Abstract
Olanzapine and clozapine, two of the most efficacious second-generation antipsychotic drugs (SGAs), are known to cause serious metabolic side effects. Despite their clinical utility, the epigenetic basis of these metabolic side-effects remains poorly understood. This exploratory study investigated whether histone methylation is associated with metabolic disorders following chronic SGA treatment. Rats were treated with olanzapine or clozapine for 9 weeks and then sacrificed 2 h after the final treatment. After evaluating the metabolic parameters, Chromatin immunoprecipitation (ChIP)-deep sequencing was conducted on liver tissue pooled from twelve samples per group to quantify histone H3K4me2 methylation and transcriptional changes. Gene ontology term enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were used to explore shared functional pathways of genes with differential histone methylation. Key findings revealed that both olanzapine and clozapine induced widespread changes in hepatic histone methylation, particularly hypermethylation at H3K4me2 across genes involved in lipid and glucose metabolism, insulin signalling, and adipogenesis. Olanzapine- and clozapine-treated rats displayed increased H3K4me2 levels at numerous gene loci and at distinct genomic regions. These findings suggest the importance of monitoring metabolic parameters in psychiatric patients and potential novel strategies to mitigate SGA-induced metabolic side effects.
1. Introduction
Second-generation antipsychotics drugs (SGAs) are used as first-line treatment for psychotic disorders including schizophrenia, bipolar disorder, and other mental disorders [1,2,3]. Olanzapine is one of the most frequently prescribed SGAs, with enhanced tolerability and efficacy compared to first-generation antipsychotics and other SGAs (such as quetiapine and ziprasidone) [4,5]. Clozapine is the only antipsychotic drug with proven efficacy for treatment-resistant schizophrenia therapy [6,7]. However, olanzapine and clozapine are two SGAs with the most severe metabolic side effects, including obesity, dyslipidaemia, hypertriglyceridemia, glucose dysregulation, insulin resistance, and type 2 diabetes mellitus [8]. These side effects not only pose serious risks for cardiovascular disease and premature death but also contribute to poor medication adherence, a major challenge in the treatment of psychotic disorders, since discontinuing antipsychotic therapy markedly increases the likelihood of relapse [8,9]. Therefore, a deeper understanding of the molecular pathways underlying SGA-induced metabolic side effects is essential for improving the safety and effectiveness of psychiatric treatments. Uncovering these pathways could provide potentials for the development of targeted interventions to mitigate adverse outcomes while preserving therapeutic benefits.
Epigenetic regulation, such as histone modification, plays an important role in the progression of metabolic disorders [10]. Modifications at the special site of lysine (K) residues on the histone N-terminal tail influence the Chromatin structure and gene expression; for example, the methylation of K4 on histone 3 (H3K4me) is widely recognised as a marker of active transcription [11], while H3K4me-mediated epigenetic regulation is linked to metabolic status [12]. Compared to healthy individuals, those with type 2 diabetes mellitus demonstrate altered patterns in histone methylation [13]. Evidence from both animal and human studies indicates that SGAs can disrupt epigenetic homeostasis and elicit pharmacoepigenomic effects, thereby offering promising insights into their potential role in modulating the host epigenome [14,15]. Recently, olanzapine has been found to cause lipid metabolic dysfunctions through enhancing H3H4me2 and reducing H3K9me histone modification on hepatic PPARγ and related lipogenesis and adipogenesis pathways [16]. It is therefore important to investigate whether these epigenetic alterations are also associated with increased hepatic SREBP (sterol regulatory element-binding protein) signalling [17,18], another key regulator of lipid biosynthesis, which may further exacerbate insulin resistance. Supporting this, elevated HOMA-IR (homeostatic model assessment of insulin resistance) levels observed in SGA-treated animal models suggest a systemic manifestation of insulin resistance [19], potentially linked to these epigenetic changes. However, the histone modulation underlying SGA-induced metabolic abnormality remains poorly understood [14]. While both olanzapine and clozapine led to substantial body weight gain in humans, it is intriguing that clozapine, unlike olanzapine, does not elicit significant weight gain in rodent models, despite its induction of other metabolic abnormalities [17,20]. It is important to investigate whether chronic treatment of olanzapine and clozapine leads to differential effects on histone H3K4me2 modification associated with metabolic side effects in rats with or without significant weight gain, since some metabolic disturbances may partially result from SGA-induced weight gain [21,22]. Given the liver’s central role in the metabolism of carbohydrates, fats, and protein, and its importance in regulating whole-body metabolic homeostasis [23], this study focused on hepatic pathways related to metabolisms and metabolic dysfunction. These pathways were examined using bioinformatics analyses and Chromatin immunoprecipitation coupled with next-generation sequencing (ChIP-seq). It aimed to provide a novel side-by-side epigenomic comparison of histone H3K4me2 modifications induced by the two most prescribed SGAs in psychiatric practice.
