Abstract
Background: Insomnia is strongly associated with stimulant use across various populations and for a wide range of substances. It represents a significant clinical problem among individuals with stimulant use disorders, yet treatment guidelines for this specific population are limited. This gap underscores the need for a systematic review to analyze the pharmacological and non-pharmacological treatments for insomnia in individuals with stimulant use disorders. The aim of this review is to determine the efficacy, safety, and limitations of these approaches and their impact on psychiatric symptoms, stimulant use, and adverse events. Methodology: A systematic review was conducted through January–July 2025 using PubMed, Scopus, and Web of Science. The review focused on the management of chronic insomnia associated with stimulant use, including substances such as amphetamines, methylphenidate, nicotine, caffeine, and cocaine. The systematic review was structured in accordance with the PRISMA guidelines, and identified studies were assessed by title/abstract and full-text evaluation. Results: A total of twenty studies were included in the systematic review. Seven studies examined pharmacological interventions, including modafinil, naltrexone/buprenorphine-naloxone, varenicline, combination NRT, and ramelteon. Thirteen studies investigated non-pharmacological approaches, including Cognitive Behavioral Therapy (CBT), Repetitive Transcranial Magnetic Stimulation (rTMS), Electrical Vestibular Nerve Stimulation (VeNS), maximal strength training, electroacupuncture (EA), and probiotics. The majority of interventions demonstrated positive outcomes in reducing insomnia severity, with some participants achieving non-clinical levels. Commonly reported clinical symptoms related to insomnia included difficulty initiating or maintaining sleep, early morning awakening, and sleep dissatisfaction. Conclusions: Both pharmacological and non-pharmacological interventions showed promise. However, the lack of validated guidelines underscores the need for integrated therapeutic approaches that address the complex comorbidity of insomnia, stimulant use, and co-occurring psychiatric conditions.
1. Introduction
1.1. Insomnia and Sleep Disorders
Insomnia is defined as difficulty initiating or maintaining sleep or experiencing early morning awakenings despite having adequate opportunity to sleep. When these symptoms occur at least three times per week for more than three months and cause significant daytime impairment, the condition is classified as chronic insomnia disorder [1]. Epidemiological studies estimate that approximately 10% of the adult population suffers from chronic insomnia, while an additional 20% experience intermittent symptoms [2]. The disorder is associated with considerable consequences, including reduced work productivity, memory and concentration issues, increased healthcare utilization, and a higher risk for mood disorders such as major depression [3]. Stimulant use is strongly associated with sleep disturbances, including insomnia, across various populations and substances [4]. Therapeutic stimulants, such as amphetamines and methylphenidate, have been linked to delayed sleep onset and reduced sleep efficiency, particularly in pediatric patients. For example, Kidwell et al. (2015) [5] found that patients initiating stimulant therapy frequently report sleep difficulties, with younger children and adolescents being particularly vulnerable. Similarly, modafinil and armodafinil may inadvertently exacerbate insomnia due to their wake-promoting effects, despite their targeted indications. Recreational stimulants, including cocaine and methamphetamine, pose significant risks for severe sleep disruptions, as these substances drastically alter dopamine pathways and circadian rhythms, leading to fragmented or reduced sleep. Legal stimulants, such as caffeine and nicotine, also contribute to insomnia [6], prolonging sleep latency and decreasing overall sleep quality, particularly when consumed close to bedtime. Nicotine, often consumed in the form of tobacco products, has been shown to stimulate arousal, disrupt sleep architecture, and influence the quality of sleep, encompassing subjective sleep quality, sleep efficiency, and duration [7].
1.2. Most Important Categories of Stimulant Drugs
Stimulant drugs represent a diverse class of substances, distinguished by their common uses, therapeutic applications, and underlying mechanisms of action [8,9]. Among therapeutic stimulants:
- (1)
- Amphetamines (e.g., Adderall®, Teva Pharmaceutical Industries Ltd., Petah Tikva, Israel) are frequently prescribed for managing attention-deficit/hyperactivity disorder (ADHD), narcolepsy, and, in some cases, treatment-resistant depression. Their efficacy is primarily attributed to their ability to enhance dopamine and norepinephrine levels. Similarly, methylphenidate (e.g., Ritalin®, Concerta®, Janssen Pharmaceuticals Inc., Titusville, United States) is extensively utilized for ADHD and narcolepsy, exerting its effects through the inhibition of dopamine and norepinephrine reuptake.
- (2)
- Modafinil and armodafinil are specifically indicated for conditions such as narcolepsy, obstructive sleep apnea, and shift work sleep disorder, with their activity linked to increased histamine and dopamine levels.
On the contrary, recreational and illicit stimulants, including cocaine, methamphetamine, and synthetic stimulants, are primarily sought for the following euphoric effects:
- (1)
- Cocaine acts by inhibiting the reuptake of dopamine, serotonin, and norepinephrine, whereas methamphetamine both promotes dopamine release and inhibits its reuptake.
- (2)
- Synthetic designer stimulants, including methylene-dioxy-methamphetamine (MDMA and ecstasy) and synthetic cathinones (e.g., bath salts), are recreationally used for their pronounced euphoric and empathogenic effects, which result from increased serotonin, dopamine, and norepinephrine activity. Additional stimulants of note include ephedrine and pseudoephedrine, common components in decongestants and weight loss products, which act via stimulation of adrenergic receptors and norepinephrine release. Khat (Catha edulis) is a flowering plant native to East Africa and the Arabian Peninsula. The leaves and shoots of the plant contain psychoactive compounds, particularly cathinone and cathine, which act as stimulants. People commonly chew khat leaves for their amphetamine-like stimulating effects, which can lead to increased energy, alertness, and euphoria.
Legal stimulants, such as caffeine and nicotine, are among the most widely consumed stimulants globally. Caffeine promotes wakefulness by antagonizing adenosine receptors, while nicotine enhances dopamine release through stimulation of nicotinic acetylcholine receptors (Scheme 1).
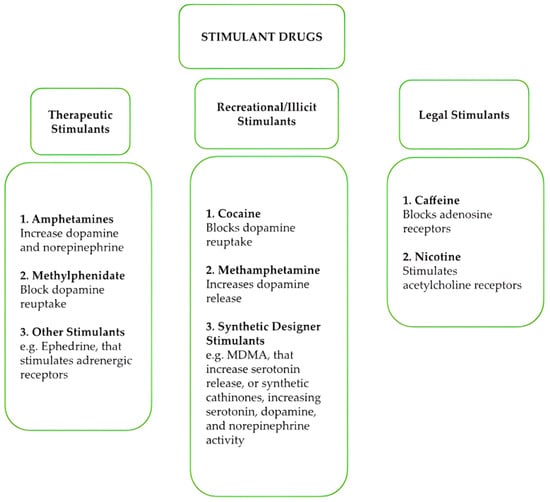
Scheme 1.
Most important stimulant drugs, classified for their characteristics and pharmacological mechanisms.
Overall, despite differences in their pharmacological mechanisms, effects such as exhilaration, enhanced self-esteem, improved mental and physical performance, increased activity, reduced appetite, extended wakefulness, sleep disorders, and euphoria are characteristic of all stimulant drugs [8,9,10]. Chronic and high-dose use of these substances is commonly associated with adverse psychological and behavioral effects, such as agitation, hostility, panic, aggression, and severe outcomes like suicidal or homicidal tendencies [10]. Paranoia, accompanied by auditory and visual hallucinations, may also manifest in certain cases, especially with prolonged misuse [1,3]. Tolerance to these substances develops rapidly, necessitating higher doses to achieve the same effects, and psychological dependence often follows [11]. Several stimulants lack recognized medical use in the United States and are classified under Schedule I due to their high potential for abuse [11]. Generally, many stimulants are classified as controlled substances due to their potential for abuse and adverse health effects. However, the classification and regulatory measures differ; e.g., amphetamine is the most common synthetic stimulant available in Europe, constituting a large and stable market worth a minimum of EUR 1.1 billion annually [12]. Its production and distribution are subject to strict regulations, and unauthorized handling is illegal. Similarly, methamphetamine is generally classified as an illicit drug, with severe penalties for production, distribution, and possession. With regard to synthetic cathinones, due to their implicit characteristics as new psychoactive substances (NPS), being sold openly as “legal” replacements for illicit drugs, their legal status changes rapidly as authorities assess their risks. The legal status of khat varies across Europe, being considered, for instance, illegal in Belgium since 2006, in Denmark since 1993, and in Sweden since 1989. Conversely, some countries may have less stringent regulations.
1.3. Guidelines Available for the Treatment of Insomnia
Several guidelines regarding the treatment of insomnia have been published in the past ten years. These include guidelines by the American College of Physicians (ACP), the American Academy of Sleep Medicine (AASM), the British Sleep Society (BSS), the German Sleep Society (GSS), and the European Sleep Research Society (ESRS), which provide evidence-based recommendations in managing chronic insomnia, emphasizing a multimodal approach, including both non-pharmacological and pharmacological treatments [13,14]. For insomnia in individuals with substance use disorders (SUDs), guidelines are limited, often highlighting the importance of addressing co-occurring conditions and avoiding medications with abuse potential, such as benzodiazepines [15]. Non-addictive alternatives like trazodone, doxepin, or gabapentin are often recommended, alongside behavioral therapies tailored to individuals with SUD [15]. Specific guidelines, such as those from the AASM and the Substance Abuse and Mental Health Services Administration (SAMHSA), underscore the necessity of integrating sleep interventions with ongoing addiction treatment to improve outcomes [14,16].
Among non-pharmacological interventions, Cognitive Behavioral Therapy for Insomnia (CBT-I) has demonstrated robust and lasting improvements in sleep outcomes [17]. Additional approaches such as acupuncture and acupressure have also shown significant efficacy in improving key sleep parameters like total sleep time, sleep latency, and sleep efficiency [18]. Recently, a brand new therapy with probiotics has emerged but remains an experimental approach for the treatment of insomnia. These beneficial microorganisms, traditionally used to support gut health, are gaining attention for their potential role in sleep regulation via the gut–brain axis [19]. The gut–brain axis (GBA) is a complex communication system linking the central nervous system and the enteric nervous system. This connection is fundamental for overall health and plays a crucial role in regulating mood, cognitive functions, and even sleep [20]. Probiotics are live microorganisms that, when administered, confer a health benefit [19]. They can influence the GBA by restoring the balance of the gut microbiota, reducing inflammation, enhancing neurotransmitter production, and strengthening the intestinal barrier. Research on the use of probiotics in mental health, a field known as psychobiotics, is rapidly growing.
2. Materials and Methods
2.1. Search Strategy
A systematic review was carried out, consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [21]. A search was performed from January to July 2025 on PubMed, Scopus, and Web of Science. The following search strategy was used: (“insomnia” OR “sleep disturbance” OR “sleep disorder” OR “chronic insomnia” OR “sleep maintenance insomnia” OR “poor sleep quality”) AND (“stimulant use” OR “stimulant abuse” OR “stimulant dependence” OR “substance use disorder” OR “chronic stimulant use” OR “amphetamine” OR “methylphenidate” OR “modafinil” OR “cocaine” OR “methamphetamine” OR “caffeine” OR “nicotine” OR “MDMA” OR “synthetic cathinones” OR “bath salts” OR “ephedrine” OR “khat”) AND (“treatment” OR “management” OR “intervention” OR “therapy” OR “pharmacological treatment” OR “behavioral treatment” OR “non pharmacological treatment”) AND (“randomized controlled trial” OR “RCT” OR “observational study” OR “cohort study” OR “case–control study” OR “qualitative study”). All titles/abstracts were examined, and full texts of potentially relevant papers were obtained. Relevant works were selected in order to have a full representation of the available literature data on the selected topic. The study was registered on PROSPERO in February 2025 with the number CRD42025641631.
