Does Vitamin D Deficiency Increase the Risk of Autism Spectrum Disorder? Linking Evidence with Theory—A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
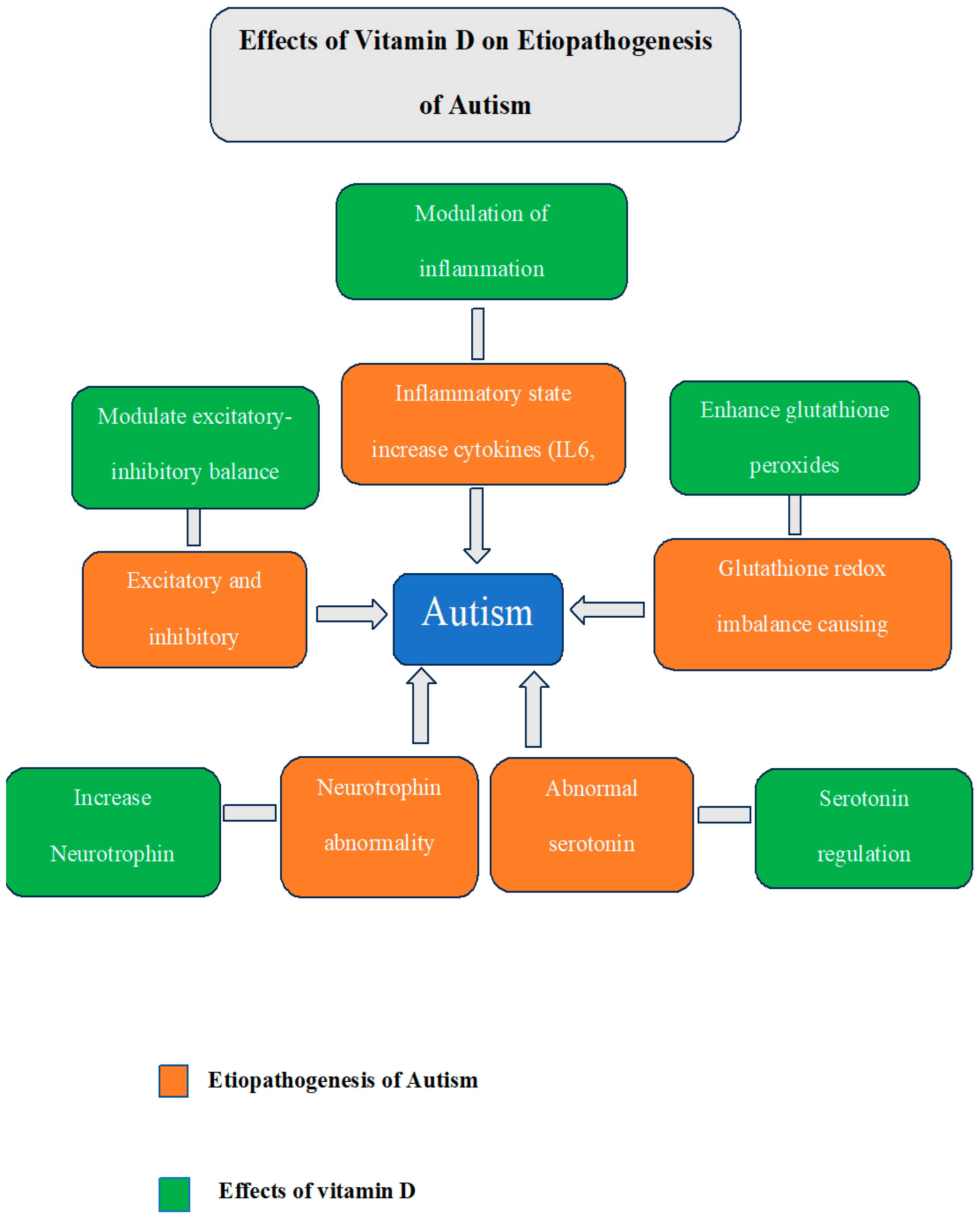
3.1. Linking Vitamin D with Autism
3.2. Evidence of the Relationship Between Vitamin D Deficiency and ASD
3.3. Evidence of Improvement with Vitamin D Supplementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, X.; Wang, M.; Hu, Y.; Xue, K.; Gu, S.; Lv, L.; Huang, S.; Xie, W. Potential role of genomic imprinted genes and brain developmental related genes in autism. BMC Med. Genom. 2020, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Primers 2020, 6, 5. [Google Scholar] [CrossRef]
- Cui, X.; Gooch, H.; Petty, A.; McGrath, J.J.; Eyles, D. Vitamin D and the brain: Genomic and non-genomic actions. Mol. Cell. Endocrinol. 2017, 453, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef]
- Cannell, J.J. Autism and vitamin D. Med. Hypotheses 2008, 70, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, H.; Liu, C.; Zhang, Y.; Wang, W.; Zou, Z.; Yang, L.; He, X.; Wu, J.; Ma, J.; et al. Research Progress on the Role of Vitamin D in Autism Spectrum Disorder. Front. Behav. Neurosci. 2022, 16, 859151. [Google Scholar] [CrossRef] [PubMed]
- Alzghoul, L. Role of Vitamin D in Autism Spectrum Disorder. Curr. Pharm. Des. 2019, 25, 4357–4367. [Google Scholar] [CrossRef] [PubMed]
- Kittana, M.; Ahmadani, A.; Stojanovska, L.; Attlee, A. The Role of Vitamin D Supplementation in Children with Autism Spectrum Disorder: A Narrative Review. Nutrients 2021, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S. Neuroimaging changes associated with vitamin D Deficiency—A narrative review. Nutr. Neurosci. 2022, 25, 1650–1658. [Google Scholar] [CrossRef]
- Mayne, P.E.; Burne, T.H. Vitamin D in Synaptic Plasticity, Cognitive Function, and Neuropsychiatric Illness. Trends Neurosci. 2019, 42, 293–306. [Google Scholar] [CrossRef]
- Kasatkina, L.A.; Gumenyuk, V.P.; Sturm, E.M.; Heinemann, A.; Bernas, T.; Trikash, I.O. Modulation of neurosecretion and approaches for its multistep analysis. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2018, 1862, 2701–2713. [Google Scholar] [CrossRef] [PubMed]
- Kasatkina, L.A.; Tarasenko, A.S.; Krupko, O.O.; Kuchmerovska, T.M.; Lisakovska, O.O.; Trikash, I.O. Vitamin D deficiency induces the excitation/inhibition brain imbalance and the proinflammatory shift. Int. J. Biochem. Cell Biol. 2020, 119, 105665. [Google Scholar] [CrossRef] [PubMed]
- Latimer, C.S.; Brewer, L.D.; Searcy, J.L.; Chen, K.-C.; Popović, J.; Kraner, S.D.; Thibault, O.; Blalock, E.M.; Landfield, P.W.; Porter, N.M. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc. Natl. Acad. Sci. USA 2014, 111, E4359-66. [Google Scholar] [CrossRef] [PubMed]
- Landel, V.; Millet, P.; Baranger, K.; Loriod, B.; Féron, F. Vitamin D interacts with Esr1 and Igf1 to regulate molecular pathways relevant to Alzheimer’s disease. Mol. Neurodegener. 2016, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Gooch, H.; Cui, X.; Anggono, V.; Trzaskowski, M.; Tan, M.C.; Eyles, D.W.; Burne, T.H.J.; Jang, S.E.; Mattheisen, M.; Hougaard, D.M.; et al. 1,25-Dihydroxyvitamin D modulates L-type voltage-gated calcium channels in a subset of neurons in the developing mouse prefrontal cortex. Transl. Psychiatry 2019, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Calcium Control of Neurotransmitter Release. Cold Spring Harb. Perspect. Biol. 2011, 4, a011353. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.B.J.; Barros, W.M.A.; da Silva, M.L.; Silva, J.M.L.; Souza, A.P.D.S.; Silva, K.G.D.; Lagranha, C.J. Impact of vitamin D on cognitive functions in healthy individuals: A systematic review in randomized controlled clinical trials. Front. Psychol. 2023, 28, 1150187. [Google Scholar] [CrossRef] [PubMed]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Krisanova, N.; Pozdnyakova, N.; Pastukhov, A.; Dudarenko, M.; Maksymchuk, O.; Parkhomets, P.; Sivko, R.; Borisova, T. Vitamin D3 deficiency in puberty rats causes presynaptic malfunctioning through alterations in exocytotic release and uptake of glutamate/GABA and expression of EAAC-1/GAT-3 transporters. Food Chem. Toxicol. 2019, 123, 142–150. [Google Scholar] [CrossRef]
