Potential Beneficial Effects of Vitamin K in SARS-CoV-2 Induced Vascular Disease?
Abstract
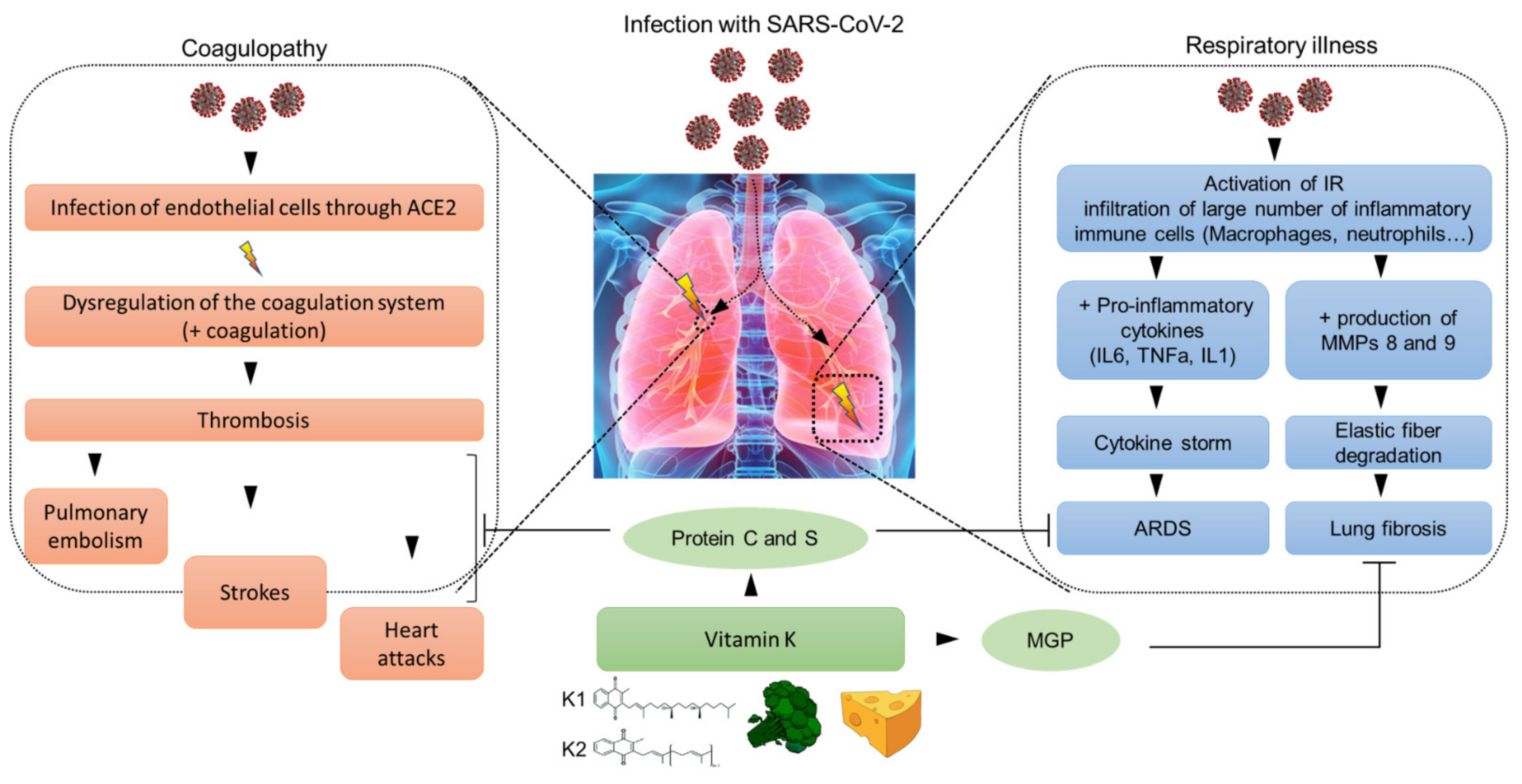
:1. Introduction
2. Respiratory Illness Associated with COVID-19
3. Coagulopathy and COVID-19
4. Vitamin K
5. Structure, Uptake and Distribution of Vitamin K
6. Vitamin K: The Coagulation Switch and Beyond
7. Using Vitamin K to Improve COVID-19 Outcomes
8. Vitamin K: An Anticoagulation Option for COVID-19?
9. Vitamin K: An Immunomodulatory Option for COVID-19?
10. Vitamin K: Vascular Health Promoter to Protect against Cardiovascular Complications and Lung Fibrosis?
11. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Weekly Operational Update on COVID-19; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020, 18, 1738–1742. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Lodigiani, C.; Iapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.D.; Sacco, C.; Alexia, B.; et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020, 191, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, S.; Coppens, M.; van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.A.; Bouman, C.C.S.; Beenen, L.F.M.; Kootte, R.S.; Heijmans, J.; et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Dofferhoff, A.S.M.; Piscaer, I.; Schurgers, L.J.; Visser, M.P.J.; van den Ouweland, J.M.W.; de Jong, P.A.; Gosens, R.; Hackeng, T.M.; van Daal, H.; Lux, P.; et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID-19. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- She, J.; Jiang, J.; Ye, L.; Hu, L.; Bai, C.; Song, Y. 2019 novel coronavirus of pneumonia in Wuhan, China: Emerging attack and management strategies. Clin. Transl. Med. 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T.; Hill, T.; Li, K.; Peters, C.J.; Tseng, C.T. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J. Virol. 2009, 83, 3039–3048. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Dassler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.Y. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients with Lung Cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, Y.; Wang, F.; Ren, H.; Zhang, S.; Shi, X.; Yu, X.; Dong, K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001343. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 1. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, B.; Qu, Y.; Chen, Y.; Xiong, J.; Feng, Y.; Men, D.; Huang, Q.; Liu, Y.; Yang, B. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 2020, 71, 1937–1942. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, T.; O’Reilly, P.; Antony, V.B.; Gaggar, A.; Thannickal, V.J. Matrix Remodeling in Pulmonary Fibrosis and Emphysema. Am. J. Respir. Cell Mol. Biol. 2016, 54, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [Green Version]
- Chen, W. A potential treatment of COVID-19 with TGF-β blockade. Int. J. Biol. Sci. 2020, 16, 1954–1955. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atallah, B.; Mallah, S.I.; AlMahmeed, W. Anticoagulation in COVID-19. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 260–261. [Google Scholar] [CrossRef]
- McGonagle, D.; O’Donnell, J.S.; Sharif, K.; Emery, P.; Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020, 2, e437–e445. [Google Scholar] [CrossRef]