2.2. Inclusion and Exclusion Criteria
Eligible studies were identified if they possessed a range of characteristics, including (1) peer-reviewed clinical/human studies; (2) at least an abstract with estimates and/or full results available; and (3) focusing on the management of chronic insomnia associated with stimulant use, including substances such as amphetamines, methylphenidate, nicotine, caffeine, and cocaine. The analysis explores the efficacy and safety of both pharmacological interventions and non-pharmacological approaches. The exclusion criteria for both selection phases were as follows: (1) non-original research (e.g., review, commentary, editorial, and book chapter); (2) no full-text article available (e.g., meeting abstract); (3) language other than English; (4) animal studies; (5) articles not focusing on patients with a specific diagnosis of stimulant use disorder (SUD); (6) articles not addressing insomnia (e.g., studies focusing solely on other sleep disorders or unrelated conditions); and (7) articles not evaluating interventions (pharmacological or non-pharmacological) for the treatment of chronic insomnia.
2.3. Research Question
The research question was formulated following the PICO framework guidelines. The population (P) includes patients affected with a stimulant (specifically amphetamine) use disorder suffering from chronic insomnia. The Intervention (I) focuses on both pharmacological and non-pharmacological interventions, while the Comparator (C) regards patients without intervention or with placebo. The outcome (O) encompasses studying all types of intervention (both pharmacological and non-pharmacological) and their efficacy for the treatment of chronic insomnia in patients affected with a stimulant use disorder. The research question was: “What are the effects of pharmacological and non-pharmacological interventions in managing chronic insomnia in individuals with stimulant use?” This systematic review reports the findings from the included studies addressing the following: the prevalence of chronic insomnia among individuals with stimulant use disorder, the patterns of insomnia symptoms (common presentations, e.g., difficulty initiating sleep, maintaining sleep, or non-restorative sleep), and severity (e.g., mild, moderate, and severe). Also associated clinical outcomes: impact on psychiatric symptoms, overall mental health, and quality of life and effects on stimulant use disorder (e.g., potential exacerbation of substance use, role in relapse, or improvements associated with insomnia treatment).
2.4. Bias Evaluation
Evaluating the risk of bias in the included studies offered important insights into the treatment of insomnia in stimulant-using populations (Supplementary Materials Table S1). Selection bias was generally low in most randomized controlled trials (RCTs), with clear descriptions of randomization procedures and appropriate allocation methods. Some studies (particularly observational or pilot designs) demonstrated a moderate to high risk of selection bias due to limited randomization details or non-randomized sampling. Performance bias varied across studies. While many RCTs implemented double-blinding or sham-controlled designs, enhancing internal validity, others lacked clear information on participant or personnel blinding, especially in behavioral or retrospective interventions. This resulted in a moderate performance bias risk in a significant number of studies. Attrition bias was low in most trials, where dropout rates were reported and appropriately handled through methods such as intention-to-treat (ITT) analyses or imputation. However, a subset of studies showed moderate risk due to incomplete handling of missing data or lack of clarity around participant retention, particularly in pilot or small-sample studies. Reporting bias was generally well controlled. The majority of studies clearly reported pre-specified outcomes, and findings were consistently aligned with the study objectives. Only a few studies showed moderate or high reporting bias, primarily due to incomplete data presentation or a lack of pre-registration, especially in observational or exploratory research. Reported limitation bias was present across the majority of studies at a moderate level, reflecting common concerns such as small sample sizes, short follow-up durations, or the use of self-reported measures. In conclusion, the overall assessment indicates a low to moderate level of bias across the reviewed studies. Aggregated data synthesis will be conducted by three members of the review team, with any discrepancies resolved through discussion with a fourth reviewer.
3. Results
From a total of 1590 articles (PubMed = 1252; Scopus = 230; Web of Science = 108), after deduplication (n = 111), a total of 1479 records were screened. Among the articles screened, 1375 were excluded by title and abstract. Specifically, 579 were non-original research (review, commentary, editorial, and book chapter), 7 were not written in English, 769 were not relevant to the subject (animal/in vitro studies, articles not focusing on patients with a specific diagnosis of stimulant use disorder, not addressing insomnia, or not evaluating interventions for the treatment of chronic insomnia), and 20 had no abstract. Of the 104 full-text articles assessed for eligibility, 84 did not match the inclusion criteria for our review (81 not relevant to the subject, 3 full-text not available); finally, 20 articles were included in the systematic review (Figure 1).
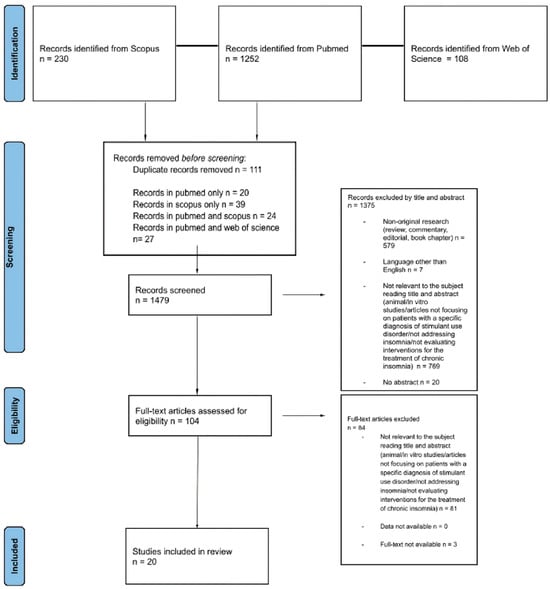
Figure 1.
PRISMA 2020 flow diagram [21]. Adapted from: The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.
Studies recorded were as follows: 15 randomized controlled trials (RCTs) (N = 15), 2 pilot studies (N = 2), 1 pilot randomized controlled trial (N = 1), 1 retrospective observational study (N = 1), and 1 observational study (N = 1). A detailed summary of the 20 articles is included in Table 1 (pharmacological interventions) and Table 2 (non-pharmacological interventions).
Demographic findings showed a mixed population in most studies. Among the included studies, the mean age ranged broadly, reflecting diverse adult populations. Gender distribution varied, with several studies reporting a predominance of males (e.g., N = 30 males [22], N = 85 males [23], N = 10 males and N = 2 females [24], 77.3% biologically male [25], N = 708 males [26]). While some studies had a female-predominant distribution (e.g., 66.3% female [27], N = 51 female, N = 49 male [28], N = 82 females, N = 38 males [29], 46% female [30], 48% female [31], 48.1% female [32], 75% female [33], N = 61 women and N = 54 men [34]). Commonly reported clinical symptoms related to insomnia included difficulty falling asleep, staying asleep, early morning awakening, and dissatisfaction with sleep [28,31,33]. Other symptoms noted were impaired vigilance and poor procedural learning as abstinence progressed [24], irregular sleep timing [25], fatigue [27,29,35], abnormal dreams [30,32], and night awakenings [30]. Symptoms of anxiety and depression were frequently co-occurring with insomnia [29,35,36]. One study also mentioned scalp discomfort, temporary headache, and nausea as mild side effects of interventions [22].
3.1. Pharmacological Interventions
A total of seven studies examined pharmacological interventions for chronic insomnia in individuals with stimulant use disorder (Table 1).
3.1.1. Modafinil
It has been found that participants treated with modafinil experienced a rebound in REM sleep following cessation of cocaine use, although they reported sleep misperception and overestimation. All participants included in the study used cocaine by smoking or intravenous route at least once each week in the past month [37]. Chronic cocaine users often report a paradoxical perception of their sleep quality; while objectively experiencing a deterioration of sleep, they subjectively describe an improvement. This phenomenon has been defined as “occult insomnia” [37].
3.1.2. Naltrexone/Buprenorphine-Naloxone
Insomnia scores were significantly lower in the Extended-Release Naltrexone (XR-NTX) group compared to the buprenorphine-naloxone (BP-NLX) group [34]. Furthermore, the increases in anxiety and depression scores were significantly associated with higher insomnia scores.
3.1.3. Nicotine Replacement Therapy (NRT)/Varenicline
Varenicline and combination NRT (NRT+) were more effective on insomnia symptoms than NRT alone at weeks 5–10 and 5–22, with VR being superior to NRT at 52 weeks [32]. The use of nicotine patches to treat nicotine dependence significantly improved sleep performance and insomnia symptoms, especially in night smokers [31]. However, another study reported that all groups (varenicline, TN, and placebo) experienced increased sleep disturbance from baseline to 1-week post-quit date, with a significantly greater increase in the varenicline and TN groups versus placebo. In particular, it has been described that existing smoking cessation treatments may exacerbate withdrawal-related sleep problems in the early phase [30].
3.1.4. Ramelteon
Significant improvements in Insomnia Severity Index (ISI) scores (from baseline 15 to 10) were recorded with ramelteon, along with significant improvements in physical and mental components [27]. Factors like older age, shorter disease duration, no alcohol intake, full-time employment, and not taking concomitant medication for insomnia positively affected the response.
3.2. Non Pharmacological Interventions
A total of thirteen studies explored non-pharmacological management of chronic insomnia associated with stimulant use (Table 2).
3.2.1. Repetitive Transcranial Magnetic Stimulation (rTMS)
High-frequency (10 Hz) rTMS applied over the left dorsolateral prefrontal cortex (DLPFC) improved cognitive performance in verbal memory, social cognition, and sleep performance, also significantly reducing craving after 5 sessions [22]. Pittsburgh Sleep Quality Index (PSQI) scores improved significantly after rTMS treatment targeting the left DLPFC, with more sessions associated with better sleep quality [23].
3.2.2. Cognitive Behavioral Therapy for Insomnia (CBT-I)
Most participants reported reductions in insomnia symptoms after group-based CBT-I, with severity decreasing to mild insomnia levels in 4 out of 5 individuals in the CBT-I group [38]. Brief Behavioral Treatment for Insomnia (BBTI) decreased the ISI median score from 18.0 to 3.0 and improved the PSQI from 11.0 to 6.0, shifting participants to non-clinical levels of insomnia [25]. CBT-I participants showed greater satisfaction with treatment and greater decreases in insomnia severity compared to the Sleep Health (SH) group, also having an indirect effect on alcohol-related consequences through its influence on insomnia symptoms [33]. While another study demonstrated that the CBT-I group showed significant improvements in sleep efficiency, sleep latency, number of awakenings, wake after sleep onset, and total sleep time compared to the control group [35]. The SH counseling incorporating CBT-I principles led to early improvements in objective sleep health, which may support abstinence and improve sleep performance [29].
3.2.3. Exercise/Physical Activity
Maximal strength training of lower extremities significantly decreased insomnia levels only in the training group, also improving neural function and reducing anxiety [39].
3.2.4. Electrical Vestibular Nerve Stimulation (VeNS)
Significant reduction in ISI scores and improvement in PSQI scores in the active VeNS group compared to the sham group, with increases in total sleep time and decreases in sleep latency based on sleep diaries [28].
3.2.5. Weighted Blankets
It has been found a significant advantage in ISI ratings for the weighted blanket intervention over the light blanket after 4 weeks, showing benefits for insomnia and sleep-related daytime symptoms [29].
3.2.6. Electro-Acupuncture (EA)
The results demonstrated an improvement in the symptoms of anxiety, depression, and insomnia, with a better curative effect on the HAMD sleep disorders score compared to the sham-EA group [36].
