Exploring the Interplay between Family History of Depression, Negative Life Events, and Social Support in First-Episode Major Depression: Insights from a Pilot Case-Control Study
Abstract
1. Introduction
1.1. Background on Depression
1.2. Diathesis–Stress Model
1.3. Importance of Family History
1.4. Role of Life Events and Social Support
1.5. Impact of Negative Life Events on Treatment Outcomes
1.6. Aim and Objectives
2. Materials and Methods
2.1. Study Design
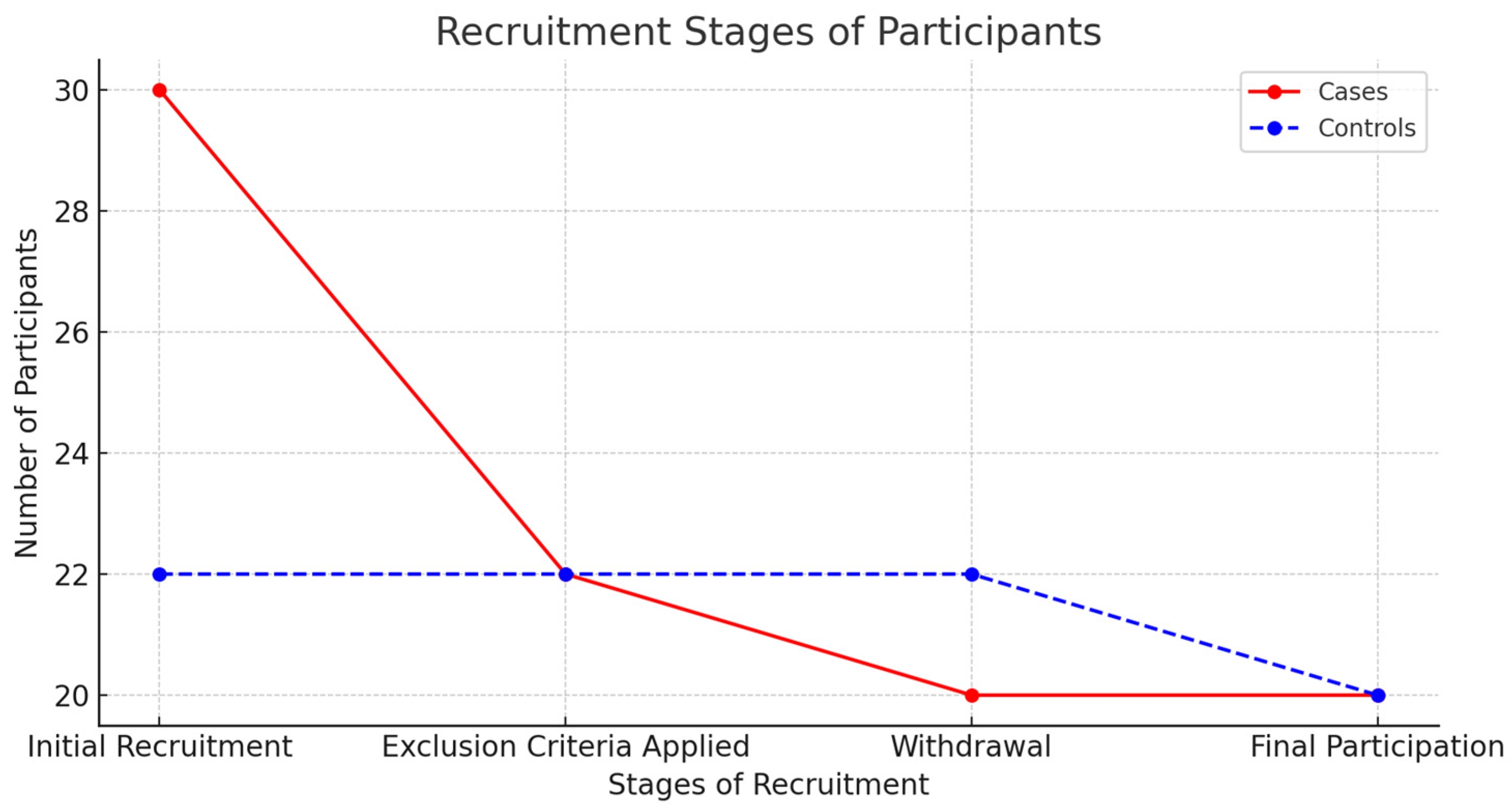
2.2. Recruitment of Participants
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Assessment Tools and Administration
2.6. Data Analysis
2.7. Follow-Up Procedures
2.8. Ethical Considerations
3. Results
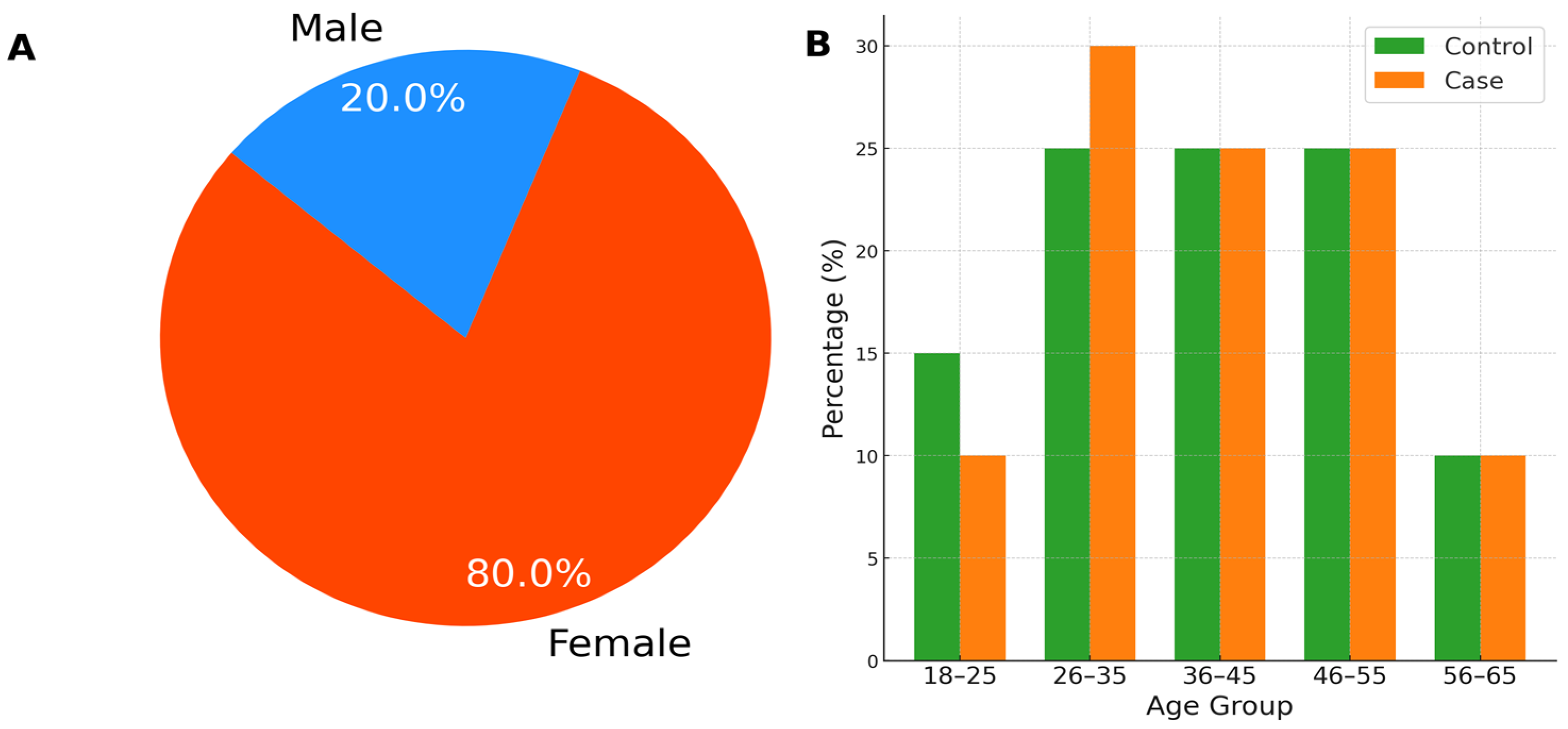
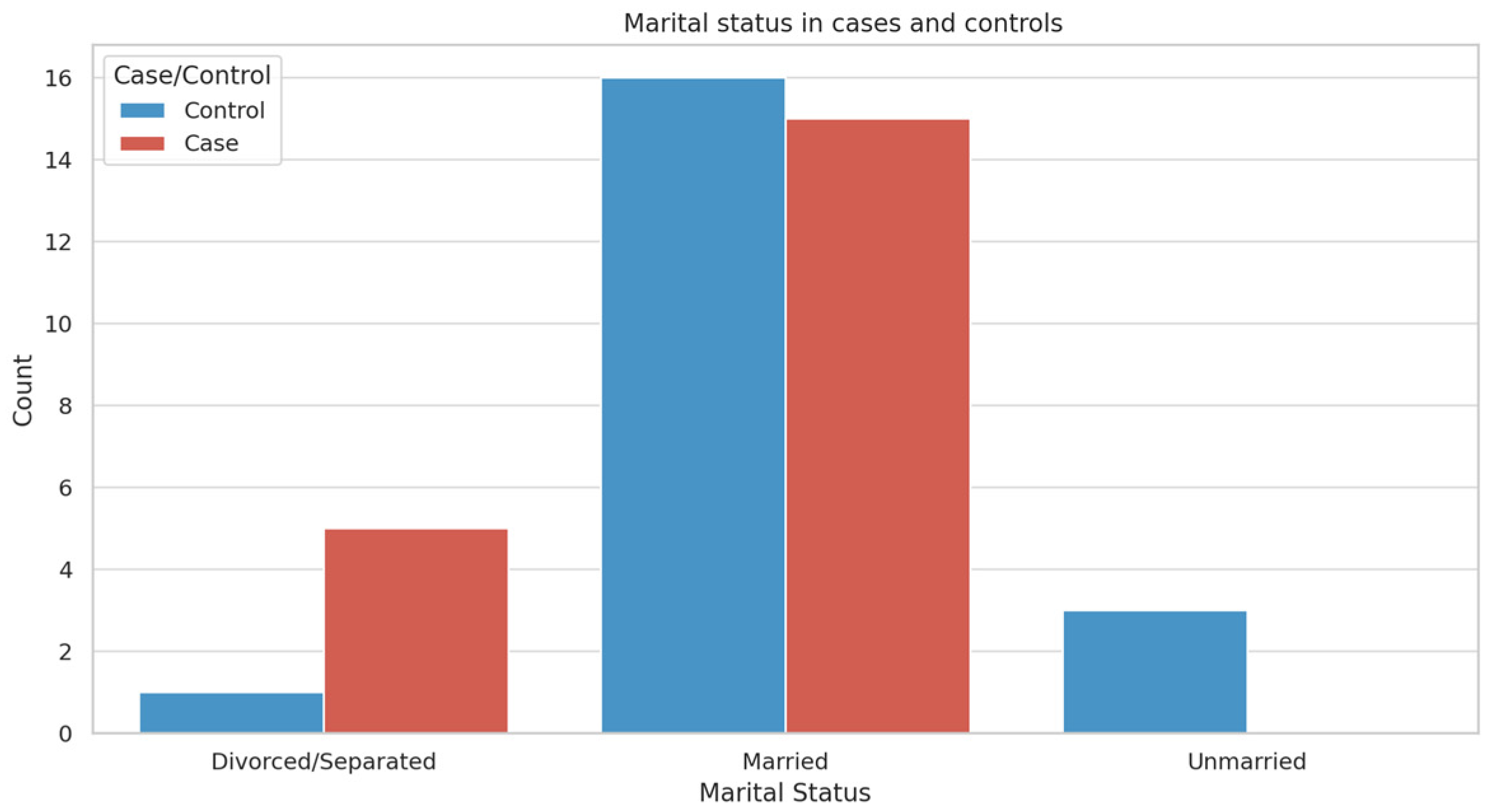
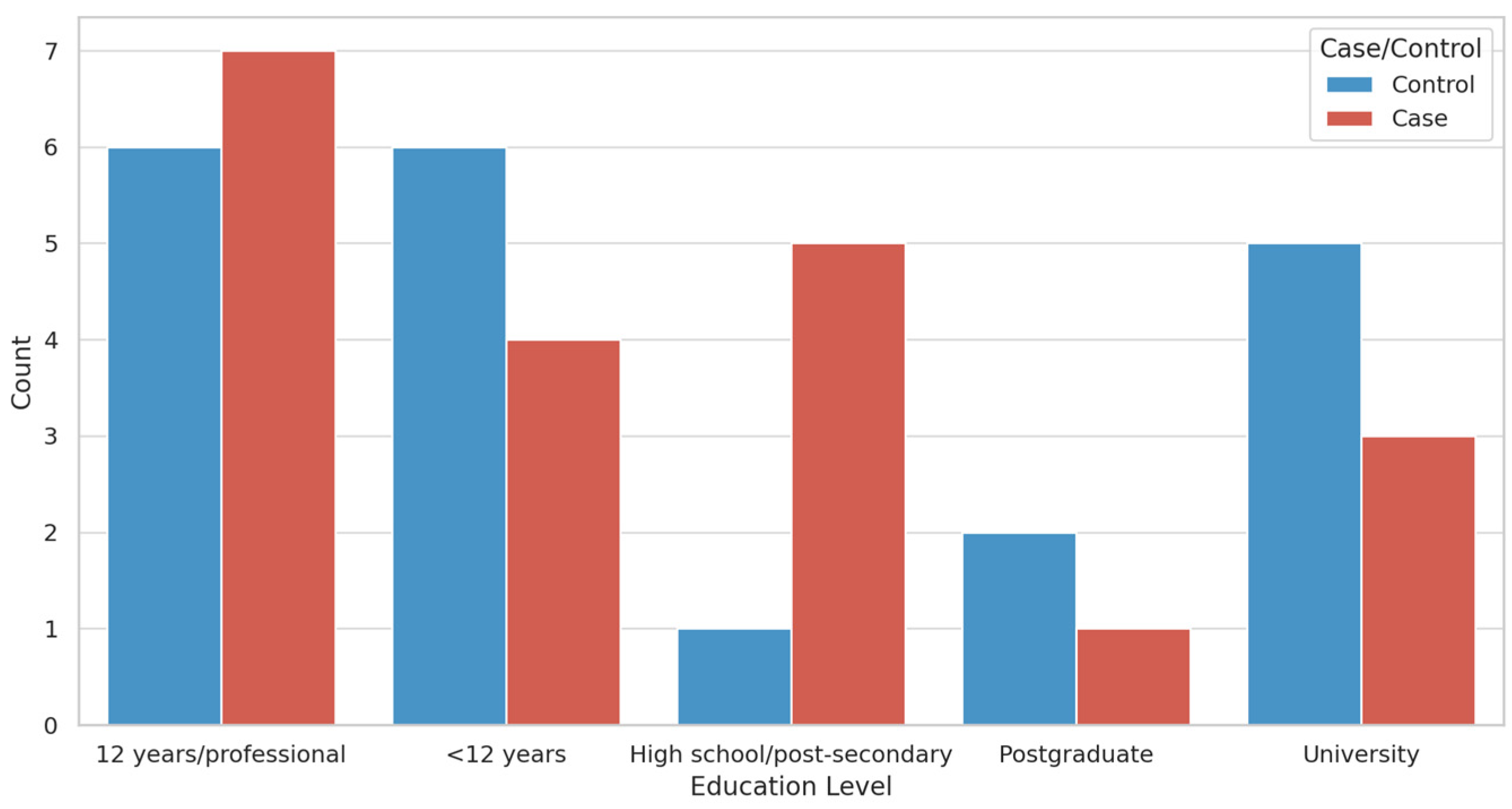
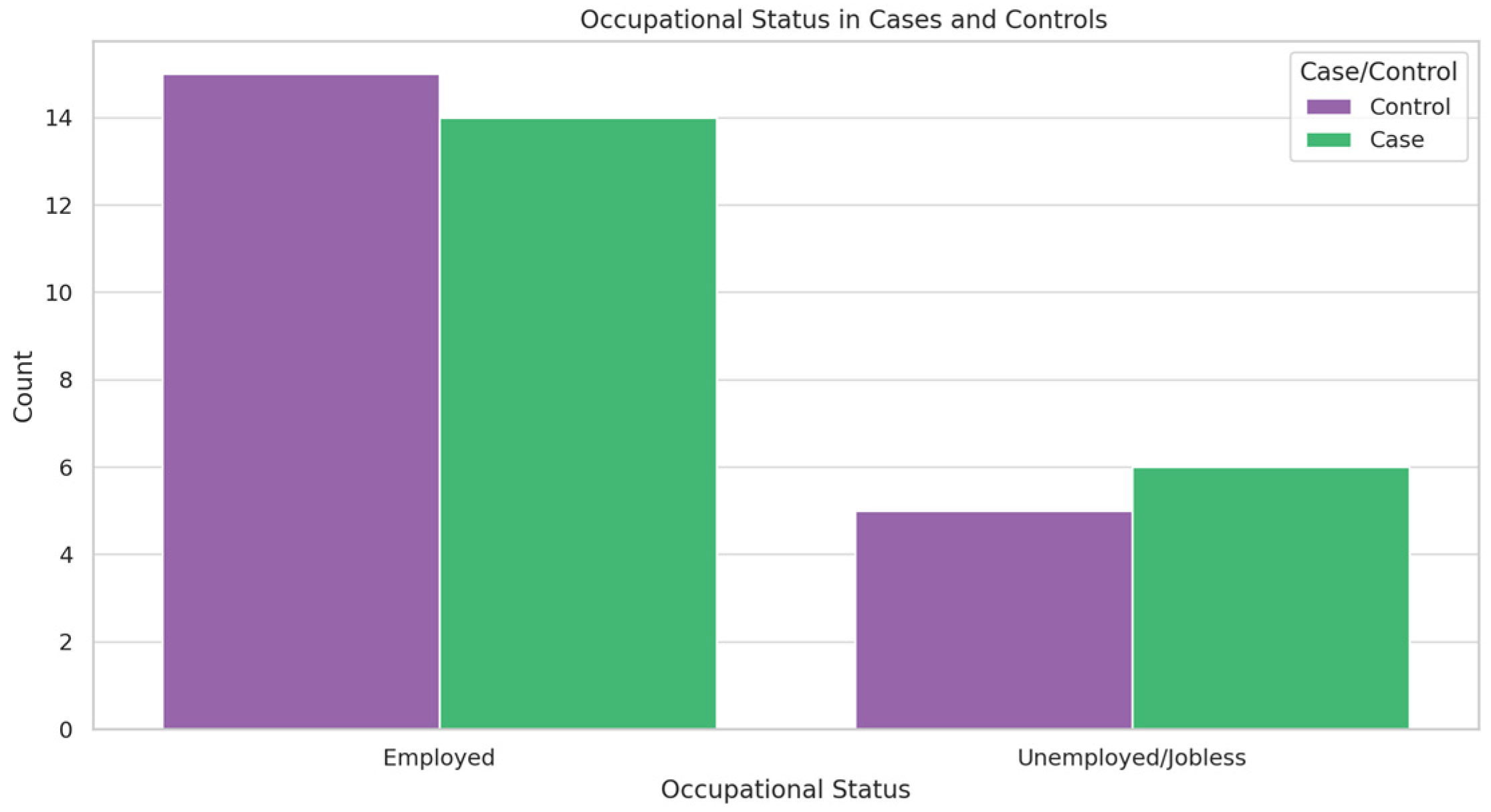
3.1. Participant Demographics
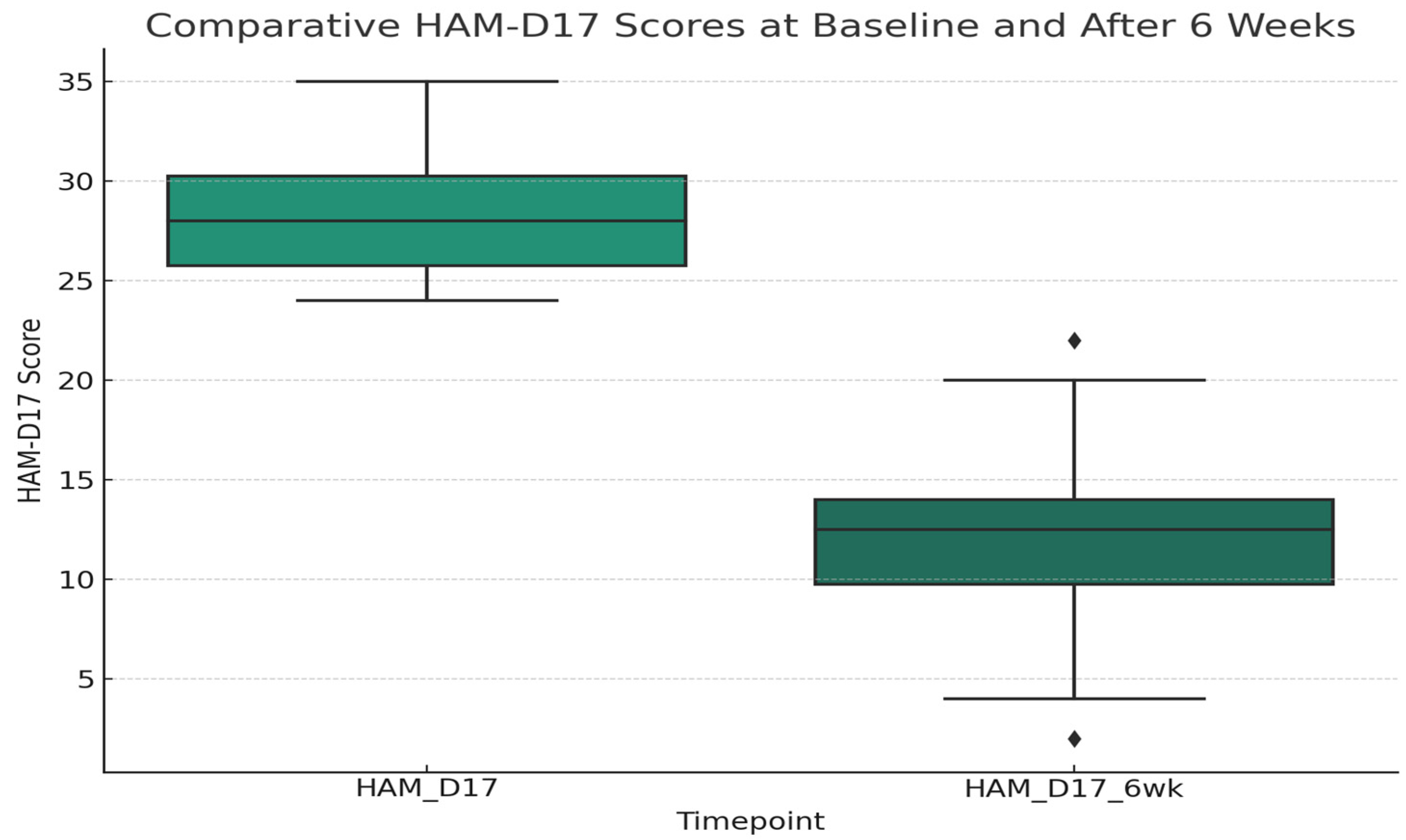
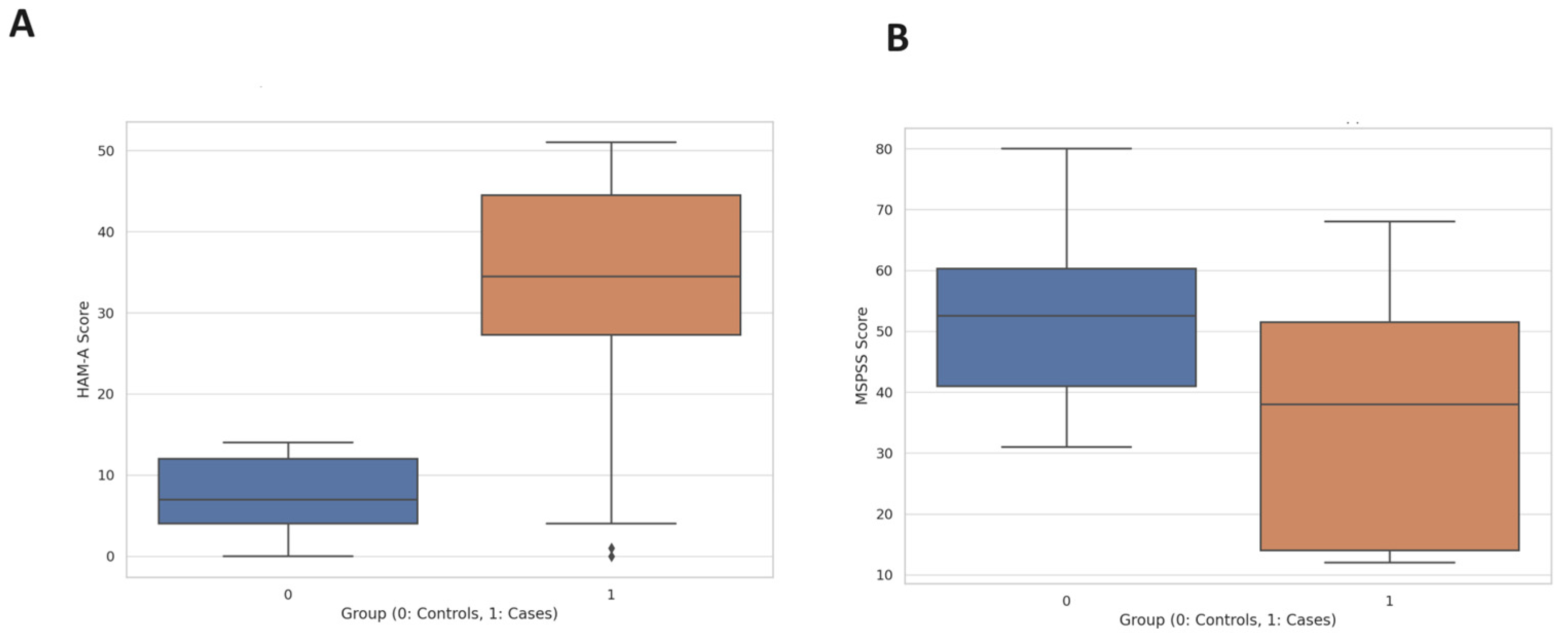
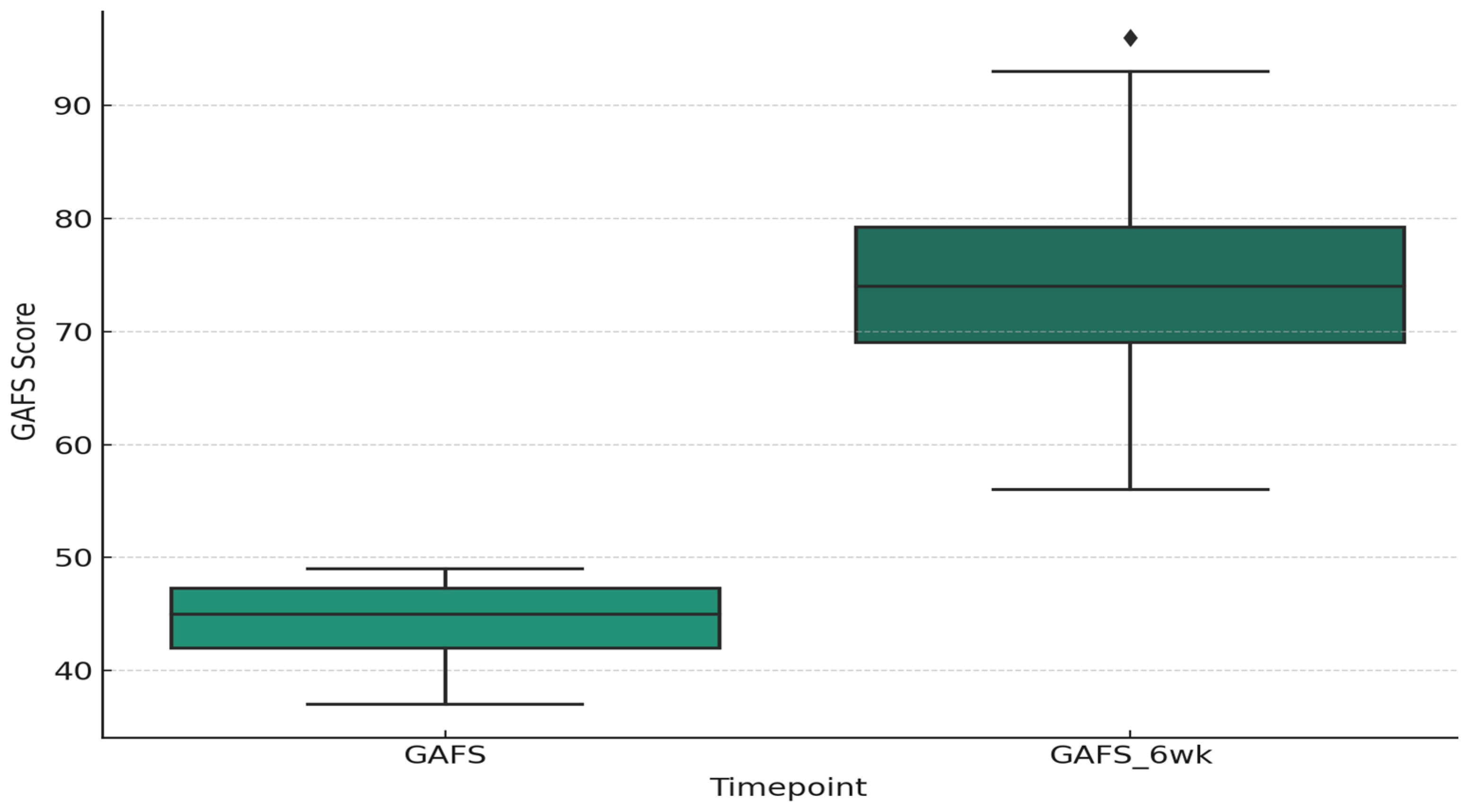
3.2. Clinical Scores and Treatment Response
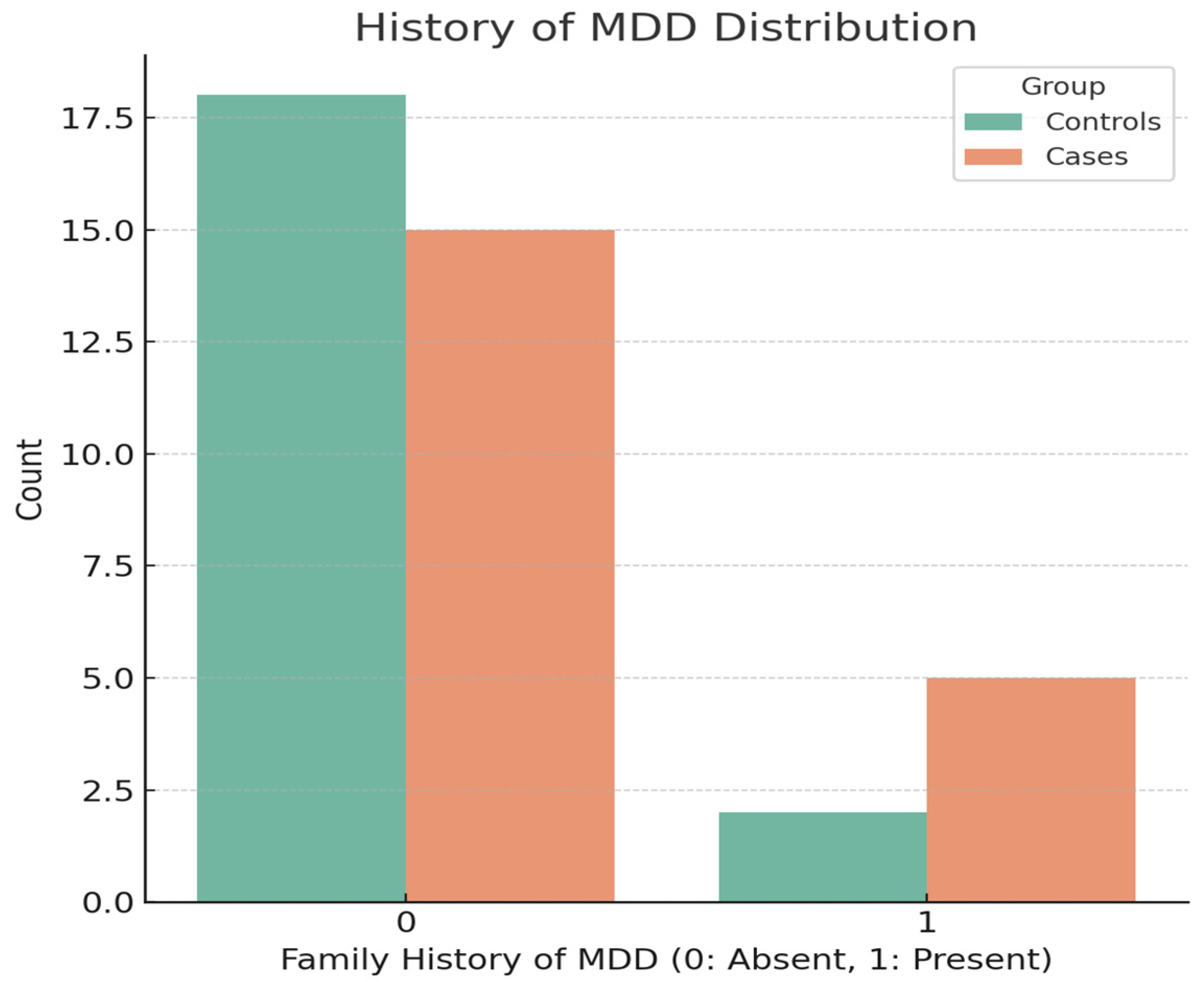
3.3. Life Events and Familial History of MDD
3.3.1. Analysis of Negative Life Events Patterns
- Personal security incidents—among individuals experiencing severe FEMD, two reported being victims of robbery, compared to one report in the control group.
- Loss events—a participant from the control group reported the loss of a pet, whereas in the group with a severe depressive episode, the death of a close relative was reported.
- Relationship breakdowns—in the severe depressive episode group, there was one report of a divorce and three instances of marital conflict. The control group did not report similar events.
- Financial and employment challenges—exclusive to the severe depressive episode group were reports of financial difficulties, including the inability to pay debts and job loss.
3.3.2. Analysis of Psychosocial Factors Influencing First Severe Depressive Episodes
3.3.3. Preliminary Findings
3.4. Life Events Exposure and Treatment Response in First Severe Depressive Episode