- Bompard, F.; Monnier, H.; Saab, I.; Tordjman, M.; Abdoul, H.; Fournier, L.; Sanchez, O.; Lorut, C.; Chassagnon, G.; Revel, M.P. Pulmonary embolism in patients with Covid-19 pneumonia. Eur. Respir. J. 2020. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Xu, Y.; Xu, Z.; Huang, Y.; Chen, S.; Li, S.; Liu, D.; Lin, Z.; Li, Y. Ventilatory Ratio in Hypercapnic Mechanically Ventilated Patients with COVID-19–associated Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 201, 1297–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients with COVID-19. Ann. Intern. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Pappas, C.; Belser, J.A.; Houser, K.V.; Zhong, W.; Wadford, D.A.; Stevens, T.; Balczon, R.; Katz, J.M.; Tumpey, T.M. Human pulmonary microvascular endothelial cells support productive replication of highly pathogenic avian influenza viruses: Possible involvement in the pathogenesis of human H5N1 virus infection. J. Virol. 2012, 86, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Farndale, R.W.; Sixma, J.J.; Barnes, M.J.; de Groot, P.G. The role of collagen in thrombosis and hemostasis. J. Thromb. Haemost. 2004, 2, 561–573. [Google Scholar] [CrossRef]
- Frantzeskaki, F.; Armaganidis, A.; Orfanos, S.E. Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks between Inflammation and Coagulation. Respiration 2017, 93, 212–225. [Google Scholar] [CrossRef]
- Esmon, C.T.; Vigano-D’Angelo, S.; D’Angelo, A.; Comp, P.C. Anticoagulation proteins C and S. Adv. Exp. Med. Biol. 1987, 214, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, A.; Rabin, J.; Menaker, J.; Madathil, R.; Galvagno, S.; Menne, A.; Chow, J.H.; Grazioli, A.; Herr, D.; Tanaka, K.; et al. Factor VIII and Functional Protein C Activity in Critically Ill Patients with Coronavirus Disease 2019: A Case Series. A A Pract. 2020, 14, e01236. [Google Scholar] [CrossRef] [PubMed]
- Dam, H. The antihaemorrhagic vitamin of the chick. Biochem. J. 1935, 29, 1273–1285. [Google Scholar] [CrossRef] [Green Version]
- Dam, H.; Schönheyder, F. A deficiency disease in chicks resembling scurvy. Biochem. J. 1934, 28, 1355–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarlane, W.D.; Graham, W.R., Jr.; Richardson, F. The fat-soluble vitamin requirements of the chick: The vitamin A and vitamin D content of fish meal and meat meal. Biochem. J. 1931, 25, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKee, R.W.; Binkley, S.B.; MacCorquodale, D.W.; Thayer, S.A.; Doisy, E.A. The Isolation of Vitamins K1 And K2. J. Am. Chem. Soc. 1939, 61, 1295. [Google Scholar] [CrossRef]
- Zetterström, R.H.C.P. Dam (1895–1976) and E. A. Doisy (1893–1986): The discovery of antihaemorrhagic vitamin and its impact on neonatal health. Acta Paediatr. 2006, 95, 642–644. [Google Scholar] [CrossRef]
- Jacobsen, B.K.; Dam, H. Vitamin K in bacteria. Biochim. Biophys. Acta 1960, 40, 211–216. [Google Scholar] [CrossRef]
- Gijsbers, B.L.; Jie, K.-S.G.; Vermeer, C. Effect of food composition on vitamin K absorption in human volunteers. Br. J. Nutr. 1996, 76, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Schurgers, L.J.; Vermeer, C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 2000, 30, 298–307. [Google Scholar] [CrossRef]
- Dismore, M.L.; Haytowitz, D.B.; Gebhardt, S.E.; Peterson, J.W.; Booth, S.L. Vitamin K content of nuts and fruits in the US diet. J. Am. Diet. Assoc. 2003, 103, 1650–1652. [Google Scholar] [CrossRef]
- Tarento, T.D.; McClure, D.D.; Talbot, A.M.; Regtop, H.L.; Biffin, J.R.; Valtchev, P.; Dehghani, F.; Kavanagh, J.M. A potential biotechnological process for the sustainable production of vitamin K1. Crit. Rev. Biotechnol. 2019, 39, 1–19. [Google Scholar] [CrossRef]