2. Materials and Methods
2.1. Animal, Drug Administration and Sample Collection
Thirty-six female Sprague Dawley rats (200 g–220 g) were obtained from the Animal Resource Centre (Perth, WA, Australia). Following a one-week acclimatisation period, the animals were randomly assigned to three groups (n = 12 per group): olanzapine (3 mg/kg, twice daily, orally, Zyprexa, Eli Lilly, Indianapolis, IN, USA), clozapine (20 mg/kg, twice daily, orally, Clozaril, Novartis, Turkey), and vehicle control (cookie dough pellet without active drugs). Antipsychotic drugs were incorporated into 300 mg cookie dough pellets composed of 30% cornflour, 30.9% sucrose, 15.5% casein, 8.4% minerals, 6.4% fibre, 6.3% gelatine, and 1.6% vitamins, mixed with an appropriate amount of water. Each rat received oral treatment twice daily (08:00 and 20:00) for a duration of nine weeks. Consumption of the medicated pellets was monitored to ensure complete ingestion. Due to aversion to clozapine-containing pellets after three days of treatment, clozapine was subsequently administered using a 1 mL syringe via the mouth. To maintain consistency in pellet intake across groups, clozapine-treated rats were also provided with an equivalent non-medicated cookie dough pellet [17]. All animals were housed individually in a temperature-controlled room (22 °C) with a 12 h light/dark cycle (lights on from 07:00 to 19:00) with ad libitum access to water and standard laboratory chow diet (3.9 kcal/g, 10% fat, 16% protein and 74% carbohydrate). Body weight, food intake, and water consumption were recorded weekly. Rats were fasted overnight before sacrifice. Two hours after final treatment, all animals were euthanized via carbon dioxide asphyxiation. Liver tissues were immediately harvested, snap-frozen in liquid nitrogen, and stored at −80 °C subsequent analysis. The metabolic parameters of rats with 9-week olanzapine and clozapine treatment in this study have been previously reported [17]. Briefly, both antipsychotics elevated fasting lipid levels; however, only olanzapine significantly increased body weight. Olanzapine-treated rats exhibited higher fasting insulin levels and HOMA-IR scores, along with mildly impaired glucose tolerance. In contrast, clozapine treatment reduced fasting plasma insulin levels but also impaired glucose tolerance. Consistent with these disturbances in glucose and lipid metabolism, both olanzapine and clozapine upregulated hepatic SREBP signalling and transcriptional expressions of their downstream target genes. Additionally, clozapine enhanced carbohydrate response element binding protein (ChREBP) signalling, independent of weight gain [17].
2.2. Chromatin Immunoprecipitation Coupled with Next-Generation Sequencing (ChIP-Seq)
Chromatin immunoprecipitation for H3K4me2 was performed using the EpiQuik™ Tissue Methyl-Histone H3-K4 ChIP Kit (#P-2009, Epigentek, Farmingdale, NY, USA) and EpiQuik™ Tissue ChIP Kit (#P-2003; Epigentek, Farmingdale, NY, USA), following the manufacturer’s instructions. Briefly, 40 mg of fine minced liver tissue, pooled from twelve samples per group, was cross-linked using 1 mL 1% formaldehyde at room temperature for 20 min, followed by homogenisation. Chromatin DNA was then sheared to an average fragment length of 200–1000 bp using a Branson 450 Digital Sonifier (Branson, MO, USA) at 20% amplitude, delivering seven pulses of 18 s each. The sheared DNA was diluted with equal-volume ChIP dilution buffer and transferred to strip wells pre-incubated with either ChIP-grade anti-dimethyl-H3-K4 or anti-normal mouse IgG (negative control) for 60 min at room temperature. Cross-linked DNA was released by 40 µL release buffer containing 1 µL Proteinase K, and reversal of cross-linking was carried out with 40 µL reverse buffer in a 65 °C dry bath for 90 min. DNA was purified using spin columns, yielding 20 µL ChIP DNA. Purified ChIP DNA for all groups was sent to the laboratory in Novogene Co. (Hong Kong) for the DNA paired-end library construction and high-throughput sequencing using an Illumina Hiseq2500 50SE platform. To ensure data reliability, quality control was performed at each stage of the process, including sample assessment, library preparation, and sequencing.
2.3. Bioinformation Analysis
Raw sequencing reads were subjected to quality control using a three-step filtering process: (1) removal of reads containing adapter sequences; (2) removal of reads with more than 10% undetermined bases (N); and (3) removal of reads in which over 50% of bases had a quality score (Q ≤ 5). Clean reads were aligned to the rattus_norvegicus reference genome Rnor_6.0 “http://asia.ensembl.org/Rattus_norvegicus/Info/Index” (accessed on 4 September 2024) using the Burrows-Wheeler Aligner (v0.7.19) software [24].
To assess the effect of chronic SGA treatment on histone modification in genes associated with metabolic side effect, only unique mapped reads without mismatch were retained for peak identification using a validated peak-calling model. Significant differential peaks in the olanzapine and clozapine groups were identified by comparison with controls using the bdgdiff subcommand in MACS2 (Log-Likelihood Ratio, LLR > 3), followed by KEGG pathway enrichment analysis with Bonferroni correction (p < 0.05) [25,26,27]. Histone methylation profiles at gene loci were visualised using Integrative Genomics Viewer (IGV2.5.0). All significantly enriched KEGG pathways related to lipid metabolism, glucose metabolism, and metabolic disorders were reported separately for each drug.
3. Results
3.1. H3K4me2 Contributes to Altered Hepatic Gene Expression Following Chronic Olanzapine and Clozapine Treatment
Deep analysis of the ChIP-Seq data found a higher percentage of genome with H3K4me2 enrichment peaking in both clozapine- and olanzapine-treated rats (Figure 1A). Furthermore, SGAs increased H3K4me2 enrichment at gene promoters on the transcription start site (TSS) and −1000 Kb, especially in olanzapine treatment (Figure 1B). To gain an initial understanding of the putative functions of genes associated with histone H3 lysine 4 methylation, we identified a total 10,145 genes with significant H3K4me2 enrichment in the clozapine and 6341 genes in the olanzapine groups. KEGG analysis showed that there were 218 pathways in the clozapine group and 214 pathways in the olanzapine group among these H3K4me2 enrichment genes, while 179 of them were activated by both olanzapine and clozapine (Figure 1C). Many of them participated in a diverse set of processes that contribute to metabolisms, including lipid metabolism, glucose metabolism, and signal pathways related insulin resistance and other metabolic disorders.