- Féron, F.; Burne, T.; Brown, J.; Smith, E.; McGrath, J.; Mackay-Sim, A.; Eyles, D. Developmental Vitamin D3 deficiency alters the adult rat brain. Brain Res. Bull. 2005, 65, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Groves, N.J.; Kesby, J.P.; Eyles, D.W.; McGrath, J.J.; Mackay-Sim, A.; Burne, T.H. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav. Brain Res. 2013, 241, 120–131. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, L.-H.; Cai, H.-L.; Li, H.-D.; Liu, Y.-P.; Tang, M.-M.; Dang, R.-L.; Zhu, W.-Y.; Xue, Y.; He, X. Neurochemical Effects of Chronic Administration of Calcitriol in Rats. Nutrients 2014, 6, 6048–6059. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.F.; Kriegstein, A.R. Is there more to gaba than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef]
- Horder, J.; Petrinovic, M.M.; Mendez, M.A.; Bruns, A.; Takumi, T.; Spooren, W.; Barker, G.J.; Künnecke, B.; Murphy, D.G. Glutamate and GABA in autism spectrum disorder—A translational magnetic resonance spectroscopy study in man and rodent models. Transl. Psychiatry 2018, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.; Brown, J.; Mackay-Sim, A.; McGrath, J.; Feron, F. Vitamin d3 and brain development. Neuroscience 2003, 118, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Liu, S.-H.; Shi, X.-J.; Fan, F.-C.; Cheng, Y. Peripheral blood neurotrophic factor levels in children with autism spectrum disorder: A meta-analysis. Sci. Rep. 2021, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Orme, R.P.; Bhangal, M.S.; Fricker, R.A. Calcitriol Imparts Neuroprotection In Vitro to Midbrain Dopaminergic Neurons by Upregulating GDNF Expression. PLoS ONE 2013, 8, e62040. [Google Scholar] [CrossRef]
- Masi, A.; Quintana, D.S.; Glozier, N.; Lloyd, A.R.; Hickie, I.B.; Guastella, A.J. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol. Psychiatry 2015, 20, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Li, X.; Zhong, Y. Inflammatory Cytokines: Potential Biomarkers of Immunologic Dysfunction in Autism Spectrum Disorders. Mediat. Inflamm. 2015, 2015, 531518. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, M.S.; Rocha, N.P.; Novaes, J.F.; Bressan, J. Vitamin D status, oxidative stress, and inflammation in children and adolescents: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The Impact of Vitamin D Levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS ONE 2015, 10, e0141770. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.G.A.; Sabico, S.; Clerici, M.; Khattak, M.N.K.; Wani, K.; Al-Musharaf, S.; Amer, O.E.; Alokail, M.S.; Al-Daghri, N.M. Vitamin D Supplementation is Associated with Increased Glutathione Peroxidase-1 Levels in Arab Adults with Prediabetes. Antioxidants 2020, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Tinkov, A.A.; Hosnedlová, B.; Kizek, R.; Ajsuvakova, O.P.; Chirumbolo, S.; Skalnaya, M.G.; Peana, M.; Dadar, M.; El-Ansary, A.; et al. The role of glutathione redox imbalance in autism spectrum disorder: A review. Free. Radic. Biol. Med. 2020, 160, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Yates, N.J.; Tesic, D.; Feindel, K.W.; Smith, J.T.; Clarke, M.W.; Wale, C.; Crew, R.C.; Wharfe, M.; Whitehouse, A.J.; Wyrwoll, C.S. Vitamin D is crucial for maternal care and offspring social behavior in rats. J. Endocrinol. 2018, 237, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Vasileva, S.; Langguth, M.; Alexander, S.; Cui, X.; Whitehouse, A.; McGrath, J.J.; Eyles, D. Developmental Vitamin D Deficiency Produces Behavioral Phenotypes of Relevance to Autism in an Animal Model. Nutrients 2019, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Tamang, M.K.; Ali, A.; Pertile, R.N.; Cui, X.; Alexander, S.; Nitert, M.D.; Palmieri, C.; Eyles, D. Developmental vitamin D-deficiency produces autism-relevant behaviours and gut-health associated alterations in a rat model. Transl. Psychiatry 2023, 13, 204. [Google Scholar] [CrossRef]
- Overeem, K.A.; Eyles, D.W.; McGrath, J.J.; Burne, T.H. The impact of vitamin D deficiency on behavior and brain function in rodents. Curr. Opin. Behav. Sci. 2016, 7, 47–52. [Google Scholar] [CrossRef]