- Davidson, R.T.; Foley, A.L.; Engelke, J.A.; Suttie, J.W. Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. J. Nutr. 1998, 128, 220–223. [Google Scholar] [CrossRef] [Green Version]
- Marles, R.J.; Roe, A.L.; Oketch-Rabah, H.A. US Pharmacopeial Convention safety evaluation of menaquinone-7, a form of vitamin K. Nutr. Rev. 2017, 75, 553–578. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, A.C.; Pavlic, A.; Petsophonsakul, P.; Halder, M.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K2 needs an RDI separate from vitamin K1. Nutrients 2020, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Teunissen, K.J.; Hamulyák, K.; Knapen, M.H.; Vik, H.; Vermeer, C. Vitamin K-containing dietary supplements: Comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 2007, 109, 3279–3283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Binkley, N.C.; Krueger, D.C.; Engelke, J.A.; Foley, A.L.; Suttie, J.W. Vitamin K supplementation reduces serum concentrations of under-gamma-carboxylated osteocalcin in healthy young and elderly adults. Am. J. Clin. Nutr. 2000, 72, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Shearer, M.J.; Mallinson, C.N.; Webster, G.R.; Barkhan, P. Clearance from plasma and excretion in urine, faeces and bile of an intravenous dose of tritiated vitamin K 1 in man. Br. J. Haematol. 1972, 22, 579–588. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Vermeer, C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim. Biophys. Acta 2002, 1570, 27–32. [Google Scholar] [CrossRef]
- Spronk, H.M.; Soute, B.A.; Schurgers, L.J.; Thijssen, H.H.; De Mey, J.G.; Vermeer, C. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats. J. Vasc. Res. 2003, 40, 531–537. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [Green Version]
- Kaneki, M.; Hedges, S.J.; Hosoi, T.; Fujiwara, S.; Lyons, A.; Ishida, N.; Nakagawa, M.; Takechi, M.; Sano, Y.; Mizuno, Y. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: Possible implications for hip-fracture risk. Nutrition 2001, 17, 315–321. [Google Scholar] [CrossRef]
- Dahlbäck, B. Blood coagulation. Lancet 2000, 355, 1627–1632. [Google Scholar] [CrossRef]
- Espana, F.; Medina, P.; Navarro, S.; Zorio, E.; Estellés, A.; Aznar, J. The multifunctional protein C system. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2005, 3, 119–131. [Google Scholar] [CrossRef]
- Olson, R.E. The function and metabolism of vitamin K. Annu. Rev. Nutr. 1984, 4, 281–337. [Google Scholar] [CrossRef] [PubMed]
- Suttie, J.W. Vitamin K-dependent carboxylase. Annu. Rev. Biochem. 1985, 54, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Danziger, J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin. J. Am. Soc. Nephrol. 2008, 3, 1504–1510. [Google Scholar] [CrossRef]
- Nelsestuen, G.L.; Zytkovicz, T.H.; Howard, J.B. The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin. J. Biol. Chem. 1974, 249, 6347–6350. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Spronk, H.M. Differential cellular effects of old and new oral anticoagulants: Consequences to the genesis and progression of atherosclerosis. Thromb. Haemost. 2014, 112, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Nelsestuen, G.L.; Suttie, J.W. Mode of action of vitamin K. Calcium binding properties of bovine prothrombin. Biochemistry 1972, 11, 4961–4964. [Google Scholar] [CrossRef]
- Ellison, E.H.; Castellino, F.J. Adsorption of vitamin K-dependent blood coagulation proteins to spread phospholipid monolayers as determined from combined measurements of the surface pressure and surface protein concentration. Biochemistry 1998, 37, 7997–8003. [Google Scholar] [CrossRef]
