Effects of a One-Day Experiential Sheep-Rearing Experience on Motivation, Anxiety, and Frontal Lobe Brain Activity in Patients with Chronic Psychiatric Disorders: A Crossover Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Methods
2.1.1. Participants
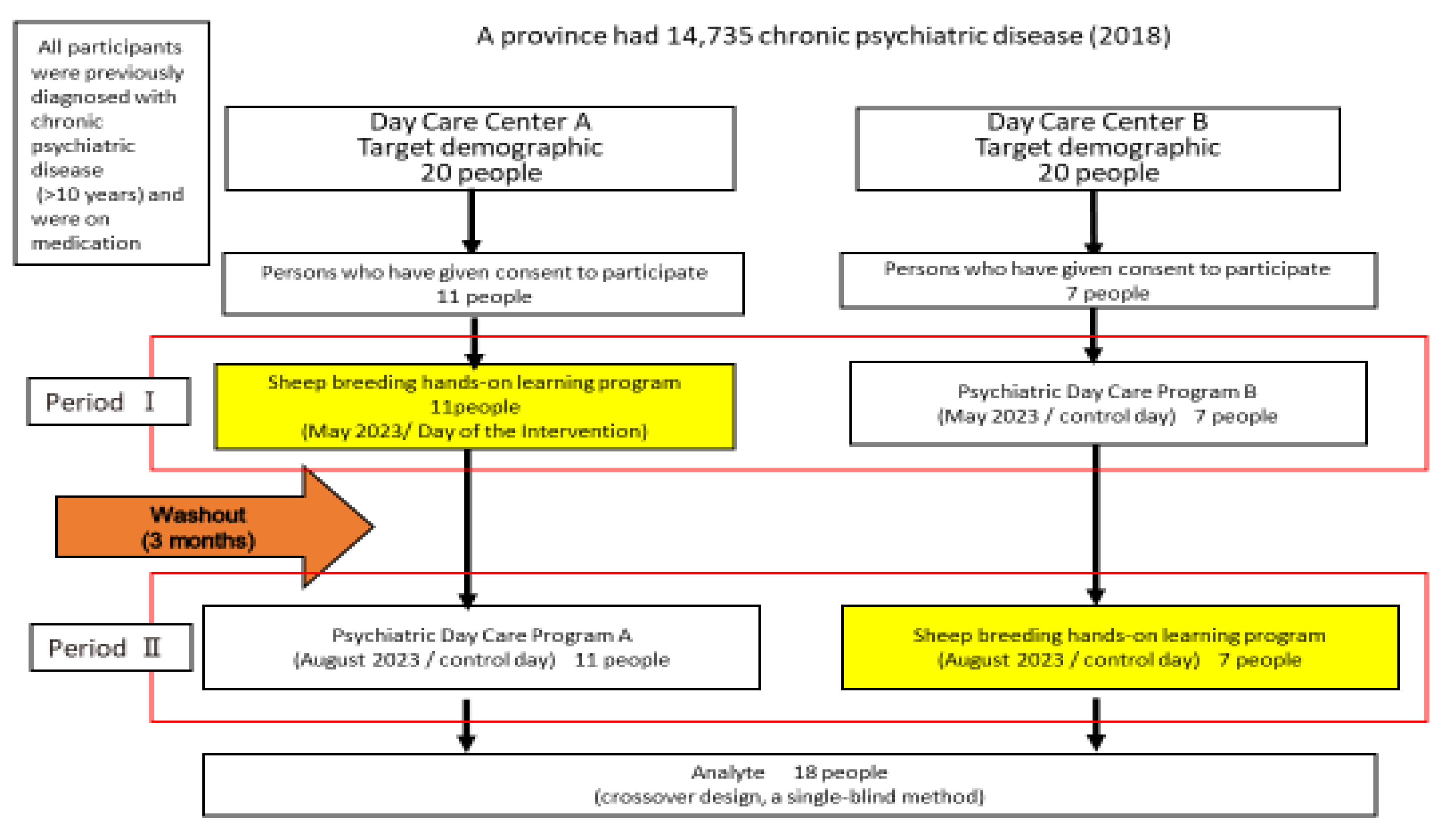
2.1.2. Study Design
2.1.3. Intervention
2.2. Survey Methodology
2.2.1. Measurement of the Motivation Score (Apathy Scale)
2.2.2. Measurement of Changes in Salivary Cortisol and Oxytocin
2.2.3. Measurement of Cerebral Blood Flow Using NIRS
2.2.4. Survey Period
2.3. Analysis
2.3.1. Identification of the Channel Location Using Standard Brain Coordinates
2.3.2. Comparison of Baseline Data on the Intervention and Control Days