- Grant, W.B.; Soles, C.M. Epidemiologic evidence for supporting the role of maternal vitamin D deficiency as a risk factor for the development of infantile autism. Derm. -Endocrinol. 2009, 1, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Zerbo, O.; Iosif, A.-M.; Delwiche, L.; Walker, C.; Hertz-Picciotto, I. Month of Conception and Risk of Autism. Epidemiology 2011, 22, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Humble, M.B.; Gustafsson, S.; Bejerot, S. Low serum levels of 25-hydroxyvitamin D (25-OHD) among psychiatric out-patients in Sweden: Relations with season, age, ethnic origin, and psychiatric diagnosis. J. Steroid Biochem. Mol. Biol. 2010, 121, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W. Vitamin D and autism: Does skin colour modify risk? Acta Paediatr. 2010, 99, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, C.; Rai, D.; Goodman, A.; Lundberg, M.; Idring, S.; Svensson, A.; Koupil, I.; Serlachius, E.; Dalman, C. Migration and autism spectrum disorder: Population-based study. Br. J. Psychiatry 2012, 201, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Van der Ven, E.; Termorshuizen, F.; Laan, W.; Breetvelt, E.J.; Van Os, J.; Selten, J.P. An incidence study of diagnosed autism-spectrum disorders among immigrants to the Netherlands. Acta Psychiatr. Scand. 2012, 128, 54–60. [Google Scholar] [CrossRef]
- Chen, J.; Xin, K.; Wei, J.; Zhang, K.; Xiao, H. Lower maternal serum 25(OH) D in first trimester associated with higher autism risk in Chinese offspring. J. Psychosom. Res. 2016, 89, 98–101. [Google Scholar] [CrossRef]
- Magnusson, C.; Kosidou, K.; Dalman, C.; Lundberg, M.; Lee, B.K.; Rai, D.; Karlsson, H.; Gardner, R.; Arver, S. Maternal vitamin D deficiency and the risk of autism spectrum disorders: Population-based study. BJPsych Open 2016, 2, 170–172. [Google Scholar] [CrossRef]
- Vinkhuyzen, A.A.E.; Eyles, D.W.; Burne, T.H.J.; Blanken, L.M.E.; Kruithof, C.J.; Verhulst, F.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational vitamin D deficiency and autism-related traits: The Generation R Study. Mol. Psychiatry 2018, 23, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Eyles, D.W.; Magnusson, C.; Newschaffer, C.J.; McGrath, J.J.; Kvaskoff, D.; Ko, P.; Dalman, C.; Karlsson, H.; Gardner, R.M. Developmental vitamin D and autism spectrum disorders: Findings from the Stockholm Youth Cohort. Mol. Psychiatry 2019, 26, 1578–1588. [Google Scholar] [CrossRef]
- Windham, G.C.; Pearl, M.; Poon, V.; Berger, K.; Soriano, J.W.; Eyles, D.; Lyall, K.; Kharrazi, M.; Croen, L.A. Maternal Vitamin D Levels During Pregnancy in Association With Autism Spectrum Disorders (ASD) or Intellectual Disability (ID) in Offspring; Exploring Non-linear Patterns and Demographic Sub-groups. Autism Res. 2020, 13, 2216–2229. [Google Scholar] [CrossRef] [PubMed]
- Sourander, A.; Upadhyaya, S.; Surcel, H.-M.; Hinkka-Yli-Salomäki, S.; Cheslack-Postava, K.; Silwal, S.; Sucksdorff, M.; McKeague, I.W.; Brown, A.S. Maternal Vitamin D Levels During Pregnancy and Offspring Autism Spectrum Disorder. Biol. Psychiatry 2021, 90, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Madley-Dowd, P.; Dardani, C.; Wootton, R.E.; Dack, K.; Palmer, T.; Thurston, R.; Havdahl, A.; Golding, J.; Lawlor, D.; Rai, D. Maternal vitamin D during pregnancy and offspring autism and autism-associated traits: A prospective cohort study. Mol. Autism 2022, 13, 44. [Google Scholar] [CrossRef]
- Fernell, E.; Bejerot, S.; Westerlund, J.; Miniscalco, C.; Simila, H.; Eyles, D.; Gillberg, C.; Humble, M.B. Autism spectrum disorder and low vitamin D at birth: A sibling control study. Mol. Autism 2015, 6, 3. [Google Scholar] [CrossRef]
- Ali, Y.; Anderson, L.N.; Smile, S.; Chen, Y.; Borkhoff, C.M.; Koroshegyi, C.; Lebovic, G.; Parkin, P.C.; Birken, C.S.; Szatmari, P.; et al. Prospective cohort study of vitamin D and autism spectrum disorder diagnoses in early childhood. Autism 2018, 23, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Niu, Q.; Eyles, D.W.; Hansen, R.L.; Iosif, A. Neonatal vitamin D status in relation to autism spectrum disorder and developmental delay in the CHARGE case–control study. Autism Res. 2019, 12, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Windham, G.C.; Pearl, M.; Anderson, M.C.; Poon, V.; Eyles, D.; Jones, K.L.; Lyall, K.; Kharrazi, M.; Croen, L.A. Newborn vitamin D levels in relation to autism spectrum disorders and intellectual disability: A case–control study in california. Autism Res. 2019, 12, 989–998. [Google Scholar] [CrossRef]
- Saad, K.; Abdel-Rahman, A.A.; Elserogy, Y.M.; Al-Atram, A.A.; Cannell, J.J.; Bjørklund, G.; Abdel-Reheim, M.K.; Othman, H.A.K.; El-Houfey, A.A.; El-Aziz, N.H.R.A.; et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr. Neurosci. 2015, 19, 346–351. [Google Scholar] [CrossRef]