3.2.7. Probiotics
The aim of the study is to evaluate the efficacy of probiotic supplements in improving insomnia but also anxiety and psychotic symptoms. Probiotics demonstrated that individuals experienced greater decreases in sleep problems at the end of the trial (Week 8) compared to the placebo group, suggesting a significant improvement in sleep quality [40].
3.3. Substance Use Disorders in Patients with Insomnia and Stimulant Use Disorder
Patients with chronic insomnia and stimulant use disorder frequently present with additional substance use. Among stimulant use disorders and substance use disorders (SUD), nicotine dependence has been reported in eight articles [24,27,30,31,32,33,38,41], Cocaine dependence has been reported in nine articles [23,24,26,37,38,39]. While Amphetamine use disorder has been described in four articles [22,36,39,40], caffeine dependence has been reported instead in four articles [27,28,33,35]. Other SUDs reported were alcohol use disorder, mentioned in two articles [25,26]; opioid dependence, in one article [34]; and cannabis use disorder in one article [33]. The prevalence of SUD among the participants in the study is described in Figure 2.

Table 1.
Overview of studies recording pharmacological management of chronic insomnia associated with stimulant drug use—a summary of the main findings.
Table 1.
Overview of studies recording pharmacological management of chronic insomnia associated with stimulant drug use—a summary of the main findings.
| 1. Nicotine | |||||||||
| Study | Study Design | Sample Features (Gender, Mean Age ± SD) | Type of Intervention | Duration of Intervention | Type of Stimulant Use Disorder and Other Substance Abuse | Additional Psychiatric Disorder | Findings | Clinical Symptoms Related to Insomnia | Notes |
| Ashare et al., 2017 [30] | Randomized controlled trial | N = 1136 treatment-seeking adult smokers; Mean age = 45.6 (SD = 11); 46% female; 55% White; smoked ≥ 10 cigarettes/day. Participants stratified by nicotine metabolite ratio (NMR) | - TN: 21 mg/day nicotine patch and varenicline: titrated to 1 mg BID for 12 weeks while placebo: matched pill + patch together with counseling | 12 weeks of treatment; sleep disturbance assessed pre-quit (baseline) and at 1-week post–target quit date | Nicotine dependence | N/A | All groups experienced increased sleep disturbance from baseline to 1-week post-TQD (p < 0.001). The increase was significantly greater in the varenicline and TN groups vs. placebo. Nicotine patch and varenicline both worsen sleep during the first week of abstinence. Existing smoking cessation treatments do not alleviate withdrawal-related sleep problems but they may exacerbate them in the early phase | Abnormal dreams, difficulty falling asleep, night awakenings, and vivid dreams | Disturbance appears to reflect both nicotine withdrawal and medication side effects. The use of adjunctive treatment like CBT can be used to target sleep during quit attempts |
| Peters et al., 2011 [31] | Randomized controlled trial | N = 385; treatment-seeking smokers (mean age ≈ 46, SD ≈ 11); 48% female; 87% White; smoked ≥ 20 cigarettes/day; inclusion required CO ≥ 10 ppm; FTND mean = 6.3 | Nicotine patch (21 mg); randomized to 0, 25, 50, or 100 mg/day naltrexone together with weekly counseling | 6 weeks (pharmacotherapy), with follow-up at weeks 1, 6, 24, and 48 post-quit. | Nicotine dependence | N/A | Night smokers (n = 135; 35%) had significantly higher pre-cessation sleep disturbance than non-night smokers (PSQI global score: 5.8 vs. 4.4; p < 0.01). The use of nicotine patches to treat nicotine dependence significantly improves sleep performance and insomnia symptoms | Difficulty initiating and maintaining sleep, reduced duration and efficiency, increased daytime dysfunction. Night smokers reported shorter sleep duration and more fragmented sleep than others | The co-occurrence of night smoking + poor sleep (PSQI > 5) predicted significantly worse smoking outcomes at weeks 6, 24, and 48 compared to those with neither issue |
| Tulloch et al., 2016 [32] | Randomized controlled trial | N = 737; Mean age = 48.6 years (SD = 10.8); 53.6% male. 59% had a lifetime psychiatric diagnosis | - NRT: nicotine patch (up to 21 mg/day) for 10 weeks - NRT+: patch (up to 35 mg/day) + gum or inhaler, flexible dose, up to 22 weeks - VR: varenicline 1 mg BID up to 24 weeks | NRT: 10 weeks; NRT+: up to 22 weeks; VR: up to 24 weeks | Nicotine dependence | Major depressive disorder 64.6%, anxiety disorders 21.4%). 59% had lifetime psychiatric diagnosis | VR and NRT+ were more effective on insomnia symptoms than NRT only at weeks 5–10 and 5–22; VR superior to NRT at 52 weeks | Sleep-related symptoms (e.g., abnormal dreams) were more frequent in the VR group (60.3%) compared to NRT+ (46.9%) and NRT (37.6%) | The VR group reported more fatigue and digestive issues. No significant differences in serious adverse events across groups |
| 2. Ramelteon | |||||||||
| Study | Study design | Sample features (gender, mean age ± SD) | Type of intervention | Duration of intervention | Type of Stimulant Use Disorder and other Substance abuse | Additional psychiatric disorder | Findings | Clinical symptoms related to insomnia | Notes |
| Uchiyama et al., 2019 [27] | Observational study | N = 1527; 1050 (68.7%) completed the 12-week observation period. Mean age: 56.8 years (SD = 19.1). 66.3% female | Ramelteon 8 mg/day administered at least 1 h before bedtime | 12 weeks. Mean duration of ramelteon therapy was 72.0 days (SD = 35.3) | Nicotine dependence/caffeine consumption (>2 cups) | Depression (19.4% of patients), anxiety disorder (9.9% of patients) | Ramelteon was associated with better response or remission. Significant improvements in ISI score (from baseline 15 to 10) with significant improvements in physical and mental components. Some factors affect the response like older age (≥75 years), shorter disease duration, no alcohol intake, being employed full-time and not taking concomitant medication for insomnia | Fatigue, anxiety, and decreased work performance | Patients in the study report concurrent disorders: hypertension (26.9%), hyperlipidemia (19.4%), diabetes (10.3%), and reflux esophagitis (7.0%). Adverse drug reactions (ADRs) occurred in 5.9% of patients, with somnolence (1.7%) and dizziness (1.5%) being the most frequent. No deaths were reported |
| 3. Modafinil | |||||||||
| Study | Study design | Sample features (gender, mean age ± SD) | Type of intervention | Duration of intervention | Type of Stimulant Use Disorder and other Substance abuse | Additional psychiatric disorder | Findings | Clinical symptoms related to insomnia | Notes |
| Hodges et al., 2017 [37] | Randomized Controlled Trial | N = 43 (44 yy ± 7 [SD]) M = 35, F = 8. Participants had a history of using cocaine for 24 ± 8 years, and were on average 7 ± 3 days abstinent from cocaine | Participants were randomized to receive either placebo or modafinil. | All participants took 4 capsules containing placebo each morning. In the modafinil group, participants received 100 mg of modafinil on Day 5, 200 mg on Day 6, and 400 mg daily | Cocaine dependence | N/A | Following cessation of cocaine use, Participants treated with modafinil experience a rebound in REM sleep with markedly shortened REM latency and longer REM sleep times confirming an improvement of insomnia | Sleep misperception and overestimation in the modafinil group | All participants reported current use of cocaine by smoked or intravenous route at least 1 time each week in the past month and a positive urine test for cocaine metabolite at screening. Chronic cocaine users experience worsening of sleep that is perceived as qualitatively improving (occult insomnia) |
| 4. Extended-release naltrexone and Buprenorphine-naloxone | |||||||||
| Study | Study design | Sample features (gender, mean age ± SD) | Type of intervention | Duration of intervention | Type of Stimulant Use Disorder and other Substance abuse | Additional psychiatric disorder | Findings | Clinical symptoms related to insomnia | Notes |
| Latif et al., 2019 [34] | Prospective randomized clinical trial | N = 115, (18 to 60 yy). Mean age = 36.4 years, N = 61 female and N = 54 male | XR-NTXvs. BP-NLX sublingual. The Mean daily dose of BP-NLX was 11.2 mg | 12-week treatment with either intramuscular injection of XR-NTX in the gluteal region every fourth week or with daily | Heroin dependence (mean [SD], 6.9 [5.8] vs. 6.7 [5.2] years)/Opioid Dependence/Amphetamine Dependence | Anxiety disorder/major depressive disorder/SUD (opioid) | Insomnia score was significantly lower in the XR-NTX group. Increases in the anxiety and depression scores were significantly associated with higher insomnia scores | Anhedonia, depression, and reduced pleasure | ISI improved in the extended-release naltrexone group. At week 16, 8 participants dropped out or failed detoxification leaving 109 participants. 29 participants completed the study. Four participants tested positive for HIV, and 86 participants (54.1%) had positive hepatitis C tests |
| 5. Cognitive Behavioral Therapy and SUD Treatment Combination | |||||||||
| Study | Study design | Sample features (gender, mean age ± SD) | Type of intervention | Duration of intervention | Type of Stimulant Use Disorder and other Substance abuse | Additional psychiatric disorder | Findings | Clinical symptoms related to insomnia | Notes |
| Sexton et al., 2021 [26] | Randomized controlled trial | N = 762 (48.14 years old (SD = 13), M = 708 Caucasian Non-Hispanic (71.6%). Participants reported heavy alcohol consumption and cocaine | Violence reduction interventions through CBT and treatment for SUD (not specified in the study) | N/A (duration of treatment not reported) | Cocaine dependence/alcohol use disorder | N/A | The SUD treatment and CBT improved insomnia assessed through ISQ | Aggressiveness, nervousness, pain sensitivity, and somatic symptoms | CBT directly addressing insomnia in populations with substance use disorder (SUD) could lessen negative behavioral outcomes. Sleep impaired individuals may demonstrate changes to threat sensitivity and poorer decision-making capacity. Heavy alcohol use, cocaine, and insomnia diagnoses were all associated with greater frequency of aggressive injury |
Abbreviations: ADHD: Attention Deficit Hyperactivity Disorder; AMP: amphetamine; AMPUD: amphetamine use disorder; CBT: Cognitive Behavioral Therapy; CUD: cannabis use disorders; MDD: major depression disorder; DSM: Diagnostic and Statistical Manual of Mental Disorders; MethAMP: methamphetamine; MPH: methylphenidate; MPHUD: methylphenidate use disorder; NA: not available; ROA: Route of Administration; SD: standard deviation; SUD: substance use disorder; N/A: Not Available; NRT: Nicotine Replacement Therapy; NRT+: Combination Nicotine Replacement Therapy; VR: varenicline; CAR: Continuous Abstinence Rate; 7PP: Point Prevalence Abstinence; XR-NTX: extended-release naltrexone; BP-NLX: buprenorphine-naloxone; CIDI: Composite International Diagnostic Interview; ISI: Insomnia Severity Index; ISQ: Insomnia Symptoms Questionnaire; MINI: Mini International Neuropsychiatric Interview; BSCS: Brief Substance Craving Scale; CCQ-N: Cocaine Craving Questionnaire-Now; HRQ: Health-Related Quality of Life.

Table 2.
Overview of studies recording non-pharmacological management of chronic insomnia associated with stimulant use—a summary of the main findings.
Table 2.