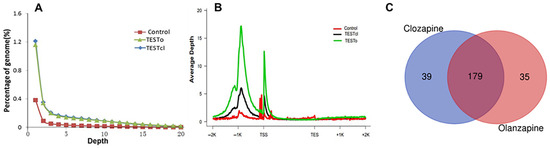
Figure 1.
Deep analysis of the cleaned H3K4me2 ChIP-Seq data and KEGG pathways intersection between olanzapine and clozapine. (A) Depth distribution of genome sequencing. (B) Depth distribution of genes and their up and down stream. Rnor_6.0 was selected as the rattus_norvegicus reference genome. The H3K4me2 modification level was calculated by the number of reads per kilobase in the mapped genomic region. (C) KEGG pathways intersection between olanzapine and clozapine. Blue, alone on clozapine; orange, alone on olanzapine; red, interaction between two drugs. Abbreviation: TSS, transcription start site; TES, transcription end site.
3.2. Olanzapine and Clozapine Differentially Modulate H3K4me2 Enrichment Across Lipid-Metabolism-Related Pathways
In the lipid metabolism pathways (Figure 2A), there was a significant increase in H3K4me2 binding on the genes related to “Fatty acid degradation”, “Glycerophospholipid metabolism”, “Glycerolipid metabolism”, and “Biosynthesis of unsaturated fatty acids” in both the olanzapine and clozapine groups; however, olanzapine showed a deeper effect on H3K4me2, binding the genes involved in these pathways. For example, the enrichment score for “Fatty acid degradation” was 5.22 in olanzapine, while it was 2.1 in clozapine. Moreover, among the 47 participating genes in this pathway, there were 24 genes peaking with H3K4me2 in olanzapine but 17 in clozapine. Intriguingly, this showed that olanzapine promoted more H3K4me2 enrichment in “Steroid biosynthesis”, “Fatty acid elongation”, “Fatty acid biosynthesis”, and “Arachidonic acid metabolism”, while clozapine had a greater H3K4me2 effect on the “Regulation of lipolysis in adipocytes”, “alpha-Linolenic acid metabolism”, and “Linoleic acid metabolism” (Figure 2A).
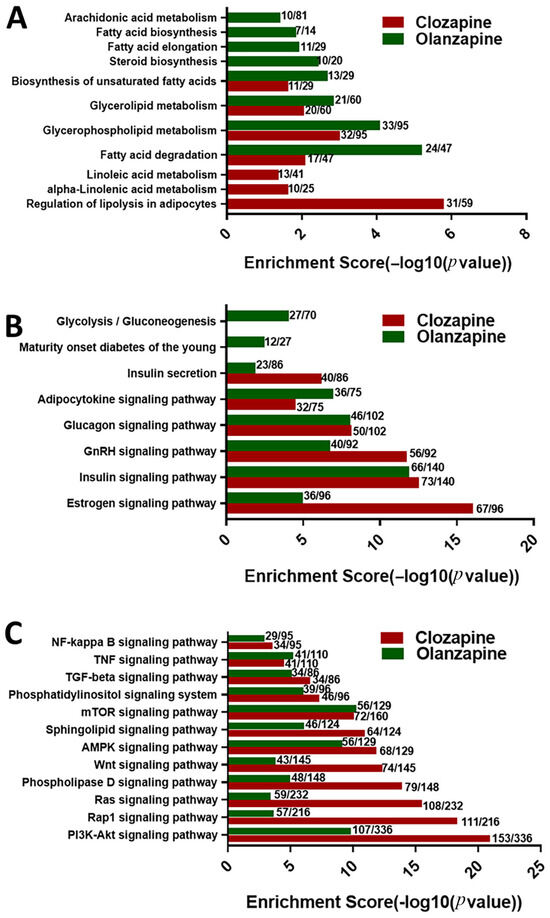
Figure 2.
Differential effects of olanzapine and clozapine on H3K4me2-modulated KEGG pathways significantly associated with (A) lipid metabolism, (B) glucose metabolism, and (C) metabolic disorders. The enrichment score is expressed as −log10 (p value), where a higher score indicates greater statistical significance of the pathway.
3.3. Modulation of Glucose Metabolism Pathways by Antipsychotic-Associated H3K4me2 Methylation
Both olanzapine and clozapine significantly increased H3K4me2 binding on the genes participating in glucose-metabolism-related pathways (Figure 2B), including the “Estrogen signaling pathway”, “Insulin signaling pathway”, “GnRH signaling pathway”, “Glucagon signaling pathway”, “Adipocytokine signaling pathway”, and “Insulin secretion”. However, only olanzapine significantly promoted H3K4me2 at gene promoters on the genes of pathways related to “Maturity onset diabetes of the young” and “Glycolysis/Gluconeogenesis” (Figure 2B).
3.4. Differential Modulation of H3K4me2 Enrichment by Olanzapine and Clozapine Affects Metabolic-Disorder-Associated Signalling Pathways
As shown in Figure 2C, all of 12 metabolic-disorder-associated signalling pathways showed significantly high H3K4me2 enrichment in both olanzapine and clozapine rats. Among these pathways, clozapine showed greater contribution not only on the number of genes, but also on the enrichment degree of H3K4me2 on the “PI3K-Akt signaling pathway”, “Rap1 signaling pathway”, “Ras signaling pathway”, “Phospholipase D signaling pathway”, “Wnt signaling pathway”, and “AMPK signaling pathway”, while olanzapine showed a higher enrichment score and number of participating genes on the “PPAR signaling pathway”.