2.3.3. Changes in the Intervention and Control Dates before and Immediately after the Start of the Intervention (Between-Group Comparisons)
2.4. Ethical Considerations
3. Results
3.1. Participants’ Characteristics
3.2. Comparison of the Salivary Oxytocin and Cortisol Levels before and after the Intervention
3.3. Test for Carryover Effect
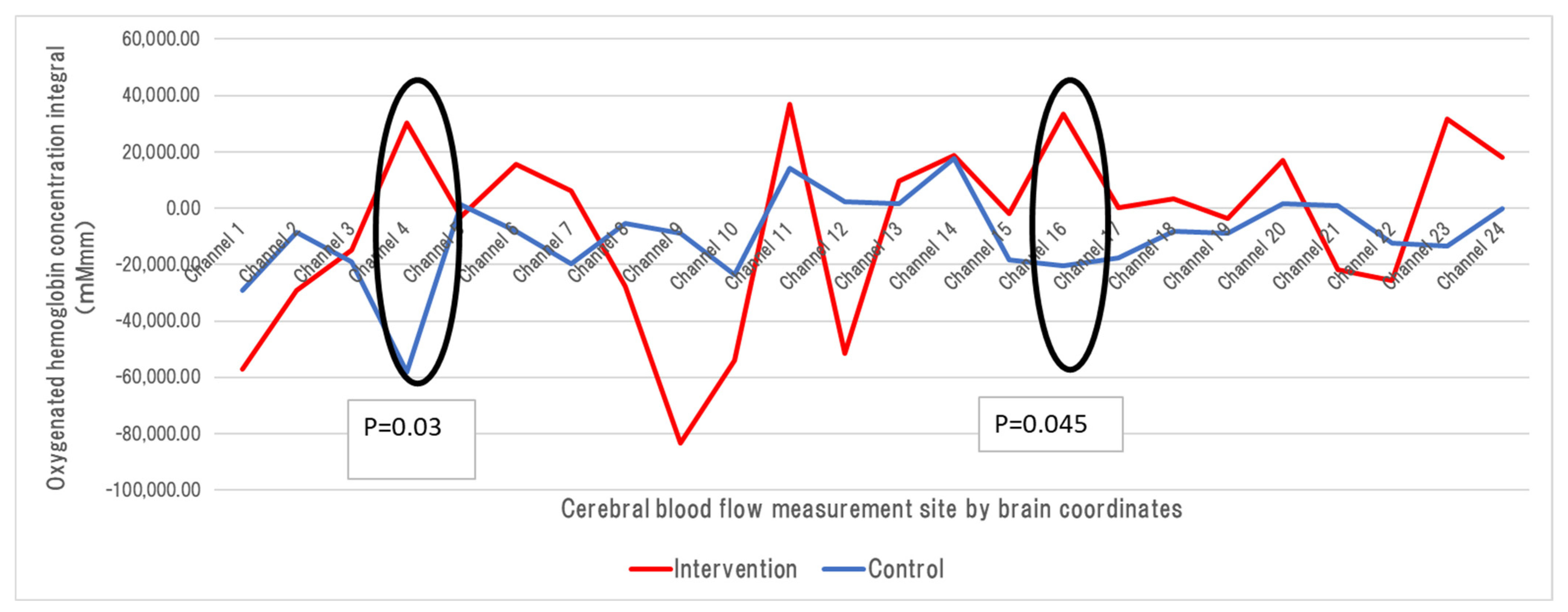
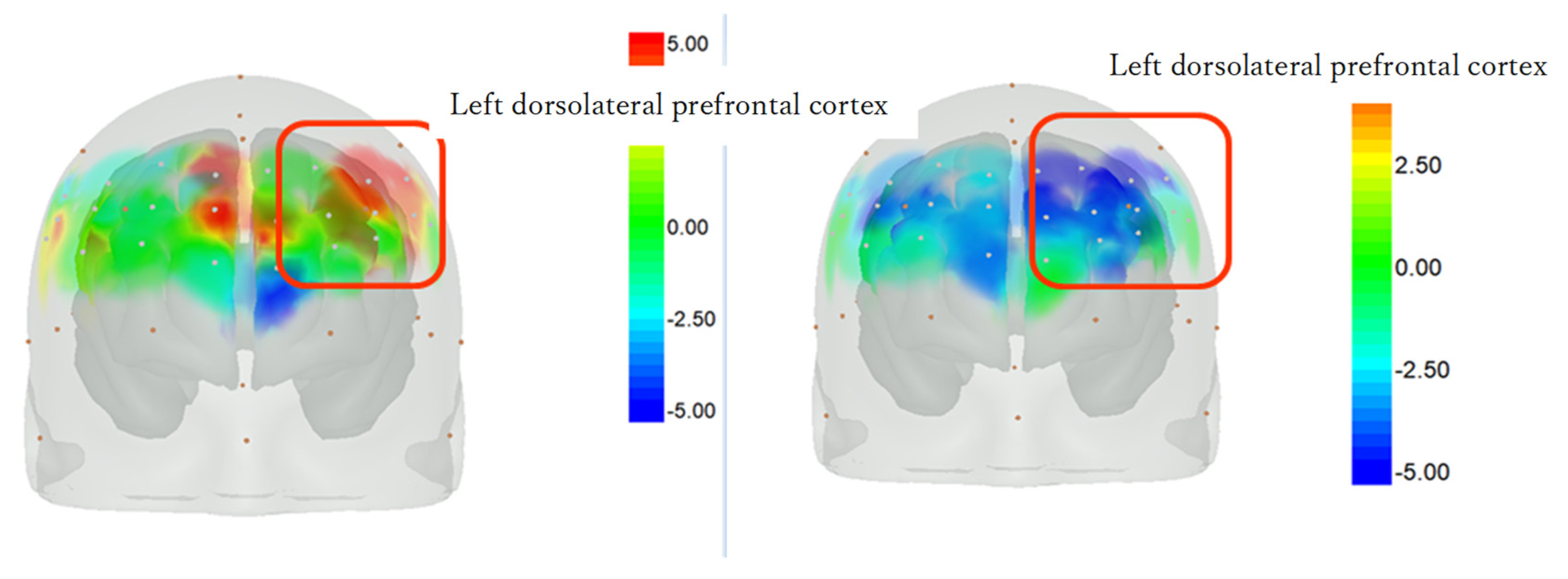
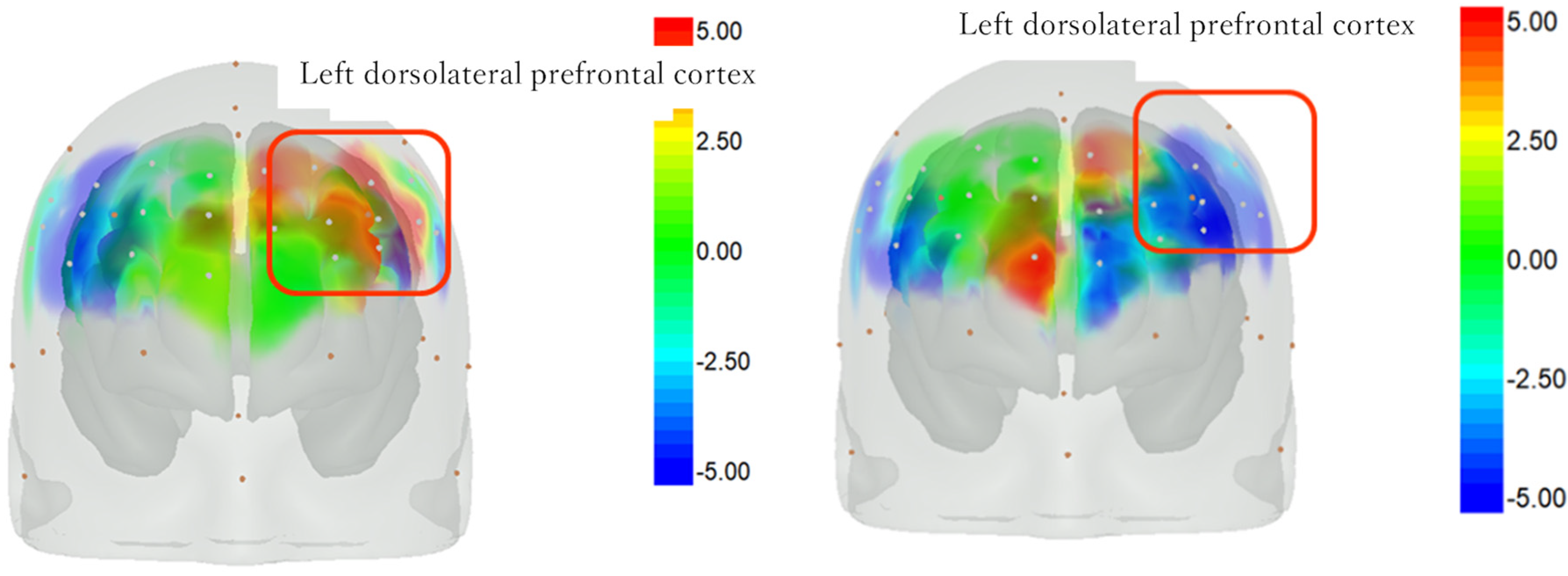
3.4. Changes in Cerebral Blood Flow on the Intervention and Control Days Using NIRS
4. Discussion
4.1. Participants’ Characteristics
4.2. Changes in the Apathy Scale Scores during the Sheep-Rearing Experience and Usual Occupational Therapy
4.3. Changes in Salivary Oxytocin and Cortisol Levels during the Sheep-Rearing Experience and Usual Occupational Therapy
4.4. Changes in Brain Activity during the Sheep-Rearing Experience and Usual Occupational Therapy
4.5. Bilateral Frontal Lobe Activation with and without an Animal Intervention
4.6. Limitations and Future Challenges
5. Conclusions
- (1)
- The fact that the sheep-rearing experience in this study showed the same apathy scale effect as the daycare program at the medical institution indicates that this project can be used as part of a psychiatric rehabilitation program.
- (2)
- Oxytocin levels increased more after the sheep-rearing experience than the regular psychiatric daycare programs. These findings indicate that including animal husbandry in employment support or the continuous experience of rearing livestock as part of psychiatric daycare programs, which also serves as employment training, may increase oxytocin levels. This increase may improve the ability of psychiatric patients to infer the psychology of others, improve their ability to perceive and recognize emotions based on the facial expressions of others and lead to better relationships with others.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabinet Office. Cabinet Office Survey on Living Conditions (FY2018). 2019. Available online: https://www8.cao.go.jp/youth/kenkyu/life/h30/pdf/s1.pdf (accessed on 20 September 2022).