Overview of studies recording non-pharmacological management of chronic insomnia associated with stimulant use—a summary of the main findings.
| Study | Study Design (Country) | Sample Features (Gender, Mean Age ± SD) | Type of Intervention | Duration of Intervention | Type of Stimulant Use Disorder | Additional Psychiatric Disorder | Findings | Clinical Symptoms Related to Insomnia | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Badrfam et al., 2024 [40] | Randomized controlled trial | N = 60 with a history of more than 3 years of methamphetamine use. Age range (18–60 yy) | Probiotic group: Probiotic capsule (Lactobacillus Acidophilus) + risperidone. Placebo group: placebo + risperidone. Risperidone dosage not specified | 8 weeks | Chronic methamphetamine use (history of >3 years). Patients were hospitalized | Psychotic disorder | For sleep quality (PSQI), there were significant interaction effects of group and time at Week 8 (t = −3.32, B = −1.83, p = 0.001, d = 0.89) such that individuals in the probiotic group experienced greater decreases in sleep problems at the end of the trial | N/A | Changes in the density and diversity of gut microbiota in chronic use of methamphetamine have been defined as contributors to anxiety symptoms and insomnia. Out of a total of 76 screened patients, 16 patients were excluded before the implementation of the study due to not having all the inclusion criteria |
| Buchanan et al., 2018 [25] | Pilot study | N = 22; median age = 46 years (range: 30–59); 77.3% biologically male; most were low income and single | BBTI: 4 sessions over 4 weeks (2 in-person, 2 by phone). Based on behavioral principles (sleep restriction, stimulus control, sleep hygiene). | 4 weeks (weekly sessions). Sleep assessed at baseline (Week 1) and post-treatment (Week 6) | Caffeine and alcohol dependence | Major depressive disorder | ISI median score decreased from 18.0 to 3.0 (p = 0.012), shifting participants from moderate/severe insomnia to non-clinical levels and PSQI improved from median 11.0 to 6.0 (p = 0.026). Sleep diary showed increased total sleep time (6.3 → 6.9 h) | Irregular sleep timing, caffeine use near bedtime, and mental hyperarousal (described as unable to empty mind) | 12 participants completed the full intervention. Dropouts (n = 10) occurred before starting therapy, mainly in the waitlist phase. Participants are HIV positive. |
| Curry et al., 2024 [28] | Randomized controlled trial | N = 100; 51 female, 49 male; mean age: 48.0 years (SD = 13.9 for active group), 48.1 years (SD = 14.1 for sham group) | VeNS delivered for 30 min prior to sleep, 5 nights a week. Active device delivered 3.5 mA output while sham device 0 mA | 8 weeks | Caffeine Dependence | Mood disorder | Significant reduction in ISI scores in the active VeNS group compared to the sham group after 8 weeks. Significant improvement in PSQI scores in the active group and increases in total sleep time and decreases in sleep latency based on sleep diaries | Difficulty falling asleep, staying asleep, early morning awakening, dissatisfaction with sleep, interference with daily functioning | Twenty-two non-anticipated AEs (n = 20 in UK) were reported during the intervention period, and one additional serious AE (minor cerebral vascular accident) that was not device related. Adherence to treatment was high. |
| Ekholm et al., 2020 [29] | Randomized controlled trial | N = 120; 82 females, 38 males. mean 39.6, range 18–77. Sleep disturbance Mean duration (years) 20.2 (SD 15.0) | Patients were randomized (1:1) to either a weighted metal chain blanket or a light plastic chain blanket | 4 weeks | Stimulant use disorder (methylphenidate) | Major depressive disorder, bipolar disorder, generalized anxiety disorder | At 4 weeks, there was a significant advantage in ISI ratings for the weighted blanket intervention over the light blanket (p < 0.001). The weighted blanket intervention showed a significant advantage for insomnia and sleep-related daytime symptoms | Fatigue, anxiety, depression, aggression, or nervousness | Participants also used hypnotics (nitrazepam zolpidem zopiclone propiomazine), sedatives (alprazolam diazepam hydroxyzine oxazepam promethazine), anticonvulsants (lamotrigine pregabalin valproate), antipsychotics (aripiprazole flupentixol olanzapine perphenazine quetiapine), antidepressants (amitriptyline) |
| Miller et al., 2021 [33] | Randomized controlled trial | Young adults (ages 18–30 years); 75% female; 73%; all college students | Cognitive Behavioral Therapy for Insomnia (CBT-I); 5 weekly sessions | 5 weeks | Nicotine Dependence and Caffeine Dependence | Depression, Anxiety Disorder, Other SUD (cannabis) | At post treatment (n = 43), CBT-I participants (M = 28.58, SD = 2.97) reported greater satisfaction with treatment than SH participants. CBT-I group reported greater decreases in insomnia severity (−9.61, SE = 0.84, p < 0.001) than those in the SH group (−6.68, SE = 0.84, p < 0.001; d = 1.20). CBT-I had an indirect effect on alcohol-related consequences through its influence on insomnia symptoms | Problems falling asleep, staying asleep, and waking too early. Dissatisfaction with sleep, distress, noticeability, and daily interference | Use of any sleep medication: trazodone, doxylamine, melatonin, OTC (e.g., diphenhydramine). Motives for substance use: social anxiety, depression, enhancement. treatment compliance is reported for the 88% of participants (25 CBT-I and 24 SH) who completed treatment diaries |
| Morgan et al., 2006 [24] | Randomized controlled trial | N = 12 (10 males, 2 females); Mean age = 39 years (SD = 7) | Procedural learning interventions (Sustained abstinence following cocaine IV self-administration: 32 mg/70 kg dose during 2 h binge sessions on Days 4–6 and/or Days 18–20) | 3 weeks | Cocaine dependence (urine positive)/nicotine dependence (92%) | N/A | Objective sleep worsened with abstinence (decrease in total sleep time and increase in latency). Cognitive performance declined with abstinence and has been reported sleep deterioration | Impaired vigilance and poor procedural learning as abstinence progressed, particularly at 14–17 days | Study identifies “occult insomnia”(dissociation between subjective vs. objective sleep): objective sleep deprivation + unawareness. Sleep spectral analysis showed increased slow-wave activity later in abstinence, possibly mimicking the feeling of deeper sleep |
| Patterson et al., 2020 [41] | Pilot randomized controlled trial | N = 29 treatment-seeking smokers; Age range: 21–65 years; Mean age ≈ 47.5 years (SD = 11.1); 52% female; 90% White; All smoked ≥ 8 cigarettes/day | SH group: 6 individual sessions combining standard smoking cessation counseling and varenicline + 20 min sessions of SH counseling, incorporating CBT-I principles (e.g., sleep hygiene, stimulus control, and sleep restriction). GH group: matched general health education (e.g., Alzheimer’s, dental care) | 15 weeks. Varenicline started 1 week before the quit date and continued for 12 weeks | Nicotine dependence | N/A | Higher sleep efficiency at baseline significantly predicted smoking cessation at end of treatment. Sleep efficiency change: +4.3% (quitters) vs. −4.9% (non-quitters), p < 0.01. These findings suggest that early improvements in objective sleep health may support abstinence and improve sleep performance | Sleep restriction, trouble falling asleep, and early awakenings | N/A |
| Pérez et al., 2020 [23] | Retrospective observational study | N = 87 patients (2 females, 85 males); mean age: 37.67 years (SD = 7.53). Participants aged 22 to 57 years | rTMS targeting the left DLPFC. 15 Hz frequency. 2400 pulses per session. Patients received 2 sessions/day for the first 5 consecutive days (10 sessions), then 2 sessions/week for the next 12 weeks | 90 days | Cocaine use disorder (urine positive) | Depression disorder, anxiety disorder | PSQI scores improved significantly after rTMS treatment, with scores significantly lower than baseline at Day 5 (5.09 ± 3.33), Day 30 (5 ± 3.13), Day 60 (5.28 ± 3.47), and Day 90 (6.12 ± 3.32) | N/A | More sessions were associated with better sleep quality. The mean number of days of cocaine use decreased significantly from 19.17 days (SD ± 11.45). 71.9% of patients were abstinent during the first 30 days and 66.1% at the end of treatment. Greater cocaine use was associated with worse sleep quality |
| Speed et al., 2022 [38] | Randomized controlled trial | N = 21 (10 in gCBT-I, 11 in SOC). Gender and specific mean age/SD not provided | Psychoeducation, sleep restriction, stimulus control, cognitive restructuring, and relaxation techniques. Comparisons of group-based Cognitive Behavioral Therapy for Insomnia to Standard of Care (SOC) | Delivered in 6 weekly 90 min group sessions | Cocaine dependence/nicotine dependence | N/A | Most participants reported reductions in insomnia symptoms while in CC treatment. Among participants with ISI scores beyond admission, insomnia severity decreased below or at the clinical cutoff for mild insomnia (ISI ≤ 8) in 4 out of 5 individuals in the gCBT-I group, compared with one out of 4 individuals in the SOC group | N/A | One participant (10%) was discharged before the intervention began. Six participants (60%) were discharged before completing the intervention; gCBT-I participants completed an average of 5 sessions |
| Su et al., 2017 [22] | Randomized controlled trial | N = 30 males; Age: 32.35 ± 4.96 years | High-frequency (10 Hz) rTMS applied over left DLPFC: 5 sessions (1/day), 8 min each, 1200 pulses per session at 80% resting motor threshold (rMT). (15 real rTMS, 15 sham) | 5 days (daily sessions); pre- and post-treatment assessments conducted within 3 weeks | Methamphetamine Use (MA) Disorder | N/A | rTMS improved cognitive performance in verbal memory, social cognition and sleep performance. After 5 sessions, real rTMS also significantly reduced craving | Mild side effects included scalp discomfort, temporary headache, and nausea | The study supports safety and efficacy of rTMS in insomnia patients with MA |
| Taylor et al., 2018 [35] | Randomized clinical trial | N = 151; active duty US Army soldiers. The mean age was 32.44 years, 82 percent were male, and 45 percent were Caucasian | Cognitive Behavioral Therapy for Insomnia Disorder (CBT-I). and included the following components: stimulus control sleep restriction, sleep hygiene, relaxation training and cognitive restructuring | 6 weeks (60 min sessions) | Caffeine dependence | Anxiety and depressive disorder | CBTi group showing significant improvements in sleep efficiency from pretreatment to post treatment compared with the control group. Similar effects were found for the components of sleep efficiency, sleep latency, number of awakenings, wake after sleep onset. CBTi group showing significant improvements for total sleep time over time compared with the control. | Fatigue, symptoms of depression, anxiety and PTSD. Reduced motivation and activity. | 68 completed the trial while 8 lost post-treatment and 5 declined to participate. 2 lost contact and 1 noncompliance with protocol |
| Unhjem et al., 2016 [39] | Randomized controlled trial | N = 24, 16 males, 8 females. Mean age: 32 ± 8 | Maximal strength training of lower extremities | TG: (85–90% of 1 RM) 3 times/week. CG: conventional clinical activities | Amphetamine dependence/cocaine dependence | Anxiety disorder/depression/schizophrenia/bipolar disorder | The TG showed improved neural function reducing anxiety and insomnia, while the CG reduced only anxiety. The level of insomnia significantly decreased only in the TG (p < 0.05). While the level of depression tended to decrease in both groups (p = 0.11 for the TG and p = 0.10 for the CG) | N/A | Of the 24 patients that were included in the study, 16 subjects completed the study period. Three patients in the TG dropped out of clinical treatment. In the CG 5 subjects dropped out; 3 patients dropped out of clinical treatment, 1 patient could not complete the testing procedure and 1 patient died from drug overdose |
| Zeng et al., 2018 [36] | Randomized controlled trial | N = 68; males. Mean age: 36.45 ± 1.57. MA abuse duration (months): 82.00 ± 66.55 | Electro-acupuncture (EA) group: 20 min. Sham electro-acupuncture (sham-EA) group: 20 min. | 4 weeks (3 times/week (Monday, Wednesday, and Friday) | Methamphetamine addictions (MA) | Anxiety Disorder/Depressive Disorder | Electro-acupuncture effectively improved symptoms of anxiety, depression and insomnia. For the HAMD sleep disorders score, the curative effect of the EA group was better than that of the sham-EA group after receiving 4 weeks of treatment | N/A | There were 4 withdrawal patients, 2 referral patients due to criminal cases, and 2 acupuncture fainting patients, which resulted in a total of 64 patients who actually completed the study (31 cases of the EA group and 33 cases of the sham-EA group) |
Abbreviations: ADHD: Attention Deficit Hyperactivity Disorder; AMP: amphetamine; AMPUD: amphetamine use disorder; CBT: Cognitive Behavioral Therapy; CUD: cannabis use disorders; MDD: major depression disorder; DSM: Diagnostic and Statistical Manual of Mental Disorders; MethAMP: methamphetamine; MPH: methylphenidate; MPHUD: methylphenidate use disorder; N/A: not available; ROA: Route of Administration; SD: standard deviation; SUD: substance use disorder; PSG = polysomnography; EEG = electroencephalogram; REM = Rapid Eye Movement sleep; SQQ = Sleep Quality Questionnaire; MST = Motor Sequence Task (sleep-dependent learning test); CDR = Cognitive Drug Research battery; VAS = Visual Analog Scale; rTMS = Repetitive Transcranial Magnetic Stimulation; ISLT = International Shopping List Task; HAMD = Hamilton Depression Scale; PSQI = Pittsburgh Sleep Quality Index; rMT = Resting Motor Threshold; TST: total sleep time; SL: sleep latency; DLPFC: left dorsolateral prefrontal cortex; TG and CG: training group and control group; VeNS: electrical vestibular nerve stimulation; BBTI: Brief Behavioral Treatment for Insomnia; SH: sleep health interventions; GH: general health interventions.