3.5. Post Hoc Power Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Depressive Disorders (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 19 November 2023).
- Vasiliu, O.; Mangalagiu, A.G.; Petrescu, B.M.; Cândea, C.A.; Tudor, C.; Vasile, D. Analysis of COVID-19-related psychiatric disorders- clinical manifestations and therapeutic considerations. Rom. J. Miliary Med. 2022, 125, 382–391. [Google Scholar] [CrossRef]
- Marcus, M.; Yasamy, M.T.; van Ommeren, M.; Chrisholm, D.; Saxena, S. Depression: A global public health concern. In WHO Department of Mental Health and Substance Abuse 2012; World Health Organisation: Geneva, Switzerland, 2012; pp. 6–8. [Google Scholar]
- Remes, O.; Mendes, J.F.; Templeton, P. Biological, Psychological, and Social Determinants of Depression: A Review of Recent Literature. Brain Sci. 2021, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, N.; Shibeshi, W.; Engidawork, E. Major Depressive Disorder: Pathophysiology and Clinical Management. J. Depress. Anxiety 2017, 6, 255–257. [Google Scholar] [CrossRef]
- Vasiliu, O. Efficacy, tolerability, and safety of toludesvenlafaxine for the treatment of major depressive disorder-A narrative review. Pharmaceuticals 2023, 16, 411. [Google Scholar] [CrossRef] [PubMed]
- Scotto, J.C.; Farisse, J. Clinical expressions of depression. Rev. Prat. 1999, 49, 701–706. [Google Scholar] [PubMed]
- Riga, S.; Riga, D.; Mihailescu, A.; Motoc, D.; Mos, L.; Schneider, F. Longevity health sciences and mental health as future medicine. Ann. N. Y. Acad. Sci. 2010, 1197, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Beekman, A.T.F.; Reynolds, C.F. 3rd. Preventing depression: A global priority. JAMA 2012, 307, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.E.; Birnbaum, H.G. The economic burden of depression in the US: Societal and patient perspectives. Expert Opin. Pharmacother. 2005, 6, 369–376. [Google Scholar] [CrossRef]
- Chaudhry, S. Depression and its Dilemma. IP J. Surg. Allied Sci. 2020, 2, 81–88. [Google Scholar] [CrossRef]
