1-based cellular responses with elevated levels of neutralizing antibodies against SARS-CoV-2 and other mutant variants [68]. A series of cationic nanocarriers such as polyethyleneimine (PEI), chitosan, and N-[1-(2,3-Dioleoyloxy) propyl]-N,N,N-trimethylammonium chloride (DOTAP) were identified as potent mucosal adjuvants. These adjuvants markedly enhanced the humoral and cellular immune response, which is attributed to increased antigen uptake and the ability of these adjuvants to activate dendritic cells [69]. A mucosal vaccine of the S1 subunit of SARS-CoV-2 encapsulated in DOTAP or poly(lactic-co-glycolic acid) (PLGA) nanoparticles was adjuvanted with a combination of IL-15 and TLR agonists such as Poly I: C (polyinosinic: polycytidylic acid) and CpG (cytosine–guanine dinucleotide). This adjuvant cocktail therapy boosted the production of dimeric IgA and IFN-α, conferring complete protection after the SARS-CoV-2 challenge in rhesus macaques [70]. Targeting specific signaling pathways, subsets of immune cells, receptors, and cytokines can shape IgA class switching, hence protecting against antigens. For instance, IL5 and TGF-β1 are associated with the expansion of IgA-secreting B lymphocytes in the murine model [71]. Similarly, intranasal administration of retinyl palmitate resulted in elevated production of IgA in the nasal mucosa and heightened protection against influenza A virus. This unequivocally highlights the role of retinoic acid derivatives in correcting IgA levels. Numerous nasal adjuvants identified to induce IgA production are summarized in Table 1.

Table 1.
Intranasal vaccine adjuvants of IgA production against respiratory pathogens.
Novel delivery systems are currently being explored to facilitate transient permeabilization of the nasal epithelial barrier to transport antigen/adjuvant to MALT [87]. Virus-like particles are genetically engineered nanoparticles that constitute multiprotein structures resembling the conformation of native viruses but devoid of the viral genome. These nanoparticle-engineered vaccines are reported to have immunological advantages over conventional vaccine platforms in terms of antigen presentation and enhanced antigen transport to draining lymph nodes. One such recent example of VLPs is the self-assembled spike RBD-ferritin nanoparticle, which demonstrated a fast clearance of virus particles in ferret models [88]. The VLPs process immune responses through the activation of pattern recognition receptors characterized by the upregulated production of proinflammatory cytokines such as TNF-α and IL-1β. This drives the DC maturation and subsequently increases the expression of co-stimulatory molecules in DCs [89]. Enveloped VLPs against SARS-CoV-2 are synthesized from VeroE6 cells, which offered the highest expression of S protein in HEK293 cells. In contrast, the nonenveloped VLPs (devoid of lipid membrane) are produced in simpler systems such as bacteria and yeast [90].
Liposomes have also garnered a special interest among scientists as they present a promising platform for antigen delivery and elicit an adjuvant effect. Moreover, the tunable nature of liposomes with hydrophilic and lipophilic composition bestows a superior advantage to tailor the desired immune response. The utility of liposomes against COVID-19 was witnessed with mRNA vaccines developed by Pfizer/BioNTech and Moderna. The liposomal vaccine platform demonstrated its versatility against other respiratory pathogens such as MERS-CoV, respiratory syncytial virus (RSV), and influenza [91]. Liposome complex prepared using phosphatidyl-β-oleoyl-γ-palmitoylethanolamine and cholesterol hemisuccinate are encapsulated with epitope peptide of M protein of MERS-CoV and CpG DNA that induced the production of antibodies [92]. Liposomal delivery of an anti-RSV peptide, RF-482, inhibited the replication of RSV. Interestingly, this study showed that the naïve liposomes also demonstrated a similar level of RSV inhibition to that of liposome-mediated delivery of RF-482 peptide [93]. This underlines the potent adjuvant effect of liposomes in inhibiting virus infections.
Nanogels are another arm of polymer-based vaccine platforms for the delivery of adjuvants, antigens, or both. Nanogels synthesized from PHEMA functionalized with pyridine exhibited an intrinsic immunomodulatory effect through the stimulation of TLR2. The adjuvant effect of PHEMA nanogels enhanced the trafficking of immune cells and induced a robust immune response in gut-mediated metabolic syndrome [94]. A pneumococcal intranasal vaccine developed from cationic cholesteryl group-bearing pullulan nanogels encapsulated with surface protein A antigen of S. pneumoniae induced the secretion of sIgA in nasal and bronchial mucosal surfaces [95]. Cationic pullulan nanogels were also utilized to deliver nontoxic Clostridium botulinum type-A neurotoxin via the intranasal route and stimulated antigen-specific mucosal IgA production [96]. The wide applicability of nanogels holds the potential to explore its intrinsic adjuvant effect against emerging respiratory pathogens and as a mucosal vaccine delivery platform [97].