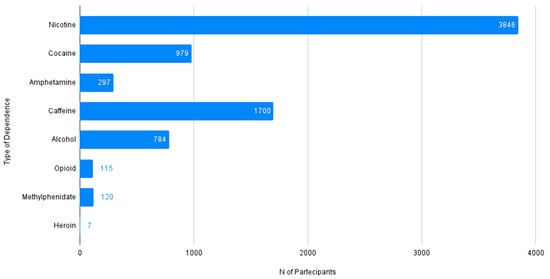
Figure 2.
Prevalence of the type of dependence reported among patients with chronic insomnia and stimulant use disorder (N Tot = 7848).
In order to quantify the co-occurring Substance Use Disorders: Nicotine Dependence (N = 3856/7848 individuals), Cocaine Dependence (N = 979/7848 individuals), Amphetamine Use Disorder (N = 297/7848 individuals), Caffeine Dependence (N = 1700/7848 individuals), Alcohol Use Disorder (N = 784/7848 individuals), Opioid Dependence (N = 115/7848 individuals), Methylphenidate use (N = 120/7848 individuals) and Heroin Dependence (N = 7/7848 individuals).
3.4. Anxiety, Depressive Disorder, and Other Psychiatric Comorbidities
Among the included studies, a proportion of patients presented comorbid psychiatric conditions, mainly anxiety and depressive disorders. Specifically, 53.2% of patients had a depressive disorder and 34% presented with an anxiety disorder, while other psychiatric disorders such as bipolar disorder (5.6%), mood disorder (3.9%), psychotic disorder (2.3%), and schizophrenia (0.9%) were less frequently reported. These comorbidities were considered in some studies as potential confounding variables, whereas in others they were not systematically assessed. Overall, comorbid psychiatric conditions emerged as a recurrent feature in the examined populations, with depressive and anxiety disorders representing the most consistently reported diagnoses.
Depressive disorder was reported in seven studies [23,29,30,32,34,35,36], anxiety disorder in eight studies [23,29,30,32,34,35,36,39], and major depressive disorder in four studies [25,29,32,34]. Bipolar disorder was reported in two studies [29,39], psychotic disorder in one study [40], and generalized anxiety disorder in one study [29]. Figure 3 describes the co-occurrence of psychiatric disorders in patients with insomnia and associated stimulant use disorder.
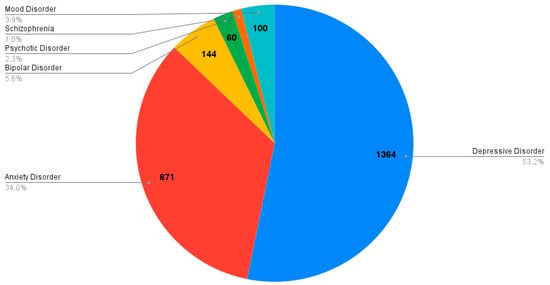
Figure 3.
Frequency of psychiatric comorbidity in patients with chronic insomnia and stimulant use disorder (N Tot = 2563).
In conclusion, the number of individuals with co-occurring psychiatric diagnoses was as follows: depressive disorder (N = 1364/2563 individuals), anxiety disorder (n = 861/2563 individuals), bipolar disorder (n = 144/2563 individuals), mood disorder (n = 100/2563 individuals), psychotic disorder (n = 60/2563 individuals) and schizophrenia (N = 24/2563 individuals).
4. Discussion
This systematic review examined 20 articles related to pharmacological and non-pharmacological interventions for chronic insomnia and associated stimulant use disorder. The study types ranged from 15 RCTs to pilot, observational, and retrospective studies (one pilot RCT, two pilot studies, one retrospective observational study, and one observational study).
4.1. Characteristics of Study Populations
The demographic characteristics of participants varied widely, reflecting both young and adult populations. Many studies reported a male predominance [22,23,24,25,26], while others showed a higher female presence [27,28,29,30,32,33,34]. The most frequently reported additional psychiatric diagnoses included depression disorder [23,29,30,32,34,35,36] and major depressive disorder [25,29,32,34]. Also, anxiety disorder [23,29,30,32,34,35,36] and generalized anxiety disorder [29] have been reported in the study population. Finally, bipolar disorder [29,39] and psychotic disorder [40].
Among stimulant use disorder and other SUD, the most commonly reported were Nicotine Dependence, reported in eight articles [24,27,30,31,32,33,38,41], cocaine dependence, found in nine articles [23,24,26,37,38,39], amphetamine use disorder, described in four articles [22,36,39,40] and caffeine dependence, mentioned in four articles [27,28,33,35]. Other SUDs reported included alcohol use disorder in two articles [25,26], opioid dependence in one article [34], and cannabis use disorder in one article [33].
4.2. Main Pharmacological Approaches to Insomnia in the Context of Stimulant Use
It is essential to acknowledge that several pharmacological options are widely used in clinical practice for insomnia in patients with stimulant use disorder [42].
- (1)
- New-generation benzodiazepine receptor agonists, known as Z-drugs (including zolpidem, zopiclone, eszopiclone, and zaleplon), demonstrated significant efficacy in the acute treatment of insomnia. Among the most relevant adverse events that emerged were an increased risk of anterograde amnesia, daytime somnolence, and fatigue [42].
- (2)
- Second-generation antipsychotics (SGAs) such as quetiapine, promazine, and perphenazine are often used for their sedative properties. While not specifically approved for primary insomnia, their sedative effects make them appealing to quickly stabilize a patient’s sleep–wake cycle. Quetiapine, for example, at doses lower than those used for psychosis, acts as a potent sedative-hypnotic. However, its use in patients with stimulant use disorder raises concerns due to potential side effects like excessive daytime sedation, weight gain, and long-term metabolic risks [43]. Chlorpromazine and perphenazine are first-generation antipsychotic drugs used to manage and treat schizophrenia, bipolar disorder, acute psychosis, and severe agitation [44]. Being a low-potency typical antipsychotic, it primarily causes dry mouth, dizziness, urine retention, blurred vision, and constipation by blocking the muscarinic receptor. When administered as intramuscular or intravenous injections, it may cause hypotension and headache. Despite being a low-potency drug, chlorpromazine can still cause extrapyramidal side effects (EPS) such as acute dystonia, akathisia, parkinsonism, and tardive dyskinesia (TD) [45].
Overall, the presence of dual diagnosis, referring to a combination of an SUD and mental disorders that occur in the same patient simultaneously, poses significant clinical and healthcare impacts and is often underdiagnosed, undertreated, and complex to manage. Indeed, among SGAs, aripiprazole was the most used drug in this specific population, either orally or by long-acting formulations, followed by risperidone with oral and long-acting formulations, clozapine, olanzapine, and quetiapine [46].
- (3)
- Among antidepressants with sedative properties used for insomnia, trazodone is one of the most commonly prescribed non-benzodiazepine hypnotics, often used at low doses for its strong sedative action. It offers an alternative to benzodiazepines with a lower risk of dependence, making it a seemingly preferable choice for patients with a history of substance use, but its long-term efficacy and side effects, such as orthostatic hypotension, must be considered [47].
- (4)
- Agomelatine, with its unique mechanism of action on melatonin and serotonin receptors, has properties that could theoretically resynchronize circadian rhythms. It is a melatonergic agonist and a 5HT2c antagonist. The melatonergic function appears to improve sleep patterns, whereas the serotonergic antagonism results in the release of norepinephrine and dopamine [48].
- (5)
- The use of benzodiazepines is widespread for insomnia, despite clear contraindications for individuals with a history of substance use disorder. Benzodiazepines enhance the effects of GABA, a neurotransmitter that inhibits nerve activity, reducing sleep onset latency and increasing sleep duration. While their short-term efficacy in reducing anxiety and inducing sleep is undeniable, the risk of dependence, tolerance, and abuse is extremely high. Their use is only recommended for ≤4 weeks due to unproven long-term efficacy in the treatment of chronic insomnia and the risk of tolerance and the potential for dependence and misuse. Prescribing benzodiazepines to patients with stimulant dependence can lead to a dangerous addiction or polydrug dependence [49].
Current guidelines recommend Cognitive Behavioral Therapy for insomnia (CBT-I) as the first-line treatment. Pharmacological recommendations by European guidelines for the treatment of insomnia disorder include positive GABAergic modulators such as short- and medium-acting benzodiazepines and “Z-drugs” (eszopiclone, zaleplon, zolpidem, and zopiclone), dual orexin receptor antagonists (DORAs; daridorexant), and melatonin receptor agonists (melatonin 2 mg prolonged release—PR). To avoid the risk of addiction and tolerance, discontinuation of benzodiazepines should be gradual, with dose reductions of 10–25% each week. CBT-I, daridorexant, eszopiclone, and melatonin were shown to facilitate the gradual discontinuation of benzodiazepines within a cross-tapered program, which can be delayed when necessary [50]. Widespread prescription of benzodiazepines, the related abuse risk, and the side effect profile, particularly in specific populations, should encourage implementation of specific training interventions in order to minimize the risk of misuse by patients and the risk of malpractice for prescribers. Short-acting BDZs are efficacious for short-term insomnia (<4 weeks), associated with a reduction in nocturnal wake time and subjective improvements in quality and depth of sleep. The risk–benefit ratio is positive in most sleep-disordered patients in the short term but is unestablished in the long term [51].