3.5. Chronic Olanzapine and Clozapine Treatment Induce Distinct Alterations in Hepatic Insulin Resistance Pathways
When comparing the genes related to the insulin resistance pathway in the liver, we observed that all membrane receptors or transmembrane carrier proteins in the liver involved in the insulin resistance pathway showed significantly higher H3K4me2 enrichment peaking in olanzapine, including tumour necrosis factor receptor (Tnfr), insulin receptor (Insr), glucose transporter 2 (Glut2), and fatty acid transport protein 5 (Fatp5) (Figure 3A). However, only a higher H3K4me2 enrichment at gene promoters on Insr was observed in clozapine (Figure 3B).
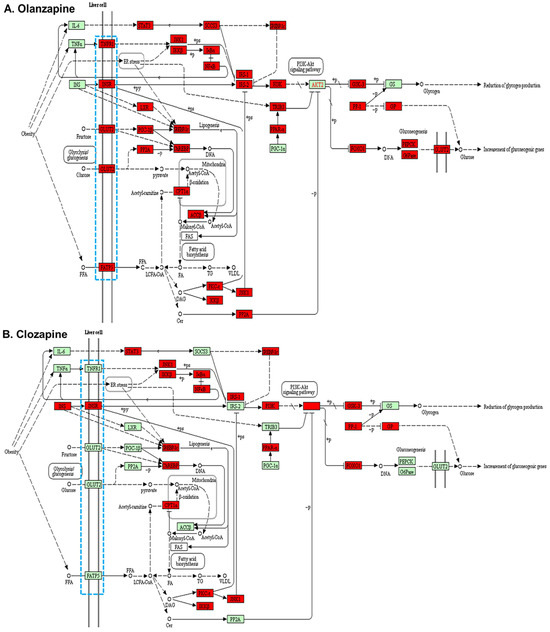
Figure 3.
Insulin resistance pathways in the liver cells of rats treated with (A) olanzapine and (B) clozapine. Blue dotted box, membrane receptors or transmembrane carrier proteins; red filled box, higher H3K4me2 binding on than control; green filled box, lower H3K4me2 binding on than the control.
3.6. Differential Effects of Olanzapine and Clozapine on Hepatic H3K4me2 Histone Modifications—ChIP-Seq Profiles
Profiles of the H3K4me2 ChIP-Seq on key genes of insulin resistance pathways in the liver were further examined (Figure 4). The results showed greater H3K4me2 peaking on the whole gene locus of these genes in olanzapine-treated rats but less in clozapine (Figure 4), especially in the glucose transporter 2 (Slc2a2), tumour necrosis factor receptor 1 (Tnfrsf1a), and long-chain fatty acid transport protein 5 (Slc27a5) (Figure 4B–D). These results indicated that olanzapine contributed more to histone H3 lysine 4 methylation, a histone active for gene transcription, peaking on the genes of the insulin resistance pathway in the liver, consistent with the more serious insulin resistance observed in the olanzapine group.
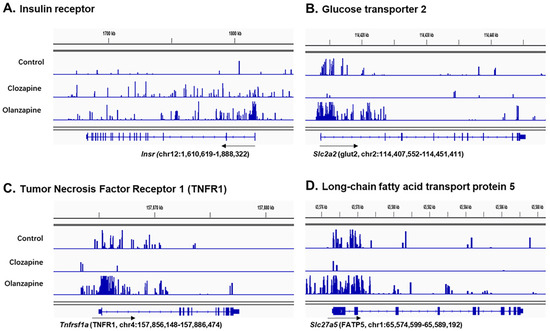
Figure 4.
Examples in snapshots of H3K4me2 binding on genes locus of the membrane receptors or transmembrane carrier proteins related to insulin resistance pathways in the liver cell in response to olanzapine and clozapine treatment. (A) Insulin receptor (Insr), (B) glucose transporter 2 (Slc2a2), (C) tumour necrosis factor receptor 1 (Tnfrsf1a), and (D) long-chain fatty acid transport protein 5 (Slc27a5). Black arrows, the directions of gene transcription.
4. Discussion
To our knowledge, this study was the first to present a novel epigenomic breadth of data linking SGA-induced metabolic side effects to histone H3K4me2 modifications, through a direct comparison between olanzapine and clozapine, the two most widely prescribed SGAs in psychiatric practice. It was interesting that a greater percentage of genomes had a higher H3K4me2, peaking in both olanzapine and clozapine treatment groups. Furthermore, in terms of the H3K4me2 enrichment genes examined by KEGG analysis, there was 214 and 218 pathways in olanzapine and clozapine, while 179 of them were activated by both olanzapine and clozapine. Most of them participated in biological processes-related metabolic disorders, such as lipid metabolism, glucose metabolism, and metabolic-disorder-related signalling pathways. H3K4 methylation is associated with various biological functions through promoting gene transcription [12]. In this study, KEGG analysis shows that 11 pathways are involved in significant H3K4me2 enrichment in signal pathways related to lipid metabolism. For instance, “Biosynthesis of unsaturated fatty acid”, “Glycerolipid metabolism”, “Glycerophospholipid metabolism”, and “Fatty acid degradation” are associated with the significant H3K4me2 enrichment in signal pathways related to lipid metabolism in both the olanzapine and clozapine groups. Previous studies have shown that most of these KEGG pathways, such as the glycerolipid metabolism, fatty acid degradation, biosynthesis of unsaturated fatty acids, and glycerophospholipid metabolism, are involved in the development of metabolic disorders [28,29,30,31]. It has been reported that the accumulation of mono-unsaturated fatty acids is necessary for the lifespan extension of H3K4me3 methyltransferase-deficient worms [32]. This study also revealed that olanzapine promoted more H3K4me2 enrichment in “Steroid biosynthesis”, “Fatty acid elongation”, “Fatty acid biosynthesis”, and “Arachidonic acid metabolism”, while clozapine had more H3K4me2 effect on “Regulation of lipolysis in adipocytes”, “alpha-Linolenic acid metabolism”, and “Linoleic acid metabolism”. This difference might be attributed to the various gene expression patterns in a cell by altering between transcriptional activation and repression, which are frequently governed by the histone modulation, including methylation and acetylation.