- Reddi, K.; Henderson, B.; Meghji, S.; Wilson, M.; Poole, S.; Hopper, C.; Harris, M.; Hodges, S.J. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine 1995, 7, 287–290. [Google Scholar] [CrossRef]
- Pan, M.H.; Maresz, K.; Lee, P.S.; Wu, J.C.; Ho, C.T.; Popko, J.; Mehta, D.S.; Stohs, S.J.; Badmaev, V. Inhibition of TNF-α, IL-1α, and IL-1β by Pretreatment of Human Monocyte-Derived Macrophages with Menaquinone-7 and Cell Activation with TLR Agonists In Vitro. J. Med. Food 2016, 19, 663–669. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Shirakawa, H.; Miura, A.; Giriwono, P.E.; Sato, S.; Ohashi, A.; Iribe, M.; Goto, T.; Komai, M. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor κB through the repression of IKKα/β phosphorylation. J. Nutr. Biochem. 2010, 21, 1120–1126. [Google Scholar] [CrossRef]
- Myneni, V.D.; Mezey, E. Immunomodulatory effect of vitamin K2: Implications for bone health. Oral Dis. 2018, 24, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Urist, M.R.; Otawara, Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem. Biophys. Res. Commun. 1983, 117, 765–771. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Lian, J.B.; Gallop, P.M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc. Natl. Acad. Sci. USA 1975, 72, 3925–3929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willems, B.A.; Vermeer, C.; Reutelingsperger, C.P.; Schurgers, L.J. The realm of vitamin K dependent proteins: Shifting from coagulation toward calcification. Mol. Nutr. Food Res. 2014, 58, 1620–1635. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Munroe, P.B.; Olgunturk, R.O.; Fryns, J.-P.; Van Maldergem, L.; Ziereisen, F.; Yuksel, B.; Gardiner, R.M.; Chung, E. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat. Genet. 1999, 21, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Piscaer, I.; Wouters, E.F.M.; Vermeer, C.; Janssens, W.; Franssen, F.M.E.; Janssen, R. Vitamin K deficiency: The linking pin between COPD and cardiovascular diseases? Respir. Res. 2017, 18, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, R.; Vermeer, C. Vitamin K deficit and elastolysis theory in pulmonary elasto-degenerative diseases. Med Hypotheses 2017, 108, 38–41. [Google Scholar] [CrossRef]
- Jono, S.; Shioi, A.; Ikari, Y.; Nishizawa, Y. Vascular calcification in chronic kidney disease. J. Bone Miner. Metab. 2006, 24, 176–181. [Google Scholar] [CrossRef]
- ERA-EDTA Council, ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: A call to action by the ERA-EDTA. Nephrol. Dial. Transplant. 2021, 36, 87–94. [Google Scholar] [CrossRef]
- Han, H.; Yang, L.; Liu, R.; Liu, F.; Wu, K.L.; Li, J.; Liu, X.H.; Zhu, C.L. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020, 58, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Kollias, A.; Kyriakoulis, K.G.; Dimakakos, E.; Poulakou, G.; Stergiou, G.S.; Syrigos, K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br. J. Haematol. 2020, 189, 846–847. [Google Scholar] [CrossRef]
- Manna, P.; Kalita, J. Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: A review. Nutrition 2016, 32, 732–739. [Google Scholar] [CrossRef]
- Campbell, A.W. Vitamin K2 in the Prevention of Cardiovascular Diseases and Diabetes. Altern. Ther. Health Med. 2017, 23, 8–10. [Google Scholar]