4.3. Summary of Findings: A Comprehensive Review of Treatment Modalities
- (1)
- In the present review, modafinil was investigated in one study. It is a non-amphetamine central nervous system stimulant and weak inhibitor of dopamine reuptake, but also it has been found that it is able to increase signaling in the hypothalamic orexin and histamine neurotransmitter pathways. Modafinil is absorbed after oral administration and cannot be administered intravenously. United States Food and Drug Administration-Approved (FDA) indications are narcolepsy, sleep–work shift disorder, and obstructive sleep apnea, but there are also off-label indications such as attention-deficit hyperactivity disorder and cocaine dependence [52]. According to the European Medicines Agency (EMA), modafinil is not approved for obstructive sleep apnea and sleep–work shift disorder but only for narcolepsy. Participants treated with modafinil experienced a rebound in REM sleep, reporting sleep misperception and overestimation. In addition, according to the study reviewed, modafinil was mainly employed in the treatment of patients with chronic insomnia and associated cocaine dependence as stimulant use disorder. Modafinil, a wake-promoting agent primarily indicated for disorders characterized by excessive daytime sleepiness such as narcolepsy, was used instead to address the complex sleep–wake alterations observed in individuals with chronic cocaine dependence during abstinence, a phenomenon called “occult insomnia.” During abstinence, these individuals often manifest a paradoxical combination of fragmented and non-restorative nocturnal sleep, coupled with marked daytime sleepiness, persistent fatigue, and cognitive deficits. Modafinil exerts its wake-promoting effects through intricate mechanisms, including increasing extracellular levels of dopamine, norepinephrine, histamine, and orexin in specific brain regions such as the hypothalamus and nucleus accumbens [53]. Modafinil was utilized to optimize the quality of daytime wakefulness. By enhancing alertness and reducing residual daytime sleepiness, modafinil can contribute to a more stable wakefulness during the day [54]. Concluding, modafinil showed better efficacy in improving sleep phase, especially if associated with a cessation of cocaine use, confirming an improvement of insomnia [37].
- (2)
- Another intervention analyzed in the study was naltrexone/buprenorphine-naloxone. Naltrexone is an opioid antagonist approved by the FDA to treat alcohol use disorder opioid dependence and is currently being studied in heroin dependence. EMA-approved indications are for management of weight and are not approved as independent therapy for opioid dependence and alcohol use disorder. Naltrexone blocks the effect of opioids and prevents opioid intoxication and physiologic dependence on opioid users [55]. Instead, buprenorphine is a synthetic opioid and partial agonist at the mu receptor used to treat chronic pain and opioid use disorder according to the indications of the FDA. When combined with buprenorphine, naltrexone selectively activates kappa receptors without stimulating the opioid receptors, allowing them to reduce compulsive cocaine use without leading to opioid addiction [56]. Naloxone operates as a competitive antagonist to the μ-opioid receptor indicated for the treatment of opioid toxicity, approved both by EMA and FDA, specifically to reverse respiratory depression from opioid use [57]. Particularly, XR-NXT and BP-NLX have been used in a population with heroin dependence, methamphetamine dependence, and opioid dependence [34], where it has been found that the insomnia score was significantly lower in the XR-NTX group compared to the BP-NLX group [34]. This intervention demonstrated that the extended formulation of naltrexone is effective in reducing the burden of insomnia. When comparing treatment outcomes between modafinil and naltrexone/buprenorphine, the latter intervention generally demonstrates superior efficacy, particularly in the population affected by methamphetamine dependence. Modafinil, a wake-promoting agent, has shown some promise in addressing stimulant use disorders, such as cocaine and methamphetamine dependence, but its role in insomnia patients is limited. Its mechanisms include alleviating withdrawal symptoms, reducing cravings, and improving cognitive function [58].
- (3)
- Varenicline acts as a partial nicotine receptor agonist similar to cytisine, demonstrating a high affinity for the α4β2 subtypes of nicotinic acetylcholine receptors. The FDA-approved indications are mainly smoking cessation and dry eye, while EMA-approved indications are only for smoking cessation [59]. Varenicline and NRT+ were more effective on insomnia symptoms than NRT alone. The use of nicotine patches significantly improved sleep performance and insomnia symptoms, especially in night smokers [31]. However, it has been observed that patients may experience increased sleep disturbance after quitting, suggesting that smoking cessation may exacerbate withdrawal-related sleep problems in the early phase. According to these results, it should be considered that this intervention in particular may initially worsen sleep quality, which should then require a more focused approach during its early phase [30,31,32].
- (4)
- Ramelteon has been used in a population with nicotine dependence and associated high caffeine consumption, showing significant improvements in ISI scores, increasing satisfaction with sleep patterns, improving the difficulty of staying asleep, and decreasing the interference with daily functioning [27]. Ramelteon acts on melatonin receptors, which are known to be involved in the modulation of the normal sleep–wake cycle. The suprachiasmatic nucleus (SCN) controls circadian rhythms of sleep and wakefulness and is the location of most of the melatonin receptors. Ramelteon binds to the MT1 and MT2 melatonin receptors in the SCN, inhibiting neuronal firing and thereby enabling the homeostatic mechanism to promote sleep. The affinity of ramelteon for the MT1 and MT2 receptors is 3 to 16 times higher than that of endogenous melatonin. Ramelteon is the only melatonin agonist currently indicated for the treatment of insomnia, according to the FDA, while it is not approved by the EMA [60]. Varenicline combined with NRT and ramelteon has demonstrated good efficacy, particularly in the study population affected by chronic insomnia and nicotine dependence as stimulant use.
- (5)
- rTMS was investigated in more studies. The use of high-frequency (10 Hz) rTMS over the left dorsolateral prefrontal cortex (DLPFC) improved cognitive performance, social cognition, and sleep performance, also significantly reducing craving after 5 sessions. PSQI scores improved significantly with more sessions associated with better sleep quality [22,23]. Intervention also reported good results addressing cocaine dependence, decreasing the mean number of days of cocaine use from 19.17 days (SD ± 11.45). 71.9% of patients were abstinent during the first 30 days and 66.1% at the end of treatment. TMS interconverts electrical and magnetic energy to induce electromagnetic phenomena. An electromagnetic coil is placed on the scalp, and an effective pulsatile magnetic field that depolarizes cortical neurons is generated for a brief duration of time. Patients who underwent stimulation of the left DLPFC showed a drop in salivary cortisol level in the morning [61]. A dysregulation of the hypothalamic–pituitary–adrenal axis (HPA) could provoke acute increases in arousal or reactions of metabolic systems during the night. These disruptions in cortisol secretion during the night may contribute to perceptions of worse sleep quality [62]. In conclusion, rTMS, by acting on the HPA, may be used to reduce the burden of insomnia. rTMS has shown promising results also in patients with stimulant use disorder, such cocaine use disorder, reducing cocaine craving and consumption with also great improvement in depressive symptoms. rTMS effectiveness has been demonstrated by the substantial decrease in the number of days per week of cocaine use after 20 sessions of treatment [63].
- (6)
- CBT-I was broadly effective: most participants reported reductions in insomnia symptoms. Brief Behavioral Treatment for Insomnia (BBTI) decreased the ISI median score from 18.0 to 3.0 and improved the PSQI from 11.0 to 6.0, substantially decreasing the impact of insomnia on daily life. CBT-I participants showed greater satisfaction with treatment and significant improvements in sleep efficiency, sleep latency, number of awakenings, wake after sleep onset, and total sleep time compared to the control group. Treatment compliance is very high and in one study reaches 88% of participants [33]. SH counseling incorporating CBT-I principles led to early improvements in objective sleep health, which may support abstinence and improve sleep performance [25,29,33,38].
- (7)
- Regarding exercise/physical activity, maximal strength training of lower extremities and VeNS significantly decreased insomnia levels, improving neural function and reducing anxiety. Weighted blankets demonstrated a significant advantage in ISI ratings over light blankets after 4 weeks, showing benefits for insomnia and sleep-related daytime symptoms [28,39,41]. Finally, EA demonstrated an improvement in the symptoms of anxiety, depression, and insomnia, with a better curative effect on HAMD sleep disorder scores [36]. Its mechanism consists of using low frequency (2 Hz) and high frequency (100 Hz) to selectively induce the release of enkephalins and dynorphins, and it has demonstrated effectiveness for the treatment of various types of pain, depression, anxiety, spinally induced muscle spasm, stroke, gastrointestinal disorders, and drug addiction [64].
- (8)
- Lastly, the use of probiotics demonstrated that individuals experienced greater decreases in sleep problems at the end of the trial (week 8) compared to the placebo group, suggesting their feasibility in improving sleep quality. Probiotic supplementation can positively impact various psychiatric symptoms such as sleep, depression, and psychotic symptoms [65].
Probiotics were utilized in individuals with methamphetamine dependence [40]. CBT was applied in contexts of nicotine, caffeine, and cocaine dependence [33,35,38,41]. The Brief Behavioral Intervention (BBTI) was employed for caffeine and alcohol dependence [25]), and VeNS was investigated only in patients with caffeine dependence [28]. Weighted blankets were explored in patients with methylphenidate use [29], and procedural learning interventions were applied in cases of cocaine and nicotine dependence [24]. TMS was utilized in the treatment of cocaine and methamphetamine dependence [22,23]. Furthermore, EA was used in methamphetamine dependence [36], and finally, lower extremity training was explored in populations with amphetamine and cocaine dependence [39].
4.4. Impact of Insomnia on Stimulant Users
Several studies noted that sleep disturbance can be exacerbated during the early phase of stimulant withdrawal or during treatment for stimulant use disorder [24,30]. Occult insomnia has been reported in two studies and refers to the dissociation between subjective and objective sleep, where objective sleep worsened with abstinence, but participants were unaware of it [24,37]. Factors like caffeine used near bedtime and mental hyperarousal were identified as contributing to sleep problems [25]. The co-occurrence of night smoking and poor sleep predicted significantly worse smoking outcomes [31]. Changes in gut microbiota were also suggested as contributors to anxiety and insomnia symptoms in chronic methamphetamine use [40].
Insomnia is characterized by persistent difficulty with sleep initiation, duration, consolidation, or quality, representing a pervasive public health concern with significant consequences. Its prevalence is notably high, impacting a substantial portion of the global population, and its chronicity often leads to a cascade of adverse health outcomes [66]. Untreated insomnia is associated with a heightened risk of various medical comorbidities, including cardiovascular diseases, metabolic disorders, and chronic pain syndromes [67]. Furthermore, it profoundly impacts mental health, frequently co-occurring with psychiatric disorders including depression, anxiety, bipolar disorder, and psychotic disorder.
The functional impairments rising from chronic insomnia extend to impaired cognitive function, reduced productivity, increased accident risk, and a significant deterioration in overall quality of life [68]. Commonly reported clinical symptoms were difficulty falling asleep, impaired vigilance, poor procedural learning, abnormal dreams, and night awakenings, along with aggressiveness and nervousness [26,28,33]. The complexity of insomnia is further compounded in populations with co-occurring SUDs, particularly those involving stimulant use. Stimulants, by their very nature, directly interfere with sleep–wake cycles and neurotransmitter systems crucial for sleep regulation. Chronic stimulant use can induce or worsen insomnia through various mechanisms, including prolonged central nervous system activation, withdrawal-related dysphoria, and disruption of circadian rhythms [69]. The occurrence of double diagnosis was very high; co-occurring SUD were commonly reported among the majority of the participants in the study, such as nicotine dependence, cocaine dependence, amphetamine use disorder, caffeine dependence, alcohol use disorder, and opioid dependence.
This bidirectional relationship creates a condition where untreated insomnia can decrease the chance of recovery from stimulant use disorder, increasing the risk of relapse, while ongoing stimulant use perpetuates sleep disturbances. Data from trend analyses, such as those from Google Trends, reports a growing public awareness and search interest in sleep problems, reflecting the rising recognition of insomnia as a significant health issue that often coexists with other conditions [70].