Despite these strong indications, only a single intranasal vaccine technology exists against respiratory infections. The slow advancement in mucosal immunization is due to the lack of safe vaccine adjuvants and challenges associated with quantifying the metrics of neutralizing IgA in nasal secretion. The rapid mucociliary clearance, entrapment of antigens in mucus, enzymatic degradation, and physical barrier of nasal epithelium further worsen the challenges [98]. Next-generation nasal vaccines should target the inductive site of mucosal immune cells to trigger an acute innate and adaptive response rather than tolerance. Additionally, the needle-less approach provides a safe, low-cost, patient-compliant, and efficient alternative for large-scale immunization, especially in the case of a global pandemic.
6. Conclusions
The COVID-19 pandemic underscored the necessity of an effective prophylactic strategy against homologous and heterologous viruses. Numerous reports support the enhanced local and systemic levels of IgA via intranasal vaccines. Current research is advancing to identify novel adjuvants and delivery platforms to overcome the mucosal barrier and prolong vaccine exposure in the nasal cavity. A comprehensive understanding of the mechanism of antigen processing and subsequent orchestrating events of IgA secretion is warranted to improve the efficacy and hasten the regulatory burden on intranasal vaccine approval.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author is grateful to the Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Boston, MA 02115, USA.
Conflicts of Interest
The author declare no conflict of interest.
Abbreviations
PPRs—pathogen recognition receptors, PAMPs—pathogen associated molecular pattern, LPS—lipopolysaccharide, TLR—toll-like receptor, STING—stimulator of interferon gene, poly I:C—polyinosine polycytidylic acid, RIG-I—retinoic acid inducible gene, MDA5—melanoma differentiation-associated gene, Treg—T-regulatory cells, NALT—nasal-associated lymphoid tissue, MALT—mucosal-associated lymphoid tissue.
References
- Kiyono, H.; Fukuyama, S. Nalt-versus Peyer’s-Patch-Mediated Mucosal Immunity. Nat. Rev. Immunol. 2004, 4, 699–710. [Google Scholar] [CrossRef]
- Elad, D.; Wolf, M.; Keck, T. Air-Conditioning in the Human Nasal Cavity. Respir. Physiol. Neurobiol. 2008, 163, 121–127. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H., 3rd; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446.e14. [Google Scholar] [CrossRef] [PubMed]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 Entry Factors Are Highly Expressed in Nasal Epithelial Cells Together with Innate Immune Genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Gallo, O.; Locatello, L.G.; Mazzoni, A.; Novelli, L.; Annunziato, F. The Central Role of the Nasal Microenvironment in the Transmission, Modulation, and Clinical Progression of SARS-CoV-2 Infection. Mucosal Immunol. 2021, 14, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Yesilkaya, H.; Manco, S.; Kadioglu, A.; Terra, V.S.; Andrew, P.W. The Ability to Utilize Mucin Affects the Regulation of Virulence Gene Expression in Streptococcus Pneumoniae. FEMS Microbiol. Lett. 2008, 278, 231–235. [Google Scholar] [CrossRef]
- Briles, D.E.; Novak, L.; Hotomi, M.; van Ginkel, F.W.; King, J. Nasal Colonization with Streptococcus Pneumoniae Includes Subpopulations of Surface and Invasive Pneumococci. Infect. Immun. 2005, 73, 6945–6951. [Google Scholar] [CrossRef] [PubMed]