- Pérez-Wehbe, A.I.; Perestelo-Pérez, L.; Bethencourt- Pérez, J.M.; Cuéllar-Pompa, L.; Peñate-Castro, W. Treatment-resistant depression: A systematic review of systematic reviews. Int. J. Clin. Health Psychol. 2014, 14, 145–153. [Google Scholar] [CrossRef][Green Version]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Hammen, C. Risk Factors for Depression: An Autobiographical Review. Annu. Rev. Clin. Psychol. 2018, 14, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Colodro-Conde, L.; Couvy-Duchesne, B.; Zhu, G.; Coventry, W.L.; Byrne, E.M.; Gordon, S.; Wright, M.; Montgomery, G.W.; Madden, P.A.; Ripke, S.; et al. A direct test of the diathesis-stress model for depression. Mol. Psychiatry 2017, 23, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Patten, S.B. Major depression epidemiology from a diathesis-stress conceptualization. BMC Psychiatry 2013, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Tennant, C. Life Events, Stress and Depression: A Review of Recent Findings. Aust. N. Z. J. Psychiatry 2002, 36, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, T.; Kim, H.C. Involvement of Genetic and Environmental Factors in the Onset of Depression. Exp. Neurobiol. 2013, 22, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.J. The Synergistic Influence of Life Experiences and Cultural Nuances on Development of Depression: A Cognitive Behavioral Perspective. Issues Ment. Health Nurs. 2020, 41, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Docherty, A.R.; Edwards, A.C.; Yang, F.; Peterson, R.E.; Sawyers, C.; Adkins, D.E.; Moore, A.A.; Webb, B.T.; Bacanu, S.A.; Flint, J.; et al. Age of onset and family history as indicators of polygenic risk for major depression. Depress. Anxiety 2017, 34, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Navarro, P.; Xia, C.; Amador, C.; Fernandez-Pujals, A.M.; Thomson, P.A.; Campbell, A.; Nagy, R.; Clarke, T.-K.; Hafferty, J.D.; et al. Shared Genetics and Couple-Associated Environment Are Major Contributors to the Risk of Both Clinical and Self-Declared Depression. EBioMedicine 2016, 14, 161–167. [Google Scholar] [CrossRef]
- van Sprang, E.D.; Maciejewski, D.F.; Milaneschi, Y.; Elzinga, B.M.; Beekman, A.T.F.; Hartman, C.A.; van Hemert, A.M.; Penninx, B.W.J.H. Familial risk for depressive and anxiety disorders: Associations with genetic, clinical, and psychosocial vulnerabilities. Psychol. Med. 2020, 52, 696–706. [Google Scholar] [CrossRef]