- Van Ballegooijen, A.J.; Cepelis, A.; Visser, M.; Brouwer, I.A.; van Schoor, N.M.; Beulens, J.W. Joint Association of Low Vitamin D and Vitamin K Status with Blood Pressure and Hypertension. Hypertension 2017, 69, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Cozzolino, M.; Mangano, M.; Galassi, A.; Ciceri, P.; Messa, P.; Nigwekar, S. Vitamin K in chronic kidney disease. Nutrients 2019, 11, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cranenburg, E.C.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.; Dalmeijer, G.W.; Westerhuis, R.; Magdeleyns, E.J.; Herfs, M.; Vermeer, C.; Laverman, G.D. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shea, M.K.; O’Donnell, C.J.; Hoffmann, U.; Dallal, G.E.; Dawson-Hughes, B.; Ordovas, J.M.; Price, P.A.; Williamson, M.K.; Booth, S.L. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am. J. Clin. Nutr. 2009, 89, 1799–1807. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.D.; Moore, H.B.; Yaffe, M.B.; Moore, E.E. ISTH interim guidance on recognition and management of coagulopathy in COVID-19: A comment. J. Thromb. Haemost. 2020. [Google Scholar] [CrossRef] [PubMed]
- Thachil, J. The versatile heparin in COVID-19. J. Thromb. Haemost. 2020, 18, 1020–1022. [Google Scholar] [CrossRef] [Green Version]
- Harr, J.N.; Moore, E.E.; Chin, T.L.; Ghasabyan, A.; Gonzalez, E.; Wohlauer, M.V.; Sauaia, A.; Banerjee, A.; Silliman, C.C. Postinjury hyperfibrinogenemia compromises efficacy of heparin-based venous thromboembolism prophylaxis. Shock 2014, 41, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turshudzhyan, A. Anticoagulation Options for Coronavirus Disease 2019 (COVID-19)-Induced Coagulopathy. Cureus 2020, 12, e8150. [Google Scholar] [CrossRef] [PubMed]
- Schutgens, R.E. DOAC in COVID-19: Yes or No? HemaSphere 2021, 5, e526. [Google Scholar] [CrossRef] [PubMed]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef]
- Testa, S.; Prandoni, P.; Paoletti, O.; Morandini, R.; Tala, M.; Dellanoce, C.; Giorgi-Pierfranceschi, M.; Betti, M.; Battista Danzi, G.; Pan, A. Direct oral anticoagulant plasma levels’ striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: The Cremona experience. J. Thromb. Haemost. 2020, 18, 1320–1323. [Google Scholar] [CrossRef]
- Burstyn-Cohen, T.; Heeb, M.J.; Lemke, G. Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J. Clin. Investig. 2009, 119, 2942–2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fair, D.S.; Marlar, R.A.; Levin, E.G. Human endothelial cells synthesize protein S. Blood 1986, 67, 1168–1171. [Google Scholar] [CrossRef] [Green Version]
- Stern, D.; Brett, J.; Harris, K.; Nawroth, P. Participation of endothelial cells in the protein C-protein S anticoagulant pathway: The synthesis and release of protein S. J. Cell Biol. 1986, 102, 1971–1978. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Shearer, M.J.; Hamulyák, K.; Stöcklin, E.; Vermeer, C. Effect of vitamin K intake on the stability of oral anticoagulant treatment: Dose-response relationships in healthy subjects. Blood 2004, 104, 2682–2689. [Google Scholar] [CrossRef]
- Paliani, U.; Filippucci, E.; Gresele, P. Significant potentiation of anticoagulation by flu-vaccine during the season 2001–2002. Haematologica 2003, 88, 599–600. [Google Scholar] [PubMed]