- Roche, F.M.; Meehan, M.; Foster, T.J. The Staphylococcus Aureus Surface Protein SasG and Its Homologues Promote Bacterial Adherence to Human Desquamated Nasal Epithelial Cells. Microbiology 2003, 149, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.; Brégeon, F.; Mège, J.-L.; Rolain, J.-M.; Blin, O. Staphylococcus Aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef]
- Mantis, N.J.; Forbes, S.J. Secretory IgA: Arresting Microbial Pathogens at Epithelial Borders. Immunol. Investig. 2010, 39, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Janoff, E.N.; Fasching, C.; Orenstein, J.M.; Rubins, J.B.; Opstad, N.L.; Dalmasso, A.P. Killing of Streptococcus Pneumoniae by Capsular Polysaccharide-Specific Polymeric IgA, Complement, and Phagocytes. J. Clin. Investig. 1999, 104, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA Dominates the Early Neutralizing Antibody Response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Shikina, T.; Hiroi, T.; Iwatani, K.; Jang, M.H.; Fukuyama, S.; Tamura, M.; Kubo, T.; Ishikawa, H.; Kiyono, H. IgA Class Switch Occurs in the Organized Nasopharynx- and Gut-Associated Lymphoid Tissue, but Not in the Diffuse Lamina Propria of Airways and Gut. J. Immunol. 2004, 172, 6259–6264. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s Complex Roles in Immunity and Mucosal Homeostasis in the Gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Cinicola, B.L.; Pulvirenti, F.; Capponi, M.; Bonetti, M.; Brindisi, G.; Gori, A.; De Castro, G.; Anania, C.; Duse, M.; Zicari, A.M. Selective IgA Deficiency and Allergy: A Fresh Look to an Old Story. Medicina 2022, 58, 129. [Google Scholar] [CrossRef]
- Abreu, R.B.; Clutter, E.F.; Attari, S.; Sautto, G.A.; Ross, T.M. IgA Responses Following Recurrent Influenza Virus Vaccination. Front. Immunol. 2020, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Martinot, M.; Oswald, L.; Parisi, E.; Etienne, E.; Argy, N.; Grawey, I.; De Briel, D.; Zadeh, M.M.; Federici, L.; Blaison, G.; et al. Immunoglobulin Deficiency in Patients with Streptococcus Pneumoniae or Haemophilus Influenzae Invasive Infections. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2014, 19, 79–84. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Yamamoto, T.; Watanabe, S. Association between Selective IgA Deficiency and COVID-19. J. Clin. Biochem. Nutr. 2020, 67, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Jolliff, C.R.; Cost, K.M.; Stivrins, P.C.; Grossman, P.P.; Nolte, C.R.; Franco, S.M.; Fijan, K.J.; Fletcher, L.L.; Shriner, H.C. Reference Intervals for Serum IgG, IgA, IgM, C3, and C4 as Determined by Rate Nephelometry. Clin. Chem. 1982, 28, 126–128. [Google Scholar] [CrossRef]
- Zervou, F.N.; Louie, P.; Stachel, A.; Zacharioudakis, I.M.; Ortiz-Mendez, Y.; Thomas, K.; Aguero-Rosenfeld, M.E. SARS-CoV-2 Antibodies: IgA Correlates with Severity of Disease in Early COVID-19 Infection. J. Med. Virol. 2021, 93, 5409–5415. [Google Scholar] [CrossRef]
- Liew, F.Y.; Russell, S.M.; Appleyard, G.; Brand, C.M.; Beale, J. Cross-Protection in Mice Infected with Influenza A Virus by the Respiratory Route Is Correlated with Local IgA Antibody Rather than Serum Antibody or Cytotoxic T Cell Reactivity. Eur. J. Immunol. 1984, 14, 350–356. [Google Scholar] [CrossRef]
- Asahi-Ozaki, Y.; Yoshikawa, T.; Iwakura, Y.; Suzuki, Y.; Tamura, S.-I.; Kurata, T.; Sata, T. Secretory IgA Antibodies Provide Cross-Protection against Infection with Different Strains of Influenza B Virus. J. Med. Virol. 2004, 74, 328–335. [Google Scholar] [CrossRef]
- Ainai, A.; Tamura, S.-I.; Suzuki, T.; van Riet, E.; Ito, R.; Odagiri, T.; Tashiro, M.; Kurata, T.; Hasegawa, H. Intranasal Vaccination with an Inactivated Whole Influenza Virus Vaccine Induces Strong Antibody Responses in Serum and Nasal Mucus of Healthy Adults. Hum. Vaccin. Immunother. 2013, 9, 1962–1970. [Google Scholar] [CrossRef]