This study shows that 12 signal transduction processes are associated with significant H3K4me2 enrichment in pathways linked to metabolic regulation in both olanzapine- and clozapine-treated rats. This study showed the greater enrichment degree of H3K4me2 and gene amount by clozapine treatment in most of the pathways, such as the “PI3K-Akt signaling pathway”, “Rap1 signaling pathway”, “Ras signaling pathway”, “Phospholipase D signaling pathway”, “Wnt signaling pathway”, and “AMPK signaling pathway”. Consistent with these findings, a previous study reported that clozapine activated the AMPK-ACC-CPT1 pathway in the rat prefrontal cortex, demonstrating its effect on the AMPK-related lipid regulatory system in the brain [33]. Additionally, Zeng and colleagues reported that clozapine protects PC12 cells from corticosterone-induced cell death by modulating activity of the PI3K/Akt/FoxO3a pathway [34]. This result suggests that both olanzapine and clozapine engage multiple signal transduction pathways implicated in metabolic disorders.
This study also uncovered some divergent patterns of H3K4me2 enrichment between the two drugs. For example, olanzapine increased enrichment at genes involved in fatty acid elongation and biosynthesis and PPAR signalling, while clozapine more strongly affected pathways related to lipolysis and essential fatty acid metabolism. Additionally, clozapine-treated samples exhibited greater H3K4me2 enrichment in several signalling pathways, including PI3K–Akt, Rap1, Ras, Wnt, and AMPK. These findings are consistent with previous reports showing that olanzapine epigenetically activates hepatic lipogenic transcription programmes via increased H3K4me2 binding at Pparg, Srebp-1, thereby promoting adipogenesis and lipid accumulation in the liver. In contrast, clozapine has been shown to impair Akt kinase activation and inhibits AMPK signalling, which may contribute to glucose dysregulation and hepatic steatosis, often without significant weight gain [16,35]. These divergent effects are particularly notable given that both drugs are associated with dyslipidaemia, yet only olanzapine consistently induces obesity in rodent models [17,20]. One possible explanation is that olanzapine’s effects on hepatic lipogenic transcription are more pronounced and directly linked to weight gain, whereas clozapine’s impact may primarily disrupt glucose and lipid metabolism through altered signalling pathways, leading to steatosis without necessarily promoting obesity.
In terms of the glucose metabolism, this study revealed that both olanzapine and clozapine significantly increased H3K4me2 binding on the signalling pathways of oestrogen, insulin, GnRH, glucagon, and adipocytokine. A precious study has shown that oestrogen and insulin are critical regulators of energy balance and glucose homeostasis [36,37,38,39,40]. Specifically, oestrogen receptor-α (ERα) interacts with and enhances the activity of the mixed lineage leukaemia gene (MLL2), a histone methyltransferase (HMT) that specifically methylates H3K4, thereby promoting epigenetic activation of gene expression [41,42]. Vanderkruk and colleagues reported that reduced H3K4 methylation leads to diminished insulin production, impaired glucose-responsiveness, and increases transcriptional entropy, indicative of a loss of β-cell maturity [43].
This study demonstrates that olanzapine and clozapine have different effects on H3K4 methylation of the insulin resistance pathway in the liver. For example, although both drugs target the same number of genes, olanzapine shows increased H3K4me2 binding at Tnfr, Insr, Glut2, and Fatp5, whereas clozapine exhibits elevated H3K4me2 binding only at Insr. The differences between olanzapine and clozapine in their impact on the insulin resistance pathway may reflect the diverse biological processes involved in antipsychotic-induced diabetes. Notably, olanzapine appears to influence a broader set of H3K4me2 genes in the insulin resistance. Alternatively, the broader effects of olanzapine on H3K4 methylation within the insulin resistance pathway may be partly secondary effects of weight gain in olanzapine-treated rats, whereas clozapine’s effects appear largely independent of weight. It has been reported that weight gain induced by chronic olanzapine treatment could further lead to insulin resistance, while clozapine more directly impairs insulin secretion regardless of weight gain in rats [17,44]. These pharmacometabolic distinctions underscore the need to disentangle primary drug effects from secondary metabolic consequences on histone methylation in further studies.
It is worth acknowledging several limitations inherent to the exploratory nature of this study, which may affect the robustness of the findings. Notably, sequencing was performed on pooled samples without biological replicates; this would undermine the statistical power in differential H3K4me2 enrichment analysis. Furthermore, the absence of gene or protein expression analyses, along with the lack of confirmatory ChIP-PCR validation [24], warrants caution in interpreting the results. This is particularly relevant for novel observations, such as clozapine-induced H3K4me2 enrichment in genes involved in linoleic/α-linolenic acid metabolism and olanzapine-associated changes in steroid biosynthesis, which require targeted follow-up studies for validation.