4.5. Influence of Anxiety and Depressive Disorders on Therapeutic Response
The presence of psychiatric comorbidities, particularly anxiety and depressive disorders, may significantly influence treatment outcomes. Patients with such conditions are often at higher risk of poor adherence, reduced response to pharmacological interventions, and greater functional impairment compared to those without psychiatric disorders. Evidence from late-life depression suggests that comorbid anxiety is associated with slower treatment response and increased relapse risk, underlining the negative prognostic value of such conditions [71]. Similarly, in the context of insomnia, the presence of anxiety or depressive disorders has been shown to moderate treatment outcomes: partial interventions, such as cognitive therapy alone or behavioral therapy alone, may be less effective, whereas full cognitive-behavioral therapy for insomnia (CBT-I) maintains its efficacy even in the presence of psychiatric comorbidities [72].
A more recent study has extended these findings, demonstrating that CBT-I applied to patients with generalized anxiety disorder not only improves sleep but also produces significant reductions in anxiety and depressive symptoms, supporting the dual benefit of integrated interventions [73]. Moreover, clinical recommendations emphasize that insomnia should be addressed as a distinct condition even when it co-occurs with depression or anxiety, as targeted treatment may improve both sleep and psychiatric outcomes [74]. Taken together, these results suggest that psychiatric comorbidities can negatively affect outcomes if not properly managed but also highlight the potential of integrated or transdiagnostic approaches in improving prognosis.
4.6. Clinical Implications of Insomnia and Stimulant Use Management
Given the interconnected nature of insomnia and stimulant use, an integrated treatment approach is recommended. Addressing sleep disturbances in this vulnerable population is not only about improving sleep quality; it is a critical component aiming at reducing psychiatric symptom burden, preventing relapse, and enhancing overall well-being.
While pharmacological options such as ramelteon show promise, and certain agents like modafinil may require careful consideration due to mixed effects on insomnia, non-pharmacological interventions, particularly CBT-I and rTMS, appear to be consistently effective and safe with minimal risk of adverse events. The high adherence rates often observed in non-pharmacological interventions further support their feasibility in this population. Addressing underlying psychiatric comorbidities and other substance dependencies is also crucial, as these factors significantly influence both insomnia presentation and treatment outcomes.
It is important to highlight that the analyzed treatments have shown efficacy only in specific clinical contexts and depending on the type of SUD. For instance, modafinil has been primarily used in patients with cocaine dependence, while naltrexone/buprenorphine has been applied mainly in individuals with nicotine and opioid dependence. Similarly, non-pharmacological interventions such as TMS, CBT-I, or probiotics have been studied in subgroups with different substance dependencies (e.g., nicotine, caffeine, methylphenidate). This underscores that the effectiveness of each treatment may vary significantly depending on the type of substance abused, suggesting the need for a personalized and substance-specific therapeutic approach.
5. Strengths and Limitations of the Study
This systematic review investigates both pharmacological and non-pharmacological interventions for the management of chronic insomnia, specifically within the context of individuals with stimulant use disorder. By synthesizing data from 20 original studies, this review highlights the significance of understanding the different range of available treatments and their efficacy in this complex population. The novelty of the findings underscores the urgent need for healthcare providers to recognize the interconnected nature of insomnia and stimulant use, with the aim of contributing to developing integrated management strategies. Given the substantial health risks associated with chronic insomnia and stimulant use, including exacerbation of psychiatric symptoms and increased risk of relapse, the data presented in this study provide an essential foundation for developing strategies aimed at improving patient care, treatment protocols, and psychoeducation. However, certain limitations must be acknowledged. There is a potential for publication bias, as the review primarily included studies published in English, which may have resulted in the exclusion of relevant research available in other languages. Moreover, the heterogeneity of study designs, stimulant types, and intervention protocols across the included articles makes direct comparisons challenging and limits the generalizability of findings. The varied outcome measures and assessment tools used for insomnia further complicate data synthesis. Some studies had relatively small sample sizes or lacked robust control groups, which might impact the strength of their conclusions. These factors may limit the overall generalizability of the findings to the broader population of individuals with chronic insomnia and stimulant use disorder. Nonetheless, the results derived from this review can be used for future research and to underline the need for careful monitoring and focused interventions for individuals with chronic insomnia and stimulant use disorder.
6. Conclusions
In conclusion, the management of insomnia in individuals with stimulant use disorder requires an integrated and individualized treatment strategy targeting both sleep disturbances and the underlying addiction. Among pharmacological options, ramelteon emerged as a promising agent due to its favorable safety profile and effectiveness in improving sleep indices. Conversely, while modafinil demonstrated potential benefits in reducing craving and promoting abstinence, its paradoxical effects on sleep underscore the need for patient-specific monitoring. Non-pharmacological interventions, particularly CBT-I and rTMS, consistently showed significant efficacy with minimal side effects and high adherence. Their capacity to improve both sleep and craving suggests that they may be employed as therapeutic intervention in this population. Other innovative strategies such as EA, weighted blankets, VeNS, and probiotics offer additional, non-invasive approaches that may be especially useful in patients with high psychiatric comorbidity or sensitivity to medications.
Insomnia is not only a secondary symptom but also a critical concern, especially in patients with stimulant dependence. Its persistence is associated with higher anxiety and depression scores, increased relapse risk, and poorer quality of life. Therefore, insomnia should be carefully assessed and treated rather than being considered a background symptom. When employing these interventions, always consider that the treatment response is influenced by demographic and clinical factors, including age, comorbid substance use, employment status, and prior sleep medication use, suggesting that applying the same schedule to all patients is not the solution. Tailoring interventions to individual profiles and combining pharmacological and non-pharmacological strategies with regular monitoring offers the most effective approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/psychiatryint6040121/s1, Table S1: risk of bias assessment table.
Author Contributions
Conceptualization: S.C., G.M. and L.P.; writing—original draft preparation, S.C. and P.D.G.; writing—review and editing, S.C., G.M., L.P., M.A., A.M., P.D.G. and S.C. independently extracted and collected relevant data; A.M. contributed to the analysis of the results and discussed with G.M., L.P. and M.A. possible issues and disagreements during the revision of the paper. The risk of bias will be measured independently by two members of the team (P.D.G. and A.M.) supervised by S.C. and G.M. using the “Assessing risk of bias in included studies” on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
G.M. has been a consultant and/or a speaker and/or has received research grants from Angelini, Doc Generici, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Servier and Recordati. S.C. has been a consultant and/or a speaker for Angelini, Lundbeck, and Otsuka. All other Authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
- Dubois, J.M.; Rouen, A.; Léger, D. Insomnie: Définitions, épidémiologie et évolution avec l’âge [Insomnia: Definitions, epidemiology and changes with age]. Rev. Prat. 2024, 74, 260–265. [Google Scholar] [PubMed]
- Morin, C.M.; Jarrin, D.C. Epidemiology of Insomnia: Prevalence, Course, Risk Factors, and Public Health Burden. Sleep. Med. Clin. 2022, 17, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.; Roehrs, T. Insomnia: Epidemiology, characteristics, and consequences. Clin. Cornerstone 2003, 5, 5–15. [Google Scholar] [CrossRef] [PubMed]
- KPavlova, M.; Latreille, V. Sleep Disorders. Am. J. Med. 2019, 132, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, K.M.; Van Dyk, T.R.; Lundahl, A.; Nelson, T.D. Stimulant Medications and Sleep for Youth With ADHD: A Meta-analysis. Pediatrics 2015, 136, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Ogeil, R.P.; Phillips, J.G. Commonly used stimulants: Sleep problems, dependence and psychological distress. Drug Alcohol. Depend. 2015, 153, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Wanjari, A.; Sinha, A.H. Effects of Nicotine on the Central Nervous System and Sleep Quality in Relation to Other Stimulants: A Narrative Review. Cureus 2023, 15, e49162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farzam, K.; Faizy, R.M.; Saadabadi, A. Stimulants. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- United Nations Office on Drugs and Crime (UNODC). Stimulants. 2019. Available online: https://wdr.unodc.org/wdr2019/en/stimulants.html (accessed on 21 January 2025).
- Drug Enforcement Administration (DEA). Stimulants. 2020. Available online: https://www.dea.gov/sites/default/files/2020-06/Stimulants-2020.pdf (accessed on 21 January 2025).
- Food and Drug Administration (FDA). Prescription Stimulant Medications. 2024. Available online: https://www.fda.gov/drugs/information-drug-class/prescription-stimulant-medications (accessed on 21 January 2025).
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report 2024: Trends and Developments. 2024. Available online: https://www.emcdda.europa.eu/publications/european-drug-report/2024_en (accessed on 21 January 2025).
- Riemann, D.; Espie, C.A.; Altena, E.; Arnardottir, E.S.; Baglioni, C.; Bassetti, C.L.A.; Bastien, C.; Berzina, N.; Bjorvatn, B.; Dikeos, D.; et al. The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023. J Sleep Res 2023, 32, e14035. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J.; Buysse, D.J.; Krystal, A.D.; Neubauer, D.N.; Heald, J.L. Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep. Med. 2017, 13, 307–349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chakravorty, S.; Vandrey, R.G.; He, S.; Stein, M.D. Sleep Management Among Patients with Substance Use Disorders. Med. Clin. North. Am. 2018, 102, 733–743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Substance Abuse And Mental Health Services Administration (SAMHSA). Treating Sleep Problems of People in Recovery From Substance Use Disorders. 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK571029/ (accessed on 21 January 2025).
- Rios, P.; Cardoso, R.; Morra, D.; Nincic, V.; Goodarzi, Z.; Farah, B.; Harricharan, S.; Morin, C.M.; Leech, J.; Straus, S.E.; et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: An overview of reviews. Syst. Rev. 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Q.H.; Liu, Y.J.; Yang, G.; Zhu, M.Y.; Yang, J.H.; Li, L.; Yan, X.M.; Liu, Q.L.; Yue, J.H.; Li, X.L.; et al. Efficacy and safety of non-pharmacological therapies for primary insomnia: A network meta-analysis. Front. Neurol. 2025, 16, 1607903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA). PRISMA Statement. 2024. Available online: http://www.prisma-statement.org/ (accessed on 21 January 2025).