- See, R.H.; Zakhartchouk, A.N.; Petric, M.; Lawrence, D.J.; Mok, C.P.Y.; Hogan, R.J.; Rowe, T.; Zitzow, L.A.; Karunakaran, K.P.; Hitt, M.M.; et al. Comparative Evaluation of Two Severe Acute Respiratory Syndrome (SARS) Vaccine Candidates in Mice Challenged with SARS Coronavirus. J. Gen. Virol. 2006, 87, 641–650. [Google Scholar] [CrossRef]
- Taylor, H.P.; Dimmock, N.J. Mechanism of Neutralization of Influenza Virus by Secretory IgA Is Different from That of Monomeric IgA or IgG. J. Exp. Med. 1985, 161, 198–209. [Google Scholar] [CrossRef]
- Sánchez Montalvo, A.; Gohy, S.; Rombaux, P.; Pilette, C.; Hox, V. The Role of IgA in Chronic Upper Airway Disease: Friend or Foe? Front. Allergy 2022, 3, 852546. [Google Scholar] [CrossRef]
- Cerutti, A. The Regulation of IgA Class Switching. Nat. Rev. Immunol. 2008, 8, 421–434. [Google Scholar] [CrossRef]
- Kumar Bharathkar, S.; Parker, B.W.; Malyutin, A.G.; Haloi, N.; Huey-Tubman, K.E.; Tajkhorshid, E.; Stadtmueller, B.M. The Structures of Secretory and Dimeric Immunoglobulin A. Elife 2020, 9, e56098. [Google Scholar] [CrossRef]
- Boyaka, P.N. Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J. Immunol. 2017, 199, 9–16. [Google Scholar] [CrossRef]
- Phalipon, A.; Cardona, A.; Kraehenbuhl, J.P.; Edelman, L.; Sansonetti, P.J.; Corthésy, B. Secretory Component: A New Role in Secretory IgA-Mediated Immune Exclusion in Vivo. Immunity 2002, 17, 107–115. [Google Scholar] [CrossRef]
- Bidgood, S.R.; Tam, J.C.H.; McEwan, W.A.; Mallery, D.L.; James, L.C. Translocalized IgA Mediates Neutralization and Stimulates Innate Immunity inside Infected Cells. Proc. Natl. Acad. Sci. USA 2014, 111, 13463–13468. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Bumpus, T.; McCarthy, E.A.; Corthésy, B.; Mantis, N.J. Transient Suppression of Shigella Flexneri Type 3 Secretion by a Protective O-Antigen-Specific Monoclonal IgA. MBio 2011, 2, e00042-11. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef]
- Corthésy, B. Multi-Faceted Functions of Secretory IgA at Mucosal Surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed]
- Turula, H.; Wobus, C.E. The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity. Viruses 2018, 10, 237. [Google Scholar] [CrossRef]
- Mathias, A.; Corthésy, B. Recognition of Gram-Positive Intestinal Bacteria by Hybridoma- and Colostrum-Derived Secretory Immunoglobulin A Is Mediated by Carbohydrates. J. Biol. Chem. 2011, 286, 17239–17247. [Google Scholar] [CrossRef]
- Huang, J.; Guerrero, A.; Parker, E.; Strum, J.S.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Site-Specific Glycosylation of Secretory Immunoglobulin A from Human Colostrum. J. Proteome Res. 2015, 14, 1335–1349. [Google Scholar] [CrossRef]
- Boullier, S.; Tanguy, M.; Kadaoui, K.A.; Caubet, C.; Sansonetti, P.; Corthésy, B.; Phalipon, A. Secretory IgA-Mediated Neutralization of Shigella Flexneri Prevents Intestinal Tissue Destruction by Down-Regulating Inflammatory Circuits. J. Immunol. 2009, 183, 5879–5885. [Google Scholar] [CrossRef]
- Weltzin, R.; Traina-Dorge, V.; Soike, K.; Zhang, J.-Y.; Mack, P.; Soman, G.; Drabik, G.; Monath, T.P. Intranasal Monoclonal IgA Antibody to Respiratory Syncytial Virus Protects Rhesus Monkeys against Upper and Lower Respiratory Tract Infection. J. Infect. Dis. 1996, 174, 256–261. [Google Scholar] [CrossRef]
- Renegar, K.B.; Small, P.A.J. Passive Transfer of Local Immunity to Influenza Virus Infection by IgA Antibody. J. Immunol. 1991, 146, 1972–1978. [Google Scholar] [PubMed]