- Zalar, B.; Blatnik, A.; Maver, A.; Klemenc-Ketis, Z.; Peterlin, B. Family History As an Important Factor for Stratifying Participants in Genetic Studies of Major Depression. Balkan J. Med. Genet. 2018, 21, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, I.H.; Joormann, J.; Foland-Ross, L.C. Understanding Familial Risk for Depression. Perspect. Psychol. Sci. 2014, 9, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.E.; Boyce, T.; Chesney, M.A.; Cohen, S.; Folkman, S.; Kahn, R.L.; Syme, S.L. Socioeconomic status and health: The challenge of the gradient. Am. Psychol. 1994, 49, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Murphy, M.L.M.; Prather, A.A. Ten Surprising Facts About Stressful Life Events and Disease Risk. Annu. Rev. Psychol. 2019, 70, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Avenevoli, S.; Merikangas, K.R. Implications of high-risk family studies for prevention of depression. Am. J. Prev. Med. 2006, 31 (Suppl. 1), S126–S135. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.C.; Carroll, D.; Der, G. Negative life events and symptoms of depression and anxiety: Stress causation and/or stress generation. Anxiety Stress Coping 2015, 28, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Marum, G.F.; Clech-Aas, J.; Nes, R.B.; Raanaas, R.K. The relationship between negative life events, psychological distress and life satisfaction: A population-based study. Qual. Life Res. 2013, 23, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Paykel, E.S. Life events, Social support and depression. Acta Psychiatr. Scand. 1994, 89, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Gariépy, G.; Honkaniemi, H.; Quesnel-Vallée, A. Social support and protection from depression: Systematic review of current findings in Western countries. Br. J. Psychiatry 2016, 209, 284–293. [Google Scholar] [CrossRef]
- Kendler, K.S.; Markowski, L.M.; Prescott, C.A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 1999, 156, 837–841. [Google Scholar] [CrossRef]