Despite the limitation that this study did not measure the plasma drug levels or dopamine D2 receptor occupancy, our previous pharmacokinetic study using the same administration method demonstrated that an oral dose of 1 mg/kg olanzapine in rats reached a peak plasma concentration of 276.5 ng/mL within 6 h, with an elimination half-life of 3.5 h [45]. In humans, olanzapine’s pharmacokinetic profile is marked by achieving a peak plasma concentration of 156.9 ng/mL around the same time following a therapeutic oral dose [46]. Similarly, a 20 mg/kg oral dose of clozapine in rats yielded a peak serum concentration of 926.6 ng/mL with a half-life of 2.89 h [47], whereas clinically effective plasma levels in humans range from 250 to 550 ng/mL for the optimal treatment of schizophrenia [48]. Kapur et al. reported that single doses of clozapine (5–15 mg/kg) and olanzapine (1–2 mg/kg) in rodents produced dopamine D2 receptor occupancies comparable to those seen in clinical settings, although the drugs’ half-life in rodents are 4–6 times shorter than in humans [49]. Considering the shorter half-life of these drugs in rats, both drugs were administered twice daily in this study. Therefore, the rat dosages used in this study, clozapine (20 mg/kg, twice daily) and olanzapine (3 mg/kg, twice daily), are therefore considered clinically relevant. However, drug or metabolite levels in plasma and liver were not measured at the end of the 9-week treatment, limiting our ability to directly correlate drug concentrations with H3K4me2 changes. It is worth noting that this study was conducted in heathy rats, free from disease-related confounds. Therefore, future studies should validate these findings in animal models for schizophrenia, such as prenatal Poly I:C animal models. The results are promising, showing modulation of histone H3K4me2 in signalling pathways related to metabolic disorders induced by antipsychotics; however, further studies are needed to determine whether these epigenetic changes translate to alterations in functional protein expression. Additionally, further mechanistic studies are crucial to explore whether blocking histone H3K4me2 can reverse the observed effects. Such insights could have important clinical implications for preventing and treating metabolic disorders caused by these antipsychotics and enhancing the overall therapeutic efficacy of antipsychotic treatment.
Author Contributions
Conceptualisation, J.L., Y.S. and C.D.; methodology, J.L. and Y.S.; validation, Y.S. and C.D.; formal analysis, J.L. and Y.S.; investigation, J.L., Y.S., N.P. and C.D.; resources, C.D.; data curation, Y.S.; writing—original draft preparation, J.L.; writing—review and editing, J.L., Y.S., N.P. and C.D.; visualisation, Y.S.; project administration, C.D.; funding acquisition, C.D., J.L. and Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Australian National Health and Medical Research Council (NHMRC) Project grant to C.D. and J.L., grant number APP1104184. J.L. was also supported by an NHMRC Early Career Fellowship Award, grant number APP1125937. Y.S. was supported by the Joint Funds for the Innovation of Science and Technology, Fujian Province (grant number 2020Y9144) and Fujian Provincial Health Technology Project (grant number 2021CXB014). The APC was funded by NHMRC grant number APP1104184.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (2013), and approved by the Institutional Review Board of the Animal Ethics Committee, University of Wollongong (Approval Code: AE12/26; Approval date: 17 April 2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequence data that support the findings of this study have been deposited in the National Center for Biotechnology Information (NCBI) with identification number GSE288060: “https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE288060 (accessed on 30 January 2025)”.
Conflicts of Interest
NP received an honorarium for Boehringer Ingelheim’s Regional CIAS CME Series. CD and JL received research grants from NHMRC (CD and JL: APP1104184; JL: APP1125937). YS received research grants from the Innovation of Science and Technology, Fujian Province (2020Y9144) and Fujian Provincial Health Technology Project (2021CXB014). The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ACC | acetyl-CoA carboxylase |
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| ChIP | chromatin immunoprecipitation |
| ChIP-Seq | chromatin immunoprecipitation coupled with next-generation sequencing |
| ChREBP | carbohydrate response element binding protein |
| CPT1 | carnitine palmitoyl transferase 1 |
| ERα | oestrogen receptor-α |
| Fatp5 | fatty acid transport protein 5 |
| FoxO3a | Forkhead box O3a |
| Glut2 | glucose transporter 2 |
| GnRH | gonadotropin-releasing hormone |
| H3K4me | methylation of K4 on histone 3 |
| HOMA-IR | homeostasis model assessment—insulin resistance |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| Insr | insulin receptor |
| PI3K | phosphoinositide 3-kinase |
| PPAR | peroxisome proliferator-activated receptor |
| Rap1 | Ras-associated protein 1 |
| SGAs | second-generation antipsychotic drugs |
| SREBP | sterol regulatory element-binding protein |