- Silvey, K.J.; Hutchings, A.B.; Vajdy, M.; Petzke, M.M.; Neutra, M.R. Role of Immunoglobulin A in Protection against Reovirus Entry into Murine Peyer’s Patches. J. Virol. 2001, 75, 10870–10879. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, A.B.; Helander, A.; Silvey, K.J.; Chandran, K.; Lucas, W.T.; Nibert, M.L.; Neutra, M.R. Secretory Immunoglobulin A Antibodies against the Sigma1 Outer Capsid Protein of Reovirus Type 1 Lang Prevent Infection of Mouse Peyer’s Patches. J. Virol. 2004, 78, 947–957. [Google Scholar] [CrossRef]
- Reboldi, A.; Arnon, T.I.; Rodda, L.B.; Atakilit, A.; Sheppard, D.; Cyster, J.G. IgA Production Requires B Cell Interaction with Subepithelial Dendritic Cells in Peyer’s Patches. Science 2016, 352, aaf4822. [Google Scholar] [CrossRef]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular Mechanism and Function of CD40/CD40L Engagement in the Immune System. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Boyaka, P.N.; Ohmura, M.; Fujihashi, K.; Koga, T.; Yamamoto, M.; Kweon, M.-N.; Takeda, Y.; Jackson, R.J.; Kiyono, H.; Yuki, Y.; et al. Chimeras of Labile Toxin One and Cholera Toxin Retain Mucosal Adjuvanticity and Direct Th Cell Subsets via Their B Subunit. J. Immunol. 2003, 170, 454–462. [Google Scholar] [CrossRef]
- Brereton, C.F.; Sutton, C.E.; Ross, P.J.; Iwakura, Y.; Pizza, M.; Rappuoli, R.; Lavelle, E.C.; Mills, K.H.G. Escherichia Coli Heat-Labile Enterotoxin Promotes Protective Th17 Responses against Infection by Driving Innate IL-1 and IL-23 Production. J. Immunol. 2011, 186, 5896–5906. [Google Scholar] [CrossRef]
- Mattsson, J.; Schön, K.; Ekman, L.; Fahlén-Yrlid, L.; Yrlid, U.; Lycke, N.Y. Cholera Toxin Adjuvant Promotes a Balanced Th1/Th2/Th17 Response Independently of IL-12 and IL-17 by Acting on Gsα in CD11b+ DCs. Mucosal Immunol. 2015, 8, 815–827. [Google Scholar] [CrossRef]
- Cerutti, A.; Cols, M.; Gentile, M.; Cassis, L.; Barra, C.M.; He, B.; Puga, I.; Chen, K. Regulation of Mucosal IgA Responses: Lessons from Primary Immunodeficiencies. Ann. N. Y. Acad. Sci. 2011, 1238, 132–144. [Google Scholar] [CrossRef]
- Poddighe, D.; Capittini, C. The Role of HLA in the Association between IgA Deficiency and Celiac Disease. Dis. Markers 2021, 2021, 8632861. [Google Scholar] [CrossRef]
- Schussler, E.; Beasley, M.B.; Maglione, P.J. Lung Disease in Primary Antibody Deficiencies. J. Allergy Clin. Immunol. Pract. 2016, 4, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Rundles, C. The Many Faces of Common Variable Immunodeficiency. Hematol. Am. Soc. Hematol. Educ. Progr. 2012, 2012, 301–305. [Google Scholar] [CrossRef]
- Abdelkader, A.; Wahba, A.A.; El-tonsy, M.; Zewail, A.A.; Shams Eldin, M. Recurrent Respiratory Infections and Vitamin A Levels: A Link? It Is Cross-Sectional. Medicine 2022, 101, e30108. [Google Scholar] [CrossRef]
- Soares-Mota, M.; Silva, T.A.; Gomes, L.M.; Pinto, M.A.S.; Mendonça, L.M.C.; Farias, M.L.F.; Nunes, T.; Ramalho, A.; Zaltman, C. High Prevalence of Vitamin A Deficiency in Crohn’s Disease Patients According to Serum Retinol Levels and the Relative Dose-Response Test. World J. Gastroenterol. 2015, 21, 1614–1620. [Google Scholar] [CrossRef]
- Surman, S.L.; Jones, B.G.; Rudraraju, R.; Sealy, R.E.; Hurwitz, J.L. Intranasal Administration of Retinyl Palmitate with a Respiratory Virus Vaccine Corrects Impaired Mucosal IgA Response in the Vitamin A-Deficient Host. Clin. Vaccine Immunol. 2014, 21, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Mistry, D.; Stockley, R.A. IgA1 Protease. Int. J. Biochem. Cell Biol. 2006, 38, 1244–1248. [Google Scholar] [CrossRef]