- Hewlett, E.; Moran, V. Making Mental Health Count: The Social and Economic Costs of Neglecting Mental Health Care; OECD Health Policy Studies; OCED Publishing: Paris, France, 2014. [Google Scholar] [CrossRef]
- Ikebuchi, E. Negative symptoms revisited—Toward the recovery of persons with schizophrenia. Seishin Shinkeigaku Zasshi 2015, 117, 179–194. (In Japanese) [Google Scholar] [PubMed]
- Green, M.F.; Kern, R.S.; Braff, D.L.; Mintz, J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr. Bull. 2000, 26, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Barch, D.M. The relationships among cognition, motivation, and emotion in schizophrenia: How much and how little we know. Schizophr. Bull. 2005, 31, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Wölwer, W.; Frommann, N.; Halfmann, S.; Piaszek, A.; Streit, M.; Gaebel, W. Remediation of impairments in facial affect recognition in schizophrenia: Efficacy and specificity of a new training program. Schizophr. Res. 2005, 80, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Penn, D.L.; Roberts, D.L.; Combs, D.; Sterne, A. Best practices: The development of the social cognition and interaction training program for schizophrenia spectrum disorders. Psychiatr. Serv. 2007, 58, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Horan, W.P.; Kern, R.S.; Shokat-Fadai, K.; Sergi, M.J.; Wynn, J.K.; Green, M.F. Social cognitive skills training in schizophrenia: An initial efficacy study of stabilized outpatients. Schizophr. Res. 2009, 107, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Combs, D.R.; Tosheva, A.; Penn, D.L.; Basso, M.R.; Wanner, J.L.; Laib, K. Attentional-shaping as a means to improve emotion perception deficits in schizophrenia. Schizophr. Res. 2008, 105, 68–77. [Google Scholar] [CrossRef][Green Version]
- Roberts, D.L.; Penn, D.L. Social cognition and interaction training (SCIT) for outpatients with schizophrenia: A preliminary study. Psychiatry Res. 2009, 166, 141–147. [Google Scholar] [CrossRef]
- Levinson, B.M. The dog as a “co-therapist”. Ment. Hyg. 1962, 46, 59–65. [Google Scholar]
- Kovács, Z.; Kis, R.; Rózsa, S.; Rózsa, L. Animal-assisted therapy for middle-aged schizophrenic patients living in a social institution. A pilot study. Clin. Rehabil. 2004, 18, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Bulucz, J.; Kis, R.; Simon, L. An exploratory study of the effect of animal-assisted therapy on nonverbal communication in three schizophrenic patients. Anthrozoös 2006, 19, 353–364. [Google Scholar] [CrossRef]
- Nathans-Barel, I.; Feldman, P.; Berger, B.; Modai, I.; Silver, H. Animal-assisted therapy ameliorates anhedonia in schizophrenia patients. A controlled pilot study. Psychother. Psychosom. 2005, 74, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Villalta-Gil, V.; Roca, M.; Gonzalez, N.; Domènec, E.; Cuca; Escanilla, A.; Asensio, M.R.; Esteban, M.E.; Ochoa, S.; Haro, J.M. Dog-assisted therapy in the treatment of chronic schizophrenia inpatients. Anthrozoös 2009, 22, 149–159. [Google Scholar] [CrossRef]
- Kamioka, H.; Okada, S.; Tsutani, K.; Park, H.; Okuizumi, H.; Handa, S.; Oshio, T.; Park, S.; Kitayuguchi, J.; Abe, T.; et al. Effectiveness of animal-assisted therapy: A systematic review of randomized controlled trials. Complement. Ther. Med 2004, 22, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Fortuny, J.R.; Guzmán, S.; Macías, C.; Bowen, J.; García, M.L.; Orejas, O.; Molins, F.; Tvarijonaviciute, A.; Cerón, J.J.; et al. Animal assisted therapy (AAT) program as a useful adjunct to conventional psychosocial rehabilitation for patients with schizophrenia: Results of a small-scale randomized controlled trial. Front. Psychol. 