- Santini, Z.I.; Koyanagi, A.; Tyrovolas, S.; Mason, C.; Haro, J.M. The association between social relationships and depression: A systematic review. J. Affect. Disord. 2015, 175, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Monroe, S.M.; Kupfer, D.J.; Frank, E.F. Life stress and treatment course of recurrent depression: I. Response during index episode. J. Consult. Clin. Psychol. 1992, 60, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Buckman, J.E.J.; Saunders, R.; Arundell, L.L.; Oshi, I.D.; Cohen, Z.D.; O’Driscoll, C.; Barnett, P.; Stott, J.; Ambler, G.; Gilbody, S.; et al. Life Events and Treatment Prognosis for Depression: A Systematic Review and Individual Patient Data Meta-Analysis. J. Affect. Disord. 2022, 299, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M. Weighted versus Unweighted Life Event Scores: Is there a Difference? J. Hum. Stress 1983, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Nierenberg, A.A.; DeCecco, L.M. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: A focus on treatment-resistant depression. J. Clin. Psychiatry 2001, 62 (Suppl. 16), 5–9. [Google Scholar] [PubMed]
- Kendler, K.S.; Kessler, R.C.; Walters, E.E.; MacLean, C.; Neale, M.C.; Heath, A.C.; Eaves, L.J. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am. J. Psychiatry 1995, 152, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Hammen, C. Stress and depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Paykel, E.S.; Cooper, Z.; Ramana, R.; Hayhurst, H. Life Events, Social Support and Marital Relationships in the Outcome of Severe Depression. Psychol. Med. 1996, 26, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Monroe, S.M.; Reid, M.W. Life Stress and Major Depression. Curr. Dir. Psychol. Sci. 2009, 18, 68–72. [Google Scholar] [CrossRef]
- Monroe, S.M.; Slavich, G.M.; Gotlieb, I.H. Life stress and family history for depression: The moderating role of past depressive episodes. J. Psychiatr. Res. 2014, 49, 90–95. [Google Scholar] [CrossRef]
- Friedberg, A.; Malefakis, D. Resilience, Trauma, and Coping. Psychodyn. Psychiatry 2022, 50, 382–409. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Smith, G.D. Resilience to depression: Implication for psychological vaccination. Front. Psychiatry 2023, 14, 1071859. [Google Scholar] [CrossRef]