- Vidarsson, G.; Overbeeke, N.; Stemerding, A.M.; van den Dobbelsteen, G.; van Ulsen, P.; van der Ley, P.; Kilian, M.; van de Winkel, J.G.J. Working Mechanism of Immunoglobulin A1 (IgA1) Protease: Cleavage of IgA1 Antibody to Neisseria Meningitidis PorA Requires de Novo Synthesis of IgA1 Protease. Infect. Immun. 2005, 73, 6721–6726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Q.; Fan, J. Biological significance of IgA1 proteases. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi = J. Biomed. Eng. = Shengwu Yixue Gongchengxue Zazhi 2011, 28, 423–428. [Google Scholar]
- Breedveld, A.; van Egmond, M. IgA and FcαRI: Pathological Roles and Therapeutic Opportunities. Front. Immunol. 2019, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Kirkeby, L.; Rasmussen, T.T.; Reinholdt, J.; Kilian, M. Immunoglobulins in Nasal Secretions of Healthy Humans: Structural Integrity of Secretory Immunoglobulin A1 (IgA1) and Occurrence of Neutralizing Antibodies to IgA1 Proteases of Nasal Bacteria. Clin. Diagn. Lab. Immunol. 2000, 7, 31–39. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.C.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M.; et al. Systemic and Mucosal IgA Responses Are Variably Induced in Response to SARS-CoV-2 MRNA Vaccination and Are Associated with Protection against Subsequent Infection. Mucosal Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef]
- Apostólico, J.D.S.; Lunardelli, V.A.S.; Coirada, F.C.; Boscardin, S.B.; Rosa, D.S. Adjuvants: Classification, Modus Operandi, and Licensing. J. Immunol. Res. 2016, 2016, 1459394. [Google Scholar] [CrossRef]
- Aoshi, T. Modes of Action for Mucosal Vaccine Adjuvants. Viral Immunol. 2017, 30, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 Vaccines: From Bench to Bed. EBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef]
- Bricker, T.L.; Darling, T.L.; Hassan, A.O.; Harastani, H.H.; Soung, A.; Jiang, X.; Dai, Y.-N.; Zhao, H.; Adams, L.J.; Holtzman, M.J.; et al. A Single Intranasal or Intramuscular Immunization with Chimpanzee Adenovirus-Vectored SARS-CoV-2 Vaccine Protects against Pneumonia in Hamsters. Cell Rep. 2021, 36, 109400. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A Single Dose of an Adenovirus-Vectored Vaccine Provides Protection against SARS-CoV-2 Challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef]
- Jangra, S.; Landers, J.J.; Rathnasinghe, R.; O’Konek, J.J.; Janczak, K.W.; Cascalho, M.; Kennedy, A.A.; Tai, A.W.; Baker, J.R.; Schotsaert, M.; et al. A Combination Adjuvant for the Induction of Potent Antiviral Immune Responses for a Recombinant SARS-CoV-2 Protein Vaccine. Front. Immunol. 2021, 12, 729189. [Google Scholar] [CrossRef]
- Alfagih, I.M.; Aldosari, B.; AlQuadeib, B.; Almurshedi, A.; Alfagih, M.M. Nanoparticles as Adjuvants and Nanodelivery Systems for mRNA-Based Vaccines. Pharmaceutics 2021, 13, 45. [Google Scholar] [CrossRef]
- Sui, Y.; Li, J.; Zhang, R.; Prabhu, S.K.; Andersen, H.; Venzon, D.; Cook, A.; Brown, R.; Teow, E.; Velasco, J.; et al. Protection against SARS-CoV-2 Infection by a Mucosal Vaccine in Rhesus Macaques. JCI Insight 2021, 6, e148494. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, L.; Morzycka-Wroblewska, E.; Smith, J.R.; Kagnoff, M.F. Cytokine-Induced Differentiation of IgA B Cells: Studies Using an IgA Expressing B-Cell Lymphoma. Immunology 1992, 76, 235–241. [Google Scholar] [PubMed]
- Cao, M.; Sasaki, O.; Yamada, A.; Imanishi, J. Enhancement of the Protective Effect of Inactivated Influenza Virus Vaccine by Cytokines. Vaccine 1992, 10, 238–242. [Google Scholar] [CrossRef]