| Tnfr | tumour necrosis factor receptor |
References
- Vermeulen, J.; Van Rooijen, G.; Doedens, P.; Numminen, E.; Van Tricht, M.; De Haan, L. Antipsychotic medication and long-term mortality risk in patients with schizophrenia; a systematic review and meta-analysis. Psychol. Med. 2017, 47, 2217–2228. [Google Scholar] [CrossRef]
- Nesvåg, R.; Hartz, I.; Bramness, J.G.; Hjellvik, V.; Handal, M.; Skurtveit, S. Mental disorder diagnoses among children and adolescents who use antipsychotic drugs. Eur. Neuropsychopharmacol. 2016, 26, 1412–1418. [Google Scholar] [CrossRef]
- Munkholm, K.; Jørgensen, K.J.; Paludan-Müller, A.S. Continuing antipsychotic medication for patients with psychotic depression in remission. J. Am. Med. Assoc. 2019, 322, 2443. [Google Scholar] [CrossRef]
- Citrome, L.; McEvoy, J.P.; Todtenkopf, M.S.; McDonnell, D.; Weiden, P.J. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: The past, present, and future. Neuropsychiatr. Dis. Treat. 2019, 15, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Huhn, M.; Nikolakopoulou, A.; Schneider-Thoma, J.; Krause, M.; Samara, M.; Peter, N.; Arndt, T.; Bäckers, L.; Rothe, P.; Cipriani, A.; et al. Comparative Efficacy and Tolerability of 32 Oral Antipsychotics for the Acute Treatment of Adults with Multi-Episode Schizophrenia: A Systematic Review and Network Meta-Analysis. Focus (Am. Psychiatr. Publ.) 2020, 18, 443–455. [Google Scholar] [CrossRef]
- Pandey, A.; Kalita, K.N. Treatment-resistant schizophrenia: How far have we traveled? Front. Psychiatry 2022, 13, 994425. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Agid, O.; Crespo-Facorro, B.; de Bartolomeis, A.; Fagiolini, A.; Seppälä, N.; Howes, O.D. A Guideline and Checklist for Initiating and Managing Clozapine Treatment in Patients with Treatment-Resistant Schizophrenia. CNS Drugs 2022, 36, 659–679. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, T.; AMcCutcheon, R.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef]
- Tondo, L.; Baldessarini, R.J. Discontinuing psychotropic drug treatment. BJPsych Open 2020, 6, e24. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Lin, Z.-J.; Li, C.-C.; Lin, X.; Shan, S.-K.; Guo, B.; Zheng, M.-H.; Li, F.; Yuan, L.-Q.; Li, Z.-H. Epigenetic regulation in metabolic diseases: Mechanisms and advances in clinical study. Signal Transduct. Target. Ther. 2023, 8, 98. [Google Scholar] [CrossRef]
- Morgan, M.A.J.; Shilatifard, A. Reevaluating the roles of histone-modifying enzymes and their associated chromatin modifications in transcriptional regulation. Nat. Genet. 2020, 52, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Lo, Y.-C.; Kao, C.-F. H3K4 Methylation in Aging and Metabolism. Epigenomes 2021, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Mannar, V.; Boro, H.; Patel, D.; Agstam, S.; Dalvi, M.; Bundela, V. Epigenetics of the Pathogenesis and Complications of Type 2 Diabetes Mellitus. Eur. Endocrinol. 2023, 19, 46–53. [Google Scholar] [CrossRef]
- Frond, D.; Rettig, A.; Burghardt, K. Epigenetic insights of olanzapine-induced insulin resistance. Epigenomics 2025, 17, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Swathy, B.; Banerjee, M. Understanding epigenetics of schizophrenia in the backdrop of its antipsychotic drug therapy. Epigenomics 2017, 9, 721–736. [Google Scholar] [CrossRef]
- Su, Y.; Liu, X.; Lian, J.; Deng, C. Epigenetic histone modulations of PPARγ and related pathways contribute to olanzapine-induced metabolic disorders. Pharmacol. Res. 2020, 155, 104703. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Lian, J.; Hu, C.-H.; Huang, X.-F.; Deng, C. Time-dependent changes and potential mechanisms of glucose-lipid metabolic disorders associated with chronic clozapine or olanzapine treatment in rats. Sci. Rep. 2017, 7, 2762. [Google Scholar] [CrossRef]
- Fernø, J.; Vik-Mo, A.O.; Jassim, G.; Håvik, B.; Berge, K.; Skrede, S.; Gudbrandsen, O.A.; Waage, J.; Lunder, N.; Mørk, S.; et al. Acute clozapine exposure in vivo induces lipid accumulation and marked sequential changes in the expression of SREBP, PPAR, and LXR target genes in rat liver. Psychopharmacology 2009, 203, 73–84. [Google Scholar] [CrossRef]
- Boyda, H.N.; Tse, L.; Procyshyn, R.M.; Honer, W.G.; Barr, A.M. Preclinical models of antipsychotic drug-induced metabolic side effects. Trends Pharmacol. Sci. 2010, 31, 484–497. [Google Scholar] [CrossRef]
- Cooper, G.; Harrold, J.; Halford, J.; Goudie, A. Chronic clozapine treatment in female rats does not induce weight gain or metabolic abnormalities but enhances adiposity: Implications for animal models of antipsychotic-induced weight gain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 428–436. [Google Scholar] [CrossRef]
- Peuskens, J.; De Hert, M.; Mortimer, A. SOLIANOL Study Group. Metabolic control in patients with schizophrenia treated with amisulpride or olanzapine. Int. Clin. Psychopharmacol. 2007, 22, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Perez-Iglesias, R.; Mata, I.; Pelayo-Teran, J.M.; Amado, J.A.; Garcia-Unzueta, M.T.; Berja, A.; Martinez-Garcia, O.; Vazquez-Barquero, J.L.; Crespo-Facorro, B. Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naïve population. Schizophr. Res. 2009, 107, 115–121. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef]