- Helmreich, I.; Kunzler, A.; Chmitorz, A.; König, J.; Binder, H.; Wessa, M.; Lieb, K. Psychological interventions for resilience enhancement in adults. Cochrane Database Syst. Rev. 2017, 2017, CD012527. [Google Scholar] [CrossRef]
- Bulmash, E.; Harkness, K.L.; Stewart, J.G.; Bagby, R.M. Personality, Stressful Life Events, and Treatment Response in Major Depression. J. Consult. Clin. Psychol. 2009, 77, 1067–1077. [Google Scholar] [CrossRef]
- Fournier, J.C.; DeRubeis, R.J.; Shelton, R.C.; Hollon, S.D.; Amsterdam, J.D.; Gallop, R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J. Consult. Clin. Psychol. 2009, 77, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Steinert, C.; Hofmann, M.; Kruse, J.; Leichsenring, F. The Prospective Long-term Course of Adult Depression in General Practice and the Community: A Systematic Literature Review. J. Affect. Disord. 2014, 152–154, 65–75. [Google Scholar] [CrossRef]
- Arnau-Soler, A.; Adams, M.J.; Clarke, T.K.; MacIntyre, D.J.; Milburn, K.; Navrady, L.; Hayward, C.; McIntosh, A.; Thomson, P.A.; Scotland, G.; et al. A validation of the diathesis-stress model for depression in Generation Scotland. Transl. Psychiatry 2019, 9, 25. [Google Scholar] [CrossRef]
- Arnau-Soler, A.; Macdonald-Dunlop, E.; Adams, M.J.; Clarke, T.K.; MacIntyre, D.J.; Milburn, K.; Navrady, L.; Hayward, C.; McIntosh, A.M.; Thomson, P.A.; et al. Genome-wide by environment interaction studies of depressive symptoms and psychosocial stress in UK Biobank and Generation Scotland. Transl. Psychiatry 2019, 9, 14. [Google Scholar] [CrossRef]
- Pan, C.; Liu, L.; Cheng, S.; Yang, X.; Meng, P.; Zhang, N.; He, D.; Chen, Y.; Li, C.; Zhang, H.; et al. A multidimensional social risk atlas of depression and anxiety: An observational and genome-wide environmental interaction study. J. Glob. Health 2023, 13, 04146. [Google Scholar] [CrossRef]