- Coşkun, S.; Şimşek, Ş.; Camkurt, M.A.; Çim, A.; Çelik, S.B. Association of polymorphisms in the vitamin D receptor gene and serum 25-hydroxyvitamin D levels in children with autism spectrum disorder. Gene 2016, 588, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Basheer, S.; Natarajan, A.; van Amelsvoort, T.; Venkataswamy, M.M.; Ravi, V.; Srinath, S.; Girimaji, S.C.; Christopher, R. Vitamin D status of children with Autism Spectrum Disorder: Case-control study from India. Asian J. Psychiatry 2017, 30, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Desoky, T.; Hassan, M.H.; Fayed, H.M.; Sakhr, H.M. Biochemical assessments of thyroid profile, serum 25-hydroxycholecalciferol and cluster of differentiation 5 expression levels among children with autism. Neuropsychiatr. Dis. Treat. 2017, 13, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Altun, H.; Kurutaş, E.B.; Şahin, N.; Güngör, O.; Fındıklı, E. The Levels of Vitamin D, Vitamin D Receptor, Homocysteine and Complex B Vitamin in Children with Autism Spectrum Disorders. Clin. Psychopharmacol. Neurosci. 2018, 16, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Arastoo, A.A.; Khojastehkia, H.; Rahimi, Z.; Khafaie, M.A.; Hosseini, S.A.; Mansouri, M.T.; Yosefyshad, S.; Abshirini, M.; Karimimalekabadi, N.; Cheraghi, M. Evaluation of serum 25-Hydroxy vitamin D levels in children with autism Spectrum disorder. Ital. J. Pediatr. 2018, 44, 150. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Cannell, J.J.; Bjørklund, G.; Bhat, R.S.; Al Dbass, A.M.; Alfawaz, H.A.; Chirumbolo, S.; Al-Ayadhi, L. In the search for reliable biomarkers for the early diagnosis of autism spectrum disorder: The role of vitamin D. Metab. Brain Dis. 2018, 33, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Alzghoul, L.; Al-Eitan, L.N.; Aladawi, M.; Odeh, M.; Hantash, O.A. The Association Between Serum Vitamin D3 Levels and Autism Among Jordanian Boys. J. Autism Dev. Disord. 2019, 50, 3149–3154. [Google Scholar] [CrossRef]
- Chtourou, M.; Naifar, M.; Grayaa, S.; Hajkacem, I.; Ben Touhemi, D.; Ayadi, F.; Moalla, Y. Vitamin d status in TUNISIAN children with autism spectrum disorders. Clin. Chim. Acta 2019, 493, S619–S620. [Google Scholar] [CrossRef]
- Petruzzelli, M.G.; Marzulli, L.; Margari, F.; De Giacomo, A.; Gabellone, A.; Giannico, O.V.; Margari, L. Vitamin D Deficiency in Autism Spectrum Disorder: A Cross-Sectional Study. Dis. Markers 2020, 2020, 9292560. [Google Scholar] [CrossRef] [PubMed]
- Şengenç, E.; Kıykım, E.; Saltik, S. Vitamin D levels in children and adolescents with autism. J. Int. Med. Res. 2020, 48, 300060520934638. [Google Scholar] [CrossRef]
- Wang, T.; Shan, L.; Du, L.; Feng, J.; Xu, Z.; Staal, W.G.; Jia, F. Serum concentration of 25-hydroxyvitamin D in autism spectrum disorder: A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2016, 25, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ding, R.; Wang, J. The Association between Vitamin D Status and Autism Spectrum Disorder (ASD): A Systematic Review and Meta-Analysis. Nutrients 2020, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Iosif, A.-M.; Angel, E.G.; Ozonoff, S. Association of Maternal Prenatal Vitamin Use With Risk for Autism Spectrum Disorder Recurrence in Young Siblings. JAMA Psychiatry 2019, 76, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shan, L.; Du, L.; Wang, B.; Li, H.; Wang, W.; Wang, T.; Dong, H.; Yue, X.; Xu, Z.; et al. Clinical improvement following vitamin D3 supplementation in Autism Spectrum Disorder. Nutr. Neurosci. 2017, 20, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Azzam, H.M.; Sayyah, H.; Youssef, S.; Lotfy, H.; Abdelhamid, I.A.; Elhamed, H.A.A.; Maher, S. Autism and vitamin D. Middle East Curr. Psychiatry 2015, 22, 9–14. [Google Scholar] [CrossRef]
- Javadfar, Z.; Abdollahzad, H.; Moludi, J.; Rezaeian, S.; Amirian, H.; Foroughi, A.A.; Nachvak, S.M.; Goharmehr, N.; Mostafai, R. Effects of vitamin D supplementation on core symptoms, serum serotonin, and interleukin-6 in children with autism spectrum disorders: A randomized clinical trial. Nutrition 2020, 79–80, 110986. [Google Scholar] [CrossRef]
- Kerley, C.P.; Power, C.; Gallagher, L.; Coghlan, D. Lack of effect of vitamin D3supplementation in autism: A 20-week, placebo-controlled RCT. Arch. Dis. Child. 2017, 102, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Mazahery, H.; Conlon, C.A.; Beck, K.L.; Mugridge, O.; Kruger, M.C.; Stonehouse, W.; Camargo, C.A.; Meyer, B.J.; Jones, B.; von Hurst, P.R. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with Autism Spectrum Disorder. J. Steroid Biochem. Mol. Biol. 2019, 187, 9–16. [Google Scholar] [CrossRef]