- Bracci, L.; Canini, I.; Puzelli, S.; Sestili, P.; Venditti, M.; Spada, M.; Donatelli, I.; Belardelli, F.; Proietti, E. Type I IFN Is a Powerful Mucosal Adjuvant for a Selective Intranasal Vaccination against Influenza Virus in Mice and Affects Antigen Capture at Mucosal Level. Vaccine 2005, 23, 2994–3004. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-Z.; Xu, R.; Quan, F.-S.; Kang, S.-M.; Wang, L.; Compans, R.W. Intranasal Immunization with Influenza VLPs Incorporating Membrane-Anchored Flagellin Induces Strong Heterosubtypic Protection. PLoS ONE 2010, 5, e13972. [Google Scholar] [CrossRef]
- Del Fresno, C.; García-Arriaza, J.; Martínez-Cano, S.; Heras-Murillo, I.; Jarit-Cabanillas, A.; Amores-Iniesta, J.; Brandi, P.; Dunphy, G.; Suay-Corredera, C.; Pricolo, M.R.; et al. The Bacterial Mucosal Immunotherapy MV130 Protects Against SARS-CoV-2 Infection and Improves COVID-19 Vaccines Immunogenicity. Front. Immunol. 2021, 12, 748103. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Liu, C.; Liu, J.-Y.; Jin, Z.-L.; Jin, Z.; Xue, R.-Y.; Feng, R.; Li, G.-C.; Deng, Y.; Cheng, H.; et al. Novel Synthetic Lipopeptides as Potential Mucosal Adjuvants Enhanced SARS-CoV-2 RRBD-Induced Immune Response. Front. Immunol. 2022, 13, 833418. [Google Scholar] [CrossRef]
- Ichinohe, T.; Watanabe, I.; Ito, S.; Fujii, H.; Moriyama, M.; Tamura, S.-I.; Takahashi, H.; Sawa, H.; Chiba, J.; Kurata, T.; et al. Synthetic Double-Stranded RNA Poly(I:C) Combined with Mucosal Vaccine Protects against Influenza Virus Infection. J. Virol. 2005, 79, 2910–2919. [Google Scholar] [CrossRef]
- Mudgal, R.; Nehul, S.; Tomar, S. Prospects for Mucosal Vaccine: Shutting the Door on SARS-CoV-2. Hum. Vaccin. Immunother. 2020, 16, 2921–2931. [Google Scholar] [CrossRef]
- Moldoveanu, Z.; Love-Homan, L.; Huang, W.Q.; Krieg, A.M. CpG DNA, a Novel Immune Enhancer for Systemic and Mucosal Immunization with Influenza Virus. Vaccine 1998, 16, 1216–1224. [Google Scholar] [CrossRef]
- Pizza, M.; Giuliani, M.M.; Fontana, M.R.; Monaci, E.; Douce, G.; Dougan, G.; Mills, K.H.; Rappuoli, R.; Del Giudice, G. Mucosal Vaccines: Non Toxic Derivatives of LT and CT as Mucosal Adjuvants. Vaccine 2001, 19, 2534–2541. [Google Scholar] [CrossRef]
- Tamura, S.; Asanuma, H.; Tomita, T.; Komase, K.; Kawahara, K.; Danbara, H.; Hattori, N.; Watanabe, K.; Suzuki, Y.; Nagamine, T. Escherichia Coli Heat-Labile Enterotoxin B Subunits Supplemented with a Trace Amount of the Holotoxin as an Adjuvant for Nasal Influenza Vaccine. Vaccine 1994, 12, 1083–1089. [Google Scholar] [CrossRef]
- Sasaki, E.; Asanuma, H.; Momose, H.; Furuhata, K.; Mizukami, T.; Hamaguchi, I. Nasal Alum-Adjuvanted Vaccine Promotes IL-33 Release from Alveolar Epithelial Cells That Elicits IgA Production via Type 2 Immune Responses. PLoS Pathog. 2021, 17, e1009890. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 Vaccines Strategies: A Comprehensive Review of Phase 3 Candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Cybulski, V.; Whitacre, M.; Bess, L.S.; Livesay, M.T.; Walsh, L.; Burkhart, D.; Bazin, H.G.; Evans, J.T. Novel Lipidated Imidazoquinoline TLR7/8 Adjuvants Elicit Influenza-Specific Th1 Immune Responses and Protect Against Heterologous H3N2 Influenza Challenge in Mice. Front. Immunol. 2020, 11, 406. [Google Scholar] [CrossRef]
- Madhun, A.S.; Haaheim, L.R.; Nøstbakken, J.K.; Ebensen, T.; Chichester, J.; Yusibov, V.; Guzman, C.A.; Cox, R.J. Intranasal C-Di-GMP-Adjuvanted Plant-Derived H5 Influenza Vaccine Induces Multifunctional Th1 CD4+ Cells and Strong Mucosal and Systemic Antibody Responses in Mice. Vaccine 2011, 29, 4973–4982. [Google Scholar] [CrossRef]