- Kuo, A.; Brown, J.; Clinard, V. Effect of influenza vaccination on international normalized ratio during chronic warfarin therapy. J. Clin. Pharm. Ther. 2012, 37, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Iorio, A.; Basileo, M.; Marcucci, M.; Guercini, F.; Camilloni, B.; Paccamiccio, E.; Vecchioli, M.; Iorio, A.M. Influenza vaccination and vitamin K antagonist treatment: A placebo-controlled, randomized, double-blind crossover study. Arch. Intern. Med. 2010, 170, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Koshihara, Y.; Hoshi, K.; Shiraki, M. Vitamin K2 (menatetrenone) inhibits prostaglandin synthesis in cultured human osteoblast-like periosteal cells by inhibiting prostaglandin H synthase activity. Biochem. Pharmacol. 1993, 46, 1355–1362. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Puig, F.; Fuster, G.; Adda, M.; Blanch, L.; Farre, R.; Navajas, D.; Artigas, A. Barrier-protective effects of activated protein C in human alveolar epithelial cells. PLoS ONE 2013, 8, e56965. [Google Scholar] [CrossRef] [Green Version]
- Urawa, M.; Kobayashi, T.; D’Alessandro-Gabazza, C.N.; Fujimoto, H.; Toda, M.; Roeen, Z.; Hinneh, J.A.; Yasuma, T.; Takei, Y.; Taguchi, O.; et al. Protein S is protective in pulmonary fibrosis. J. Thromb. Haemost. 2016, 14, 1588–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhmerov, A.; Marbán, E. COVID-19 and the Heart. Circ. Res. 2020, 126, 1443–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalmeijer, G.; van der Schouw, Y.T.; Magdeleyns, E.; Ahmed, N.; Vermeer, C.; Beulens, J. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis 2012, 225, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Vermeer, C.; Magdeleyns, E.J.; Schurgers, L.J.; Beulens, J.W. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J. Nutr. Biochem. 2013, 24, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, R.J.; De Leeuw, P.W.; Kessels, A.G.; Schurgers, L.J.; Vermeer, C.; Van Engelshoven, J.M.; Kemerink, G.J.; Kroon, A.A. Calcium scores and matrix Gla protein levels: Association with vitamin K status. Eur. J. Clin. Investig. 2010, 40, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Braam, L.A.; Hoeks, A.P.; Brouns, F.; Hamulyák, K.; Gerichhausen, M.J.; Vermeer, C. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: A follow-up study. Thromb. Haemost. 2004, 91, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enomoto, N.; Suda, T.; Kono, M.; Kaida, Y.; Hashimoto, D.; Fujisawa, T.; Inui, N.; Nakamura, Y.; Imokawa, S.; Funai, K.; et al. Amount of elastic fibers predicts prognosis of idiopathic pulmonary fibrosis. Respir. Med. 2013, 107, 1608–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piscaer, I.; van den Ouweland, J.M.W.; Vermeersch, K.; Reynaert, N.L.; Franssen, F.M.E.; Keene, S.; Wouters, E.F.M.; Janssens, W.; Vermeer, C.; Janssen, R. Low Vitamin K Status Is Associated with Increased Elastin Degradation in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2019, 8, 1116. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudelko, M.; Yip, T.F.; Hei Law, G.C.; Lee, S.M.Y. Potential Beneficial Effects of Vitamin K in SARS-CoV-2 Induced Vascular Disease? Immuno 2021, 1, 17-29. https://doi.org/10.3390/immuno1010003
Kudelko M, Yip TF, Hei Law GC, Lee SMY. Potential Beneficial Effects of Vitamin K in SARS-CoV-2 Induced Vascular Disease? Immuno. 2021; 1(1):17-29. https://doi.org/10.3390/immuno1010003
Chicago/Turabian StyleKudelko, Mateusz, Tsz Fung Yip, Grace Chun Hei Law, and Suki Man Yan Lee. 2021. "Potential Beneficial Effects of Vitamin K in SARS-CoV-2 Induced Vascular Disease?" Immuno 1, no. 1: 17-29. https://doi.org/10.3390/immuno1010003
APA StyleKudelko, M., Yip, T. F., Hei Law, G. C., & Lee, S. M. Y. (2021). Potential Beneficial Effects of Vitamin K in SARS-CoV-2 Induced Vascular Disease? Immuno, 1(1), 17-29. https://doi.org/10.3390/immuno1010003