2016, 7, 631. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Gomi, Y.; Imai, I.; Shimoda, N.; Hiwatari, M.; Kato, H. Shift of motor activation areas during recovery from hemiparesis after cerebral infarction: A longitudinal study with near-infrared spectroscopy. Neurosci. Res. 2007, 59, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yahata, N.; Abe, O.; Kuwabara, H.; Inoue, H.; Takano, Y.; Iwashiro, N.; Natsubori, T.; Aoki, Y.; Takao, A.; et al. Diminished medial prefrontal activity behind autistic social judgments of incongruent information. PLoS ONE 2012, 7, e39561. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, A.M. The Pocket Guide to the DSM-5-TR™ Diagnostic Exam; American Psychiatric Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Beetz, A.; Uvnäs-Moberg, K.; Julius, H.; Kotrschal, K. Psychosocial and psychophysiological effects of human-animal interactions: The possible role of oxytocin. Front. Psychol. 2012, 3, 234. [Google Scholar] [CrossRef]
- Gehrke, E.K.; Baldwin, A.; Schiltz, P.M. Heart rate variability in horses engaged in equine-assisted activities. J. Equine. Vet. Sci. 2011, 31, 78–84. [Google Scholar] [CrossRef]
- Starkstein, S.E.; Fedoroff, J.P.; Price, T.R.; Leiguarda, R.; Robinson, R.G. Apathy following cerebrovascular lesions. Stroke 1993, 24, 1625–1630. [Google Scholar] [CrossRef]
- Okada, K.; Kobayashi, S.; Aoki, K.; Suyama, N.; Yamaguchi, S. Assessment of motivational loss in poststroke patients using the Japanese version of Starkstein’s Apathy Scale. Jpn. J. Stroke 1998, 20, 318–323. [Google Scholar] [CrossRef]
- Furlan, P.M.; DeMartinis, N.; Schweizer, E.; Rickels, K.; Lucki, I. Abnormal salivary cortisol levels in social phobic patients in response to acute psychological but not physical stress. Biol. Psychiatry 2001, 50, 254–259. [Google Scholar] [CrossRef]
- Kudielka, B.M.; Buske-Kirschbaum, A.; Hellhammer, D.H.; Kirschbaum, C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology 2004, 29, 83–98. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Pellerin, L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Peter, T.F.; Marcus, E.R. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 1986, 83, 1140–1144. [Google Scholar] [CrossRef]
- Michael, S.P.; Brian, C.W.; Douglas, R.W. The propagation of optical radiation in tissue. Ⅱ: Optical properties of tissues and resulting fluence distributions. Lasers Med. Sci. 1991, 6, 379–390. [Google Scholar]
- Singh, A.K.; Okamoto, M.; Dan, H.; Jurcak, V.; Dan, I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage 2005, 27, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Yamazaki, C.; Asano, K.; Ohe, S.; Ishida, M. Non-randomized controlled trial examining the effects of livestock on motivation and anxiety in patients with chronic psychiatric disorders. SAGE Open Med. 2023, 11, 20503121231175291. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.H. On some physiological actions of ergot. J. Physiol. 1906, 34, 163–206. [Google Scholar] [CrossRef]
- Vigneaud, V.D.; Ressler, C.; Swan, C.J.M.; Roberts, C.W.; Katsoyannis, P.G.; Gordon, S. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J. Am. Chem. Soc. 1953, 75, 4879–4880. [Google Scholar] [CrossRef]
- Skuse, D.H.; Gallagher, L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn. Sci. 2009, 13, 27–35. [Google Scholar] [CrossRef]
- Steinman, M.Q.; Duque-Wilckens, N.; Trainor, B.C. Complementary neural circuits for divergent effects of oxytocin: Social approach versus social anxiety. Biol. Psychiatry 2019, 85, 792–801. [Google Scholar] [CrossRef]
- Domes, G.; Heinrichs, M.; Michel, A.; Berger, C.; Herpertz, S.C. Oxytocin improves “mind-reading” in humans. Biol. Psychiatry 2007, 61, 731–733. [Google Scholar] [CrossRef]