- Mandelli, L.; Serretti, A. Gene-environment interaction studies in depression and suicidal behavior: An update. Neurosci. Biobehav. Rev. 2013, 37 Pt 1, 2375–2397. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, L.; Yang, J.; Han, D.; Fang, D.; Qiu, X.; Yang, X.; Qiao, Z.; Ma, J.; Wang, L.; et al. BDNF Val66Met polymorphism, life stress and depression: A meta-analysis of gene-environment interaction. J. Affect. Disord. 2018, 227, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Burcusa, S.L.; Iacomo, W.G. Risk for recurrence in depression. Clin. Psychol. Rev. 2007, 27, 959–985. [Google Scholar] [CrossRef] [PubMed]
- Spijker, J.; de Graaf, R.; Oldehinkel, A.J.; Nolen, W.A.; Ormel, J. Are the vulnerability effects of personality and psychosocial functioning on depression accounted for by subthreshold symptoms? Depress. Anxiety 2007, 24, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Ormel, J.; Oldehinkel, A.J.; Vollebergh, W. Vulnerability before, during, and after a major depressive episode: A 3-wave population-based study. Arch. Gen. Psychiatry 2004, 61, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Moraru, C.; Nafureanu, E.D.; Zavadovschi, I.; Floria, M.; Popescu, D.M. Posttraumatic stress disorder and cardiovascular disease. Rom. J. Mil. Med. 2022, 125, 97–111. [Google Scholar]
- Buist-Bouwman, M.A.; Ormel, J.; de Graaf, R.; Vollenbergh, W.A.M. Functioning after a major depressive episode: Complete or incomplete recovery? J. Affect. Disord. 2004, 82, 363–371. [Google Scholar] [CrossRef]
- van Tuijl, L.A.; Glashouwer, K.A.; Elgersma, H.J.; Bockting, C.L.H.; Penninx, B.W.J.H.; de Jong, P.J. Depression recurrence after recovery: Prognostic value of implicit and explicit self-depressed associations. Behav. Res. Ther. 2018, 107, 76–82. [Google Scholar] [CrossRef]
- Licu, M.; Ionescu, C.G.; Suciu, M.; Paun, S. Self-esteem, self-efficacy, and smoking prevalence: A cross-sectional study among military and civilian medical students. Rom. J. Mil. Med. 2023, 126, 502–511. [Google Scholar] [CrossRef]
- Petrescu, B.; Vasile, D.; Vasiliu, O.; Tudor, C.; Mangalagiu, A.; Ungureanu, D. SSRI dose escalation versus duloxetine in treatment of major depressive disorder not responding to initial SSRI. Eur. Neuropsychopharmacol. 2014, 24, S455–S456. [Google Scholar] [CrossRef]