- Mazahery, H.; Conlon, C.A.; Beck, K.L.; Mugridge, O.; Kruger, M.C.; Stonehouse, W.; Camargo, C.A., Jr.; Meyer, B.J.; Tsang, B.; Jones, B.; et al. A Randomised-Controlled Trial of Vitamin D and Omega-3 Long Chain Polyunsaturated Fatty Acids in the Treatment of Core Symptoms of Autism Spectrum Disorder in Children. J. Autism Dev. Disord. 2019, 49, 1778–1794. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Sohrabi, M.; Taheri, H.; Khodashenas, E.; Movahedi, A. Comparison of the effects of perceptual-motor exercises, vitamin D supplementation and the combination of these interventions on decreasing stereotypical behavior in children with autism disorder. Int. J. Dev. Disabil. 2020, 66, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kolachahi, S.A.; AdibSaber, F.; Elmieh, A. Effects of Vitamin D and/or Aquatic Exercise on IL-1β and IL-1RA Serum Levels and Behavior of Children with Autism Spectrum Disorder. Stud. Med. Sci. 2020, 31, 690–699. [Google Scholar] [CrossRef]
- Volkmar, F.R.; Anderson, G.M. Neurochemical perspectives on infantile autism. In Autism: Nature, Diagnosis, and Treatment; The Guilford Press: New York, NY, USA, 1989; pp. 208–224. [Google Scholar] [CrossRef]
- Schoenecker, B.; Heller, K. The involvement of dopamine (DA) and serotonin (5-HT) in stress-induced stereotypies in bank voles (Clethrionomys glareolus). Appl. Anim. Behav. Sci. 2001, 73, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.P.; Ames, B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Luo, X.; Jiang, Q.; Chen, Z.; Zhou, L.; Wang, D.; Chen, A. Vitamin D Supplementation is Beneficial for Children with Autism Spectrum Disorder: A Meta-analysis. Clin. Psychopharmacol. Neurosci. 2020, 18, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, Y.; Zhang, X.; Zhang, L.; Wu, Y.; Wang, X.; Zhu, C. The effect of vitamin D supplementation in treatment of children with autism spectrum disorder: A systematic review and meta-analysis of randomized controlled trials. Nutr. Neurosci. 2022, 25, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, Y.; Lu, Z.; Song, M.; Huang, X.; Mi, L.; Yang, J.; Cui, X. Effects of Vitamin D Supplementation on Children with Autism Spectrum Disorder: A Systematic Review and Meta-analysis. Clin. Psychopharmacol. Neurosci. 2023, 21, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, G.; Henley, K.; Green, J. Autism: Will vitamin D supplementation during pregnancy and early childhood reduce the recurrence rate of autism in newborn siblings? Med. Hypotheses 2016, 88, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Jepsen, J.R.; Sevelsted, A.; Horner, D.; Vinding, R.; Rosenberg, J.B.; Brustad, N.; Eliasen, A.; Mohammadzadeh, P.; Følsgaard, N.; et al. High-dose vitamin D3 supplementation in pregnancy and risk of neurodevelopmental disorders in the children at age 10: A randomized clinical trial. Am. J. Clin. Nutr. 2024, 119, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Dang, W.; Jiang, Z.; Jiang, Y. Exploring the Missing link between vitamin D and autism spectrum disorder: Scientific evidence and new perspectives. Heliyon 2024, 10, e36572. [Google Scholar] [CrossRef]
- Trojsi, F.; Siciliano, M.; Passaniti, C.; Bisecco, A.; Russo, A.; Lavorgna, L.; Esposito, S.; Ricciardi, D.; Monsurrò, M.R.; Tedeschi, G.; et al. Vitamin D supplementation has no effects on progression of motor dysfunction in amyotrophic lateral sclerosis (ALS). Eur. J. Clin. Nutr. 2020, 74, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, K.; Hu, T.; Wang, G.; Wang, W.; Zhang, J. Vitamin D3 Supplement Attenuates Blood–Brain Barrier Disruption and Cognitive Impairments in a Rat Model of Traumatic Brain Injury. Neuromol. Med. 2021, 23, 491–499. [Google Scholar] [CrossRef]
- Liao, X.; Liu, Y.; Fu, X.; Li, Y. Postmortem Studies of Neuroinflammation in Autism Spectrum Disorder: A Systematic Review. Mol. Neurobiol. 2020, 57, 3424–3438. [Google Scholar] [CrossRef]
- Adegoke, S.A.; Smith, O.S.; Adekile, A.D.; Figueiredo, M.S. Relationship between serum 25-hydroxyvitamin D and inflammatory cytokines in paediatric sickle cell disease. Cytokine 2017, 96, 87–93. [Google Scholar] [CrossRef]
- Qiu, S.; Qiu, Y.; Li, Y.; Cong, X. Genetics of autism spectrum disorder: An umbrella review of systematic reviews and meta-analyses. Transl. Psychiatry 2022, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, R.; Dinger, M.E.; Fatehi, R.; Taheri, M.; Ghafouri-Fard, S. Identification of miRNA-mRNA network in autism spectrum disorder using a bioinformatics method. J. Mol. Neurosci. 2021, 71, 761–766. [Google Scholar] [CrossRef] [PubMed]