- Su, H.; Zhong, N.; Gan, H.; Wang, J.; Han, H.; Chen, T.; Li, X.; Ruan, X.; Zhu, Y.; Jiang, H.; et al. High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: A randomised clinical trial. Drug Alcohol. Depend. 2017, 175, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Gómez Pérez, L.J.; Cardullo, S.; Cellini, N.; Sarlo, M.; Monteanni, T.; Bonci, A.; Terraneo, A.; Gallimberti, L.; Madeo, G. Sleep quality improves during treatment with repetitive transcranial magnetic stimulation (rTMS) in patients with cocaine use disorder: A retrospective observational study. BMC Psychiatry 2020, 20, 153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morgan, P.T.; Pace-Schott, E.F.; Sahul, Z.H.; Coric, V.; Stickgold, R.; Malison, R.T. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug Alcohol. Depend. 2006, 82, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, D.T.; McCurry, S.M.; Eilers, K.; Applin, S.; Williams, E.T.; Voss, J.G. Brief Behavioral Treatment for Insomnia in Persons Living with HIV. Behav. Sleep. Med. 2018, 16, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Sexton, M.B.; Dawson, S.; Spencer, R.J.; Phillips, D.; Reckow, J.M.; Conroy, D.A.; Winters, J.J.; Bonar, E.E.; Chermack, S.T. Relationships between insomnia and alcohol and cocaine use frequency with aggression among veterans engaged in substance use treatment. Sleep. Med. 2021, 83, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Sakamoto, S.; Miyata, K. Effect of ramelteon on insomnia severity: Evaluation of patient characteristics affecting treatment response. Sleep. Biol. Rhythms 2019, 17, 379–388. [Google Scholar] [CrossRef]
- Curry, G.; Cheung, T.; Zhang, S.D.; Logue, S.; McAnena, L.; Price, R.; Sittlington, J.J. Repeated electrical vestibular nerve stimulation (VeNS) reduces severity in moderate to severe insomnia; a randomised, sham-controlled trial; the modius sleep study. Brain Stimul. 2024, 17, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Ekholm, B.; Spulber, S.; Adler, M. A randomized controlled study of weighted chain blankets for insomnia in psychiatric disorders. J. Clin. Sleep. Med. 2020, 16, 1567–1577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashare, R.L.; Lerman, C.; Tyndale, R.F.; Hawk, L.W.; George, T.P.; Cinciripini, P.; Schnoll, R.A. Sleep Disturbance During Smoking Cessation: Withdrawal or Side Effect of Treatment? J. Smok. Cessat. 2017, 12, 63–70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peters, E.N.; Fucito, L.M.; Novosad, C.; Toll, B.A.; O’Malley, S.S. Effect of night smoking, sleep disturbance, and their co-occurrence on smoking outcomes. Psychol. Addict. Behav. 2011, 25, 312–319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tulloch, H.E.; Pipe, A.L.; Els, C.; Clyde, M.J.; Reid, R.D. Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: A randomized controlled trial. BMC Med. 2016, 14, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, M.B.; Deroche, C.B.; Freeman, L.K.; Park, C.J.; Hall, N.A.; Sahota, P.K.; McCrae, C.S. Cognitive behavioral therapy for insomnia among young adults who are actively drinking: A randomized pilot trial. Sleep 2021, 44, zsaa171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Latif, Z.E.; Šaltyte Benth, J.; Solli, K.K.; Opheim, A.; Kunoe, N.; Krajci, P.; Sharma-Haase, K.; Tanum, L. Anxiety, Depression, and Insomnia Among Adults With Opioid Dependence Treated With Extended-Release Naltrexone vs Buprenorphine-Naloxone: A Randomized Clinical Trial and Follow-up Study. JAMA Psychiatry 2019, 76, 127–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, D.J.; Peterson, A.L.; Pruiksma, K.E.; Hale, W.J.; Young-McCaughan, S.; Wilkerson, A.; Nicholson, K.; Litz, B.T.; Dondanville, K.A.; Roache, J.D.; et al. Impact of cognitive behavioral therapy for insomnia disorder on sleep and comorbid symptoms in military personnel: A randomized clinical trial. Sleep 2018, 41, zsy069. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tao, Y.; Hou, W.; Zong, L.; Yu, L. Electro-acupuncture improves psychiatric symptoms, anxiety and depression in methamphetamine addicts during abstinence: A randomized controlled trial. Medicine 2018, 97, e11905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hodges, S.E.; Pittman, B.; Morgan, P.T. Sleep Perception and Misperception in Chronic Cocaine Users During Abstinence. Sleep 2017, 40, zsw069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Speed, T.J.; Hanks, L.; Turner, G.; Gurule, E.; Kearson, A.; Buenaver, L.; Smith, M.T.; Antoine, D. A comparison of cognitive behavioral therapy for insomnia to standard of care in an outpatient substance use disorder clinic embedded within a therapeutic community: A RE-AIM framework evaluation. Trials 2022, 23, 965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unhjem, R.; Flemmen, G.; Hoff, J.; Wang, E. Maximal strength training as physical rehabilitation for patients with substance use disorder; a randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Badrfam, R.; Zandifar, A.; Hajialigol, A.; Rashidian, M.; Schmidt, N.B.; Morabito, D.; Qorbani, M.; Shahrestanaki, E.; Mehrabani Natanzi, M. Efficacy of probiotic supplements in improving the symptoms of psychosis, anxiety, insomnia, and anorexia due to amphetamine and methamphetamine use: A randomized clinical trial. Psychopharmacology 2024, 241, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Patterson, F.; Grandner, M.A.; Malone, S.K.; Pohlig, R.T.; Ashare, R.L.; Edwards, D.G. Efficacy of a sleep health intervention to optimize standard smoking cessation treatment response: Results from a pilot randomized controlled trial. J. Smok. Cessat. 2020, 15, 113–117. [Google Scholar] [CrossRef]
- De Crescenzo, F.; D’Alò, G.L.; Ostinelli, E.G.; Ciabattini, M.; Di Franco, V.; Watanabe, N.; Kurtulmus, A.; Tomlinson, A.; Mitrova, Z.; Foti, F.; et al. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: A systematic review and network meta-analysis. Lancet 2022, 400, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D. Atypical antipsychotics: Sleep, sedation, and efficacy. Prim. Care Companion J. Clin. Psychiatry 2004, 6 (Suppl. 2), 3–7. [Google Scholar] [PubMed] [PubMed Central]
- Chokhawala, K.; Stevens, L. Antipsychotic Medications. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Mann, S.K.; Marwaha, R. Chlorpromazine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Martinotti, G.; Chiappini, S.; Mosca, A.; Miuli, A.; Santovito, M.C.; Pettorruso, M.; Skryabin, V.; Sensi, S.L.; Giannantonio, M.D. Atypical Antipsychotic Drugs in Dual Disorders: Current Evidence for Clinical Practice. Curr. Pharm. Des. 2022, 28, 2241–2259. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, K.Y.; Chang, T.; Vanle, B.; Dang, J.; Steiner, A.J.; Loera, N.; Abdelmesseh, M.; Danovitch, I.; Ishak, W.W. Trazodone for Insomnia: A Systematic Review. Innov. Clin. Neurosci. 2017, 14, 24–34. [Google Scholar] [PubMed] [PubMed Central]
- Sansone, R.A.; Sansone, L.A. Agomelatine: A novel antidepressant. Innov. Clin. Neurosci. 2011, 8, 10–14. [Google Scholar] [PubMed] [PubMed Central]
- Soyka, M.; Wild, I.; Caulet, B.; Leontiou, C.; Lugoboni, F.; Hajak, G. Long-term use of benzodiazepines in chronic insomnia: A European perspective. Front. Psychiatry 2023, 14, 1212028. [Google Scholar] [CrossRef]
- Palagini, L.; Brugnoli, R.; Dell’ Osso, B.M.; Di Nicola, M.; Maina, G.; Martinotti, G.; Maruani, J.; Mauries, S.; Serafini, G.; Mencacci, C.; et al. Clinical practice guidelines for switching or deprescribing hypnotic medications for chronic insomnia: Results of European neuropsychopharmacology and sleep expert’s consensus group. Sleep. Med. 2025, 128, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, B.; Albert, U.; Atti, A.R.; Carmassi, C.; Carrà, G.; Cosci, F.; Del Vecchio, V.; Di Nicola, M.; Ferrari, S.; Goracci, A.; et al. Bridging the gap between education and appropriate use of benzodiazepines in psychiatric clinical practice. Neuropsychiatr. Dis. Treat. 2015, 11, 1885–1909. [Google Scholar] [CrossRef]
- Greenblatt, K.; Adams, N. Modafinil. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Minzenberg, M.J.; Carter, C.S. Modafinil: A review of neurochemical actions and effects on cognition. Neuropsychopharmacology 2008, 33, 1477–1502. [Google Scholar] [CrossRef] [PubMed]
- Edgar, D.M.; Seidel, W.F. Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. J. Pharmacol. Exp. Ther. 1997, 283, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Saadabadi, A. Naltrexone. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Kumar, R.; Viswanath, O.; Saadabadi, A. Buprenorphine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Jordan, M.R.; Patel, P.; Morrisonponce, D. Naloxone. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Anderson, A.L.; Reid, M.S.; Li, S.H.; Holmes, T.; Shemanski, L.; Slee, A.; Smith, E.V.; Kahn, R.; Chiang, N.; Vocci, F.; et al. Modafinil for the treatment of cocaine dependence. Drug Alcohol. Depend. 2009, 104, 133–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, D.; Saadabadi, A. Varenicline. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Zammit, G.K. Ramelteon: A novel hypnotic indicated for the treatment of insomnia. Psychiatry 2007, 4, 36–42. [Google Scholar] [PubMed] [PubMed Central]
- Rizvi, S.; Khan, A.M. Use of Transcranial Magnetic Stimulation for Depression. Cureus 2019, 11, e4736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pulopulos, M.M.; Hidalgo, V.; Puig-Perez, S.; Montoliu, T.; Salvador, A. Relationship between Cortisol Changes during the Night and Subjective and Objective Sleep Quality in Healthy Older People. Int. J. Environ. Res. Public Health 2020, 17, 1264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinotti, G.; Pettorruso, M.; Montemitro, C.; Spagnolo, P.A.; Acuti Martellucci, C.; Di Carlo, F.; Fanella, F.; di Giannantonio, M.; Brainswitch Study Group. Repetitive transcranial magnetic stimulation in treatment-seeking subjects with cocaine use disorder: A randomized, double-blind, sham-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 116, 110513. [Google Scholar] [CrossRef] [PubMed]
- Ulett, G.A.; Han, S.; Han, J.S. Electroacupuncture: Mechanisms and clinical application. Biol. Psychiatry 1998, 44, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.M.; Mishra, B.; Sojan, C. Psychobiotics: A new frontier in treatment of depression. Int. J. Pharm. Drug Anal. 2023, 11, 1–8. [Google Scholar] [CrossRef]
- Bollu, P.C.; Kaur, H. Sleep Medicine: Insomnia and Sleep. Mo. Med. 2019, 116, 68–75. [Google Scholar] [PubMed] [PubMed Central]
- Grandner, M.; Olivieri, A.; Ahuja, A.; Büsser, A.; Freidank, M.; McCall, W.V. The burden of untreated insomnia disorder in a sample of 1 million adults: A cohort study. BMC Public Health 2023, 23, 1481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, M.A.; Al-Jahdali, H. The consequences of sleep deprivation on cognitive performance. Neurosciences 2023, 28, 91–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanacek, C.; Lane, J.; Tang, Y.L. Insomnia in Patients with Substance Use Disorders: Assessment and Management. J. Psychiatr. Pract. 2025, 31, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Zitting, K.M.; Lammers-van der Holst, H.M.; Yuan, R.K.; Wang, W.; Quan, S.F.; Duffy, J.F. Google Trends reveals increases in internet searches for insomnia during the 2019 coronavirus disease (COVID-19) global pandemic. J. Clin. Sleep Med. 2021, 17, 177–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andreescu, C.; Lenze, E.J.; Dew, M.A.; Begley, A.E.; Mulsant, B.H.; Dombrovski, A.Y.; Pollock, B.G.; Stack, J.; Miller, M.D.; Reynolds, C.F. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: Controlled study. Br. J. Psychiatry 2007, 190, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, L.; Harvey, A.G.; Fortier-Brochu, É.; Beaulieu-Bonneau, S.; Eidelman, P.; Talbot, L.; Ivers, H.; Hein, K.; Lamy, M.; Soehner, A.M.; et al. Impact of comorbid anxiety and depressive disorders on treatment response to cognitive behavior therapy for insomnia. J. Consult. Clin. Psychol. 2016, 84, 659–667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jansson-Fröjmark, M.; Jacobson, K. Cognitive behavioural therapy for insomnia for patients with co-morbid generalized anxiety disorder: An open trial on clinical outcomes and putative mechanisms. Behav. Cogn. Psychother. 2021, 49, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; Bertisch, S.M.; Pelayo, R.; Watson, N.F.; Winkelman, J.W.; Zee, P.C.; Krystal, A.D. What Should Be the Focus of Treatment When Insomnia Disorder Is Comorbid with Depression or Anxiety Disorder? J. Clin. Med. 2023, 12, 1975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).