- Su, Y.; Lian, J.; Deng, C. Dataset of histone H3K4me2 modified genes in the liver of female Sprague-Dawley rats with chronic antipsychotic drugs of olanzapine or clozapine. Data Brief 2025, 59, 111425. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nusbaum, C.; Myers, R.M.; Brown, M.; Li, W.; et al. Model-based Analysis of ChIP-Seq (MACS). Genome Biol. 2008, 9, R137. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; He, Q.-Y. ChIPseeker: An R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 2015, 31, 2382–2383. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Prentki, M.; Madiraju, S.R.M. Glycerolipid Metabolism and Signaling in Health and Disease. Endocr. Rev. 2008, 29, 647–676. [Google Scholar] [CrossRef]
- Huang, W.; Cao, G.; Deng, C.; Chen, Y.; Wang, T.; Chen, D.; Cai, Z. Adverse effects of triclosan on kidney in mice: Implication of lipid metabolism disorders. J. Environ. Sci. 2023, 124, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, W.J. Unsaturated Fatty Acids, Desaturases, and Human Health. J. Med. Food 2014, 17, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Huang, Y.; Tan, X.; Wu, J.; Duan, J.; Zhang, H.; Yin, B.; Li, Y.; Zheng, P.; Wei, H.; et al. Alterations of glycerophospholipid and fatty acyl metabolism in multiple brain regions of schizophrenia microbiota recipient mice. Neuropsychiatr. Dis. Treat. 2019, 15, 3219–3229. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Schroeder, E.A.; Silva-García, C.G.; Hebestreit, K.; Mair, W.B.; Brunet, A. Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature 2017, 544, 185–190. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, S.H.; Yu, H.S.; Park, H.G.; Kang, U.G.; Ahn, Y.M.; Kim, Y.S. The effect of clozapine on the AMPK-ACC-CPT1 pathway in the rat frontal cortex. Int. J. Neuropsychopharmacol. 2012, 15, 907–917. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, X.; Bhardwaj, S.K.; Zhou, X.; Little, P.J.; Quirion, R.; Srivastava, L.K.; Zheng, W. The Atypical Antipsychotic Agent, Clozapine, Protects Against Corticosterone-Induced Death of PC12 Cells by Regulating the Akt/FoxO3a Signaling Pathway. Mol. Neurobiol. 2017, 54, 3395–3406. [Google Scholar] [CrossRef]
- Fehsel, K. Metabolic Side Effects from Antipsychotic Treatment with Clozapine Linked to Aryl Hydrocarbon Receptor (AhR) Activation. Biomedicines 2024, 12, 2294. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Aronoff, S.L.; Berkowitz, K.; Shreiner, B.; Want, L. Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. Diabetes Spectr. 2004, 17, 183–190. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Metab. 2003, 284, E671–E678. [Google Scholar] [CrossRef]
- Roland, A.V.; Moenter, S.M. Regulation of gonadotropin-releasing hormone neurons by glucose. Trends Endocrinol. Metab. 2011, 22, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Cao, H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014, 220, T47–T59. [Google Scholar] [CrossRef] [PubMed]
- Klonou, A.; Chlamydas, S.; Piperi, C. Structure, Activity and Function of the MLL2 (KMT2B) Protein Lysine Methyltransferase. Life 2021, 11, 823. [Google Scholar] [CrossRef]
- Bjune, J.-I.; Strømland, P.P.; Jersin, R.Å.; Mellgren, G.; Dankel, S.N. Metabolic and Epigenetic Regulation by Estrogen in Adipocytes. Front. Endocrinol. 2022, 13, 828780. [Google Scholar] [CrossRef] [PubMed]
- Vanderkruk, B.; Maeshima, N.; Pasula, D.J.; An, M.; McDonald, C.L.; Suresh, P.; Luciani, D.S.; Lynn, F.C.; Hoffman, B.G. Methylation of histone H3 lysine 4 is required for maintenance of beta cell function in adult mice. Diabetologia 2023, 66, 1097–1115. [Google Scholar] [CrossRef]
- Smith, G.C.; Vickers, M.H.; Cognard, E.; Shepherd, P.R. Clozapine and quetiapine acutely reduce glucagon-like peptide-1 production and increase glucagon release in obese rats: Implications for glucose metabolism and food choice behaviour. Schizophr. Res. 2009, 115, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Deng, C.; Cao, S.; Gong, J.; Wang, B.-C.; Hu, C.-H. Acute effects of oral olanzapine treatment on the expression of fatty acid and cholesterol metabolism-related gene in rats. Life Sci. 2015, 128, 72–78. [Google Scholar] [CrossRef]
- Green, W. Child & Adolescent Clinical Psychopharmacology, 3rd ed.; Lippincott Williams & Wilkins: Ambler, PA, USA, 2001. [Google Scholar]
- Manjunath, K.; Venkateswarlu, V. Validated HPLC method for determination of clozapine in rat serum and its application to pharmacokinetics. Indian J. Pharm. Sci. 2005, 67, 448–452. [Google Scholar]
- Northwood, K.; Pearson, E.; Arnautovska, U.; Kisely, S.; Pawar, M.; Sharma, M.; Vitangcol, K.; Wagner, E.; Warren, N.; Siskind, D. Optimising plasma clozapine levels to improve treatment response: An individual patient data meta-analysis and receiver operating characteristic curve analysis. Br. J. Psychiatry 2023, 222, 241–245. [Google Scholar] [CrossRef]
- Kapur, S.; VanderSpek, S.C.; Brownlee, B.A.; Nobrega, J.N. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: A suggested solution based on in vivo occupancy. J. Pharmacol. Exp. Ther. 2003, 305, 625–631. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).