| First Author/Year | Study Design | Sample Size | Trimester | Sample and Assessment Method | Vitamin D Levels ng/mL | Diagnosis | Outcome |
|---|---|---|---|---|---|---|---|
| Chen et al. (2016) [48] | Case–control China | ASD: 68 Controls: 68 | 11–13 weeks | Serum | <25—deficiency >50—sufficient | DSM-5 | Significant association (p—0.001 and OR: 3.99 (2.58–7.12) |
| Magnuss et al. 2016 [49] | Register-based total population study Sweden | N = 509 4–17 years | - | Serum | <25—deficiency | ICD-10 | Significantly increased offspring risk of ASD OR = 2.51, (95% CI 1.22–5.16) |
| Lee et al. (2019) [51] | Nested case–control Sweden | ASD: 449 Controls: 574 | 10.9 (9.3–13.0) weeks | Dried blood spot LC-MS | <25—deficiency >50—sufficient | ICD-10 and DSMIV | VD deficiency was associated with 1.58 times higher odds of ASD (95% CI: 1.00, 2.49) as compared with controls. |
| Windham, et al. (2020) [52] | Case–control California | ASD—534 Controls—421 | Mid-pregnancy | Serum LC-MS | <50—deficiency >75—sufficient | DSMIV-TR | No association OR-0.79 (95% CI 0.49–1.3) |
| Souranderetal 2021 [53] | Nested case–control study Finland | ASD—1558 Controls—1558 | First and early second trimester | Serum CMI | <30—deficiency | ICD-10 | Increased risk of ASD in VD deficiency (1.44, 95% CI 1.15–1.81, p = 0.001 |
| First Author/Year | Study Design | Sample Size | Trimester | Sample and Assessment Method | Vitamin D Levels ng/mL | Diagnosis | Outcome |
|---|---|---|---|---|---|---|---|
| Vinkhuyzen et al. (2018) [50] | Prospective cohort Netherland | ASD: 62 Controls: 3895 | Mid-gestation: 18.1–24.9 weeks Neonatal serum | Serum LC-MS | <25—deficiency >50—sufficient | SRS | Significantly high SRS scores (p = 0.001) |
| Dowd et al. (2022) [54] | Prospective cohort study UK | Mothers: 7689 | Midpoint of each trimester: 7 weeks, 20 weeks, and 34 weeks | Serum LC-MS | <25—deficiency >50—sufficient | ICD-10 SCDC | No asssociation [OR = 0.9895% CI = 0.90–1.06),] Mendelian randomization suggested no causal effect [OR = 1.08, 95% CI = 0.46–2.55)] |
| First Author/Year | Study Design | Sample Size (Both Boys and Girls) | Sample and Assessment Method | Vitamin D Levels ng/mL | Diagnosis/Outcome Measure | Outcome |
|---|---|---|---|---|---|---|
| Fernell et al. (2015) [55] | Case–control Sweden | ASD: 58 Controls: 57 | Dried blood Spot LC-MS | ASD: 24.0 ± 19.6 Controls: 31.9 ± 27.7 | DSM-5 | Significantly low VD levels in a group of newborn children, who later developed ASD. |
| Lee et al. (2019) [51] | Nested case–control Sweden | ASD: 449 Controls: 574 | Dried blood spot LC-MS | ASD: 10.28 ± 5.96 Controls: 10.64 ± 6.31 | ICD-10 and DSM-IV | VD deficiency was associated with 1.33 (CI 1.01, 1.74) times higher odds of ASD as compared with controls. |
| Schmidt et al. (2019) [57] | Case–control USA | ASD: 357 Controls: 234 | Dried blood Spot LC-MS/MS | ASD: 32.04 ± 14.96 Controls: 33.08 ± 15.72 | ADOS-2, ADI-R | No significant association OR: 0.98 (0.63–1.51). |
| Windham et al. (2019) [58] | Case–control | ASD: 563 Controls: 436 | Dried blood Spot LC-MS/MS | ASD: 34.04 ± 14.1 Controls: 33.72 ± 11.85 | DSM-IV-TR | No significant association OR: 0.96 (0.65–1.43). |
| First Author/Year | Study Design | Sample Size (Both Boys and Girls) | Sample and Assessment Method | Vitamin D Levels ng/mL | Diagnosis/Outcome Measure | Outcome |
|---|---|---|---|---|---|---|
| Vinkhuyzen et al. (2017) [50] | Prospective cohort Netherland | ASD: 62 Controls: 3895 | Serum LC-MS | - | SRS | VD-deficient individuals had significantly high SRS scores p = 0.001 |
| Ali et al. (2019) [56] | Prospective study cohort Canada | ASD: 26 Controls: 2500 | Serum: A two-step chemiluminescence assay | - | ADOS-2/DSM-IV/ DSM-5 | No increase in risk of ASD RR: 1.06 (0.95–1.18) |
| First Author | Study Design | Sample Size/Age | Sample and Assessment Method | Vitamin D Levels ng/mL | Diagnosis/Outcome Measure | Outcome |
|---|---|---|---|---|---|---|
| Saad et al. (2016) [59] | Case–control cross-sectional Egypt | ASD: 122 Controls: 100 Age ASD: 5.09 ± 1.42 year Controls: 4.88 ± 1.30 year | Serum ELISA | ASD: 18.02 ± 8.75 Controls: 42.51 ± 9.48 | DSM-IV-TR | Significantly low VD in ASD children (p ≤ 0.0001) |
| Coşkun et al. (2016) [60] | Case–control Turkey | ASD: 85 Controls: 82 Age ASD: 43.4 ± 25.3 month Controls: 47.1 ± 14.2 month | Serum ELISA | ASD: 79.5 ± 25.9 Controls: 65.1 ± 23.9 | DSM-5 | Significantly low VD in ASD children (p ≤ 0.0001) |