- Lye, M.S.; Tey, Y.Y.; Tor, Y.S.; Shahabudin, A.F.; Ibrahim, N.; Ling, K.H.; Stanslas, J.; Loh, S.-P.; Rosli, R.; Lokman, K.A.; et al. Predictors of recurrence of major depressive disorder. PLoS ONE 2020, 15, e0230363. [Google Scholar] [CrossRef]

| Variable | Coefficient | Std. Error | z-Value | p-Value | 95% CI Lower | 95% CI Upper | Odds Ratio (OR) | OR 95% CI Lower | OR 95% CI Upper |
|---|---|---|---|---|---|---|---|---|---|
| Constant | 2.8315 | 1.579 | 1.793 | 0.073 | −0.2633 | 5.9263 | N/A | N/A | N/A |
| MSPSS | −0.0838 | 0.034 | −0.469 | 0.014 | −0.1504 | −0.0172 | 0.92 | 0.86 | 0.98 |
| Family history of MDD | 1.2784 | 1.086 | 1.177 | 0.239 | −0.8502 | 3.4070 | 3.59 | 0.43 | 30.17 |
| Negative life events | 2.7274 | 1.083 | 2.518 | 0.012 | 0.06047 | 4.8501 | 15.29 | 1.06 | 127.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangalagiu, A.G.; Riga, S.; Vasiliu, O. Exploring the Interplay between Family History of Depression, Negative Life Events, and Social Support in First-Episode Major Depression: Insights from a Pilot Case-Control Study. Psychiatry Int. 2024, 5, 305-322. https://doi.org/10.3390/psychiatryint5030021
Mangalagiu AG, Riga S, Vasiliu O. Exploring the Interplay between Family History of Depression, Negative Life Events, and Social Support in First-Episode Major Depression: Insights from a Pilot Case-Control Study. Psychiatry International. 2024; 5(3):305-322. https://doi.org/10.3390/psychiatryint5030021
Chicago/Turabian StyleMangalagiu, Andrei Gabriel, Sorin Riga, and Octavian Vasiliu. 2024. "Exploring the Interplay between Family History of Depression, Negative Life Events, and Social Support in First-Episode Major Depression: Insights from a Pilot Case-Control Study" Psychiatry International 5, no. 3: 305-322. https://doi.org/10.3390/psychiatryint5030021
APA StyleMangalagiu, A. G., Riga, S., & Vasiliu, O. (2024). Exploring the Interplay between Family History of Depression, Negative Life Events, and Social Support in First-Episode Major Depression: Insights from a Pilot Case-Control Study. Psychiatry International, 5(3), 305-322. https://doi.org/10.3390/psychiatryint5030021