- Stark, F.C.; Akache, B.; Deschatelets, L.; Tran, A.; Stuible, M.; Durocher, Y.; McCluskie, M.J.; Agbayani, G.; Dudani, R.; Harrison, B.A.; et al. Intranasal Immunization with a Proteosome-Adjuvanted SARS-CoV-2 Spike Protein-Based Vaccine Is Immunogenic and Efficacious in Mice and Hamsters. Sci. Rep. 2022, 12, 9772. [Google Scholar] [CrossRef]
- Takaki, H.; Ichimiya, S.; Matsumoto, M.; Seya, T. Mucosal Immune Response in Nasal-Associated Lymphoid Tissue upon Intranasal Administration by Adjuvants. J. Innate Immun. 2018, 10, 515–521. [Google Scholar] [CrossRef]
- Young-Il, K.; Dokyun, K.; Kwang-Min, Y.; David, S.H.; Shin-Ae, L.; Casel, M.A.B.; Seung-Gyu, J.; Stephanie, K.; WooRam, J.; Chih-Jen, L.; et al. Development of Spike Receptor-Binding Domain Nanoparticles as a Vaccine Candidate against SARS-CoV-2 Infection in Ferrets. MBio 2021, 12, e00230-21. [Google Scholar] [CrossRef]
- Zepeda-Cervantes, J.; Ramírez-Jarquín, J.O.; Vaca, L. Interaction between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs. Front. Immunol. 2020, 11, 1100. [Google Scholar] [CrossRef]
- Prates-Syed, W.A.; Chaves, L.C.S.; Crema, K.P.; Vuitika, L.; Lira, A.; Côrtes, N.; Kersten, V.; Guimarães, F.E.G.; Sadraeian, M.; Barroso da Silva, F.L.; et al. VLP-Based COVID-19 Vaccines: An Adaptable Technology against the Threat of New Variants. Vaccines 2021, 9, 1409. [Google Scholar] [CrossRef]
- Attia, M.A.; Essa, E.A.; Elebyary, T.T.; Faheem, A.M.; Elkordy, A.A. Brief on Recent Application of Liposomal Vaccines for Lower Respiratory Tract Viral Infections: From Influenza to COVID-19 Vaccines. Pharmaceuticals 2021, 14, 1173. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Lee, S.I.; Bae, J.-Y.; Park, M.-S.; Lee, Y.; Kwon, H.-J. Production of a Monoclonal Antibody Targeting the M Protein of MERS-CoV for Detection of MERS-CoV Using a Synthetic Peptide Epitope Formulated with a CpG-DNA-Liposome Complex. Int. J. Pept. Res. Ther. 2019, 25, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Chaudhari, A.A.; Dennis, V.; Kirby, D.J.; Perrie, Y.; Singh, S.R. Anti-RSV Peptide-Loaded Liposomes for the Inhibition of Respiratory Syncytial Virus. Bioengineering 2018, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, M.J.; Kim, S.; Zhou, H.; Jing, T.T.; Luna, M.; Guss, J.D.; Reddy, P.; Lai, K.; Leifer, C.A.; Brito, I.L.; et al. Immunomodulatory Nanogels Overcome Restricted Immunity in a Murine Model of Gut Microbiome–Mediated Metabolic Syndrome. Sci. Adv. 2022, 5, eaav9788. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Dkhar, H.K.; Chandra, V.; Dave, S.; Nanduri, R.; Janmeja, A.K.; Agrewala, J.N.; Gupta, P. Mycobacterium Tuberculosis Modulates Macrophage Lipid-Sensing Nuclear Receptors PPARγ and TR4 for Survival. J. Immunol. 2012, 188, 5593–5603. [Google Scholar] [CrossRef] [PubMed]
- Nochi, T.; Yuki, Y.; Takahashi, H.; Sawada, S.; Mejima, M.; Kohda, T.; Harada, N.; Kong, I.G.; Sato, A.; Kataoka, N.; et al. Nanogel Antigenic Protein-Delivery System for Adjuvant-Free Intranasal Vaccines. Nat. Mater. 2010, 9, 572–578. [Google Scholar] [CrossRef]
- Chen, M.; Shou, Z.; Jin, X.; Chen, Y. Emerging Strategies in Nanotechnology to Treat Respiratory Tract Infections: Realizing Current Trends for Future Clinical Perspectives. Drug Deliv. 2022, 29, 2442–2458. [Google Scholar] [CrossRef]
- Calzas, C.; Chevalier, C. Innovative Mucosal Vaccine Formulations against Influenza A Virus Infections. Front. Immunol. 2019, 10, 1605. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).