| Basheer et al. (2017) [61] | Case–Control India | ASD: 40 Controls: 30 Age ASD: 3–12 year control: 3–12 year | Serum LC-MS/MS | ASD: 13.5 ± 4.7 Controls: 12.7 ± 4.7 | DSM-5 ADI-R | No significant difference between groups p = 0.462 OR: 0.88 (0.14–5.63) |
| Desoky et al. (2017) [62] | Case–control cross-sectional Egypt | ASD: 60 Controls: 40 Age ASD: 7.03 ± 2.34 year Controls: 7.91 ± 3.21 year | Serum ELISA | ASD: 18.63 ± 10.8 Controls: 45.9 ± 8.85 | - | Significantly low VD in ASD children (p = 0.001) |
| Altun et al. (2018) [63] | Cross-sectional Turkey | ASD: 60 Controls: 45 Age ASD: 5.8 ± 2.7 year Controls: 6.7 ± 2.5 year | Serum ELISA | ASD: 13.79 ± 1.03 | DSM-IV-TR | Significantly low VD in ASD children (p < 0.001) |
| Arastoo et al. (2018) [64] | Cross-sectional Iran | ASD: 31 Controls: 31 Age ASD: 9.17 ± 2.11 year Controls: 9.31 ± 2.09 year | Serum ELISA | ASD: 9.03 ± 4.14 Controls: 15.25 ± 7.89 | DSM-IV ADI-R | Significantly low VD in ASD children (p = 0.001) OR: 12.273 (1.447–104.101) |
| El-Ansary et al. (2018) [65] | Cross-sectional Saudi Arabia | ASD: 28 Controls: 27 Age ASD: 7.0 ± 2.34 year Controls: 7.2 ± 2.14 year | Plasma ELISA | ASD: 95.63 ± 26.63 Controls: 140.43 ± 17.68 | DSM-IV-TR | Significantly low VD in ASD children (p < 0.001) |
| Alzghoul et al. (2018) [66] | Case–control cross-sectional Jordan | ASD: 83 Controls: 106 Age ASD: 5.08 year Controls: 5.02 year | Serum LC-MS/MS | ASD: 23.4 Controls: 37.5 | DSM-5 | Significantly low VD in ASD children OR: 9.896 (4.605–21.264) |
| Chtourou et al. (2019) [67] | Case–control Tunisia | ASD: 40 Controls: 43 Age Controls: 4.76 ± 1.08 year | Serum - | ASD: 17.13 ± 9.65 Controls: 21.34 ± 8.1 | DSM-5 | No significant difference between groups (p = 0.03) |
| Petruzzelli et al. (2020) [68] | Case–control Italy | ASD: 54 Controls: 36 Age ASD: 6.87 (±3.92) year Controls: 11.28 (±4.44) year | Serum CMI | ASD: 35 (64.8) Controls: 12 (33.3) | DSM-5 ADI-R ADOS-2 | A significant association between ASD and VD deficiency (p = 0.006) |
| Sengenc¸ et al. 2020 [69] | Case–control Turkey | ASD: 100 Controls: 100 Age ASD: 5.95, 3.13 year Controls: 6.68, 3.8 year | Serum ELISA | ASD: 42.86, 19.84 Controls: 48.57, 22.36 | DSM-5 | Significantly low VD in ASD children (p = 0.037) |
| First Author | Study Design | Population Characteristics | Serum Vitamin D Estimation Method | Diagnosis | Result |
|---|---|---|---|---|---|
| Wang T et al. 2015 [70] | 11 case–control | 870 ASD patients and 782 healthy controls | Serum plasma ELISA, RIA, LC-MS/MS HPLC | DSM IV(TR) | Significant difference between the ASD group and control group (WMD = −8.63; 95% CI (−13.17, −4.09), p = 0.0002). |
| Wang Z et al. 2020 [71] | 34 studies | Total participants = 20,580 (Asia, America, Europe, Africa) | Serum, plasma, or dried blood spot ELISA, RIA, LC-MS/MS HPLC | DSM-IV, DSM-IV-TR, ADOS, ADIR, DSM-V, ICD-9, ICD-10, ICD-F84.0 | Vitamin D concentration of the ASD group was 7.46 ng/mL lower than that of the control group (95% CI: −10.26; −4.66 ng/mL, p < 0.0001. |
| 26 case–control studies | Assessing Vitamin D in children 1792 ASDs 1969 controls | ||||
| 3 case–control studies and 2 nested case–control studies of neonates | Assessing Vitamin D in neonates (2687 ASDs 3574 controls) | ||||
| 1 nested case–control | Maternal vitamin D concentration of the ASD and control groups (517 ASDs, 642 controls) | ||||
| 2 cohort studies | Investigated the OR/RR for ASD incidence after being exposed to early-life vitamin D deficiency (5442 neonates, 3957 pregnant women) |
| First Author/Year | Study Design | Type of Participants Age/Gender | Treatment Details and Duration | Change in Level of Vitamin D | ASD Severity Measure (M) | Result | |
|---|---|---|---|---|---|---|---|
| Before- After the Change | Between-Group Comparison | ||||||
| Azzam (2015) [74] | Prospective, case–control | n = 21 Age: 2–12 year M:F: 16:5 | 2000 IU/day of vitamin D3 for 6 months (n = 10) No supplement (n = 11) for cases | 47 ± 20 to 71 ± 35 (nmol/L) 69 ± 41 to 70 ± 36 (nmol/L) | CARS ATEC | Decrease Decrease | NS |
| Kerley 2017 [75] | RDBPC | n = 38 Age: <18 year M:F: 33:5 | 2000 IU vitamin D3 (n = 18) for 20 weeks Placebo (n = 20) | 58.4 ± 17.9 to 86.1 (nmol/L) | ABC SRS | Decrease Decrease | NS |
| Mazahery (2019) a New Zealand [76] | RDBPC | Age: 2.5–8 year M:F: 60:13 | 2000 IU/day (n = 28) Omega 3 (n = 28) Placebo (n = 30) 12 months | - | ABC | Decrease in hyperactivity and irritability | Significant reduction in irritability and hyperactivity (−5.2 ± 6.3 vs. −0.8 ± 5.6, p = 0.047). |
| Mazahery (2019) [77] | RDBPC | n = 73 Age: 2.5–8 year M:F: 60:13 | 2000 IU/day vitamin D3 (n = 19) omega-3 (n = 23) Vitamin D3 and omega 3 (n = 15) Placebo (n = 16) For 12 months | 68 ± 21 to an increase by 95 ± 14 (nmol/L) | SRS SPM | Decrease Decrease | NS |
| Javadfar (2020) [78] | RDBPC | n = 43 children with ASD Age: 3–13 year M:F: 36:7 | 300 IU/kg/day (Max. 6000 IU/day) of vitamin D3 for 15 weeks (n = 22) Placebo n = 21 | 8.19 ± 6.78 to 39.10 | CARS ATEC ABC-C subscale | Decrease Decrease Decrease | Significant decrease in CARS AND ATEC scores No significant difference in ABC domains |
| Moradi (2020) [79] | RDBPC | n = 100 Age: 6–9 year M:F: 100:0 | 300 IU/kg/day (max. 5000 IU/day) of vitamin D3 (n = 25) Perceptual-motor exercises (n = 25) Exercises and vitamin D3 (n = 25) Placebo (n = 25) 3 months | 12.6 to 24.36 | SS-GARS-2 | Decrease | Significant decrease in stereotypes (p = 0.01) |
| Ansari et al., 2020 [80] | RDBPC | n = 40 Age: 6–14 year M:F: 100:0 | 50,000 IU/week or 50,000 IU/2 weeks (vitamin D3) for 10 weeks (n = 10) Placebo (n = 10) | 11.12 to 31.60 | GARS-2 | Significant decrease in stereotypes | |
| First Author | Study Design | Population Characteristics | Vitamin D Supplementation | Outcome Measure | Result |
|---|---|---|---|---|---|
| Song et al., 2020 [84] | 4 RCTs 3 included in meta-analysis | - (New Zealand, China, Ireland) Age 2–10 years Both male and female | Dose range 800 IU/day to 2000 IU/day Duration 20 weeks–12 months | ABC SRS CARS DD-CGAS | No significant improvement (SMD = −0.46, 95% CI: −0.87 to −0.05; p = 0.03 |
| Li et al., 2020 [85] | 5 RCTs 3 included in meta-analysis | 349 participants New Zealand Age 2–12 years Both male and female | Dose: 2000 IU/day Duration = 5–12 months | ABC SRS CARS SPM ATEC DD-CGAS SSGARS-2 | Social interaction: No difference (pooled MD: −1.54; 95% CI: [−4.09, 1.01]; p = 0.24) Communication: No difference (pooled MD: −0.05; 95% CI: [−1.79, 1.69]; p = 0.96) Repetitive restrictive behavior: No difference (pooled MD: 0.85; 95% CI: [−0.33, 2.02]; p = 0.16) Hyperactivity: A significant difference (pooled MD: −3.20; 95% CI: [−6.06, −0.34]; p = 0.03) Irritability: No difference (pooled MD: −2.31; 95% CI: [−6.08, 1.46]; p = 0.23), |
| Zhang 2023 [86] | 8 RCTs 6 included in meta-analysis | 266 participants (New Zealand, Iran, China, Ireland) Age 2.5−14 years Both male and female | Dose range: 800 IU/day to 50,000 IU/week and 2000 IU/day Duration 10 weeks to 12 months | ABC SRS CARS | No difference in core symptoms (pooled MD: −8.74; 95% CI: −17.45, −0.03; p = 0.05) Social interaction: No difference (pooled MD: −0.07; 95% CI: −1.70, 1.57; p = 0.93) Communication: No difference (pooled MD: −0.04; 95% CI: −1.19, 1.10; p = 0.94), Stereotypes: Significant difference between the intervention and placebo groups (pooled MD: −1.39; 95% CI: −2.7, −0.07; p = 0.04) Irritability: No difference (pooled MD: −1.79; 95% CI: −4.42, −0.85; p = 0.18) Hyperactivity: No difference (pooled MD: −1.35; 95% CI: −4.37, 1.67; p = 038) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, S.; Alhejin, N.; Serafi, R.; Abu Alrahi, M.; Afifi, G.; Al-Adawi, L.; Serafi, M.; El Madhoun, N. Does Vitamin D Deficiency Increase the Risk of Autism Spectrum Disorder? Linking Evidence with Theory—A Narrative Review. Psychiatry Int. 2025, 6, 22. https://doi.org/10.3390/psychiatryint6010022
Sultan S, Alhejin N, Serafi R, Abu Alrahi M, Afifi G, Al-Adawi L, Serafi M, El Madhoun N. Does Vitamin D Deficiency Increase the Risk of Autism Spectrum Disorder? Linking Evidence with Theory—A Narrative Review. Psychiatry International. 2025; 6(1):22. https://doi.org/10.3390/psychiatryint6010022
Chicago/Turabian StyleSultan, Sadia, Noor Alhejin, Raed Serafi, Manar Abu Alrahi, Gehad Afifi, Layan Al-Adawi, Mohammed Serafi, and Nada El Madhoun. 2025. "Does Vitamin D Deficiency Increase the Risk of Autism Spectrum Disorder? Linking Evidence with Theory—A Narrative Review" Psychiatry International 6, no. 1: 22. https://doi.org/10.3390/psychiatryint6010022
APA StyleSultan, S., Alhejin, N., Serafi, R., Abu Alrahi, M., Afifi, G., Al-Adawi, L., Serafi, M., & El Madhoun, N. (2025). Does Vitamin D Deficiency Increase the Risk of Autism Spectrum Disorder? Linking Evidence with Theory—A Narrative Review. Psychiatry International, 6(1), 22. https://doi.org/10.3390/psychiatryint6010022