- Lischke, A.; Berger, C.; Prehn, K.; Heinrichs, M.; Herpertz, S.C.; Domes, G. Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology 2012, 37, 475–481. [Google Scholar] [CrossRef]
- Marsh, A.A.; Yu, H.H.; Pine, D.S.; Blair, R.J. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology 2010, 209, 225–232. [Google Scholar] [CrossRef]
- Yamasue, H.; Okada, T.; Munesue, T.; Kuroda, M.; Fujioka, T.; Uno, Y.; Matsumoto, K.; Kuwabara, H.; Mori, D.; Okamoto, Y.; et al. Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: A randomized clinical trial. Mol. Psychiatry 2020, 25, 1849–1858. [Google Scholar] [CrossRef]
- Jesso, S.; Morlog, D.; Ross, S.; Pell, M.D.; Pasternak, S.H.; Mitchell, D.G.; Kertesz, A.; Finger, E.C.; Finger, E.C. The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain 2011, 134, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Donadon, M.F.; Martin-Santos, R.; Osório, F.L. The associations between oxytocin and trauma in humans: A systematic review. Front. Pharmacol. 2018, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Shamay-Tsoory, S.G.; Abu-Akel, A. The social salience hypothesis of oxytocin. Biol. Psychiatry 2016, 79, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Izawa, S.; Suzuki, K. The comparison of salivary cortisol immunoassay kits: Correlations between salivary and plasma cortisol concentrations and comparison of immunoassay methods. Jpn. J. Complement. Altern. Med. 2007, 4, 113–118. [Google Scholar] [CrossRef][Green Version]
- LaFrance, C.; Garcia, L.J.; Labreche, J. The effect of a therapy dog on the communication skills of an adult with aphasia. J. Commun. Disord. 2007, 40, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Izawa, S.; Miki, K.; Tsuchiya, M.; Mitani, T.; Midorikawa, T.; Fuchu, T.; Komatsu, T.; Togo, F.; Togo, F. Cortisol level measurements in fingernails as a retrospective index of hormone production. Psychoneuroendocrinology 2015, 54, 24–30. [Google Scholar] [CrossRef]
- Smith, E.E.; Jonides, J. Working memory: A view from neuroimaging. Cogn. Psychol. 1997, 33, 5–42. [Google Scholar] [CrossRef]
- Miller, E.K. The prefrontal cortex: Complex neural properties for complex behavior. Neuron 1999, 22, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Kraepelin, E. Dementia Praecox and Paraphrenia; Robert, D., Ed.; Krieger Publishing: New York, NY, USA, 1919. [Google Scholar]
- Desimone, R. Neural mechanisms for visual memory and their role in attention. Proc. Natl. Acad. Sci. USA 1996, 93, 13494–13499. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Tomita, H.; Ohbayashi, M.; Nakahara, K.; Hasegawa, I.; Miyashita, Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature 1999, 401, 699–703. [Google Scholar] [CrossRef]
- Berman, K.F.; Zec, R.F.; Weinberger, D.R. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia, II: Role of neuroleptic treatment, attention, and mental effort. Arch. Gen. Psychiatry 1986, 43, 126–135. [Google Scholar] [CrossRef]
- Yoon, J.H.; Minzenberg, M.J.; Ursu, S.; Walters, R.; Wendelken, C.; Ragland, J.D.; Carter, C.S. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: Relationship with impaired cognition, behavioral disorganization, and global function. Am. J. Psychiatry 2008, 165, 1006–1014. [Google Scholar] [CrossRef]
- Weinberger, D.R.; Berman, K.F.; Zec, R.F. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia: I. Regional cerebral blood flow evidence. Arch. Gen. Psychiatry 1986, 43, 114–124. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Kramer, A.F.; McAuley, E.; Erickson, K.I.; Scalf, P. Neurocognitive aging and cardiovascular fitness: Recent findings and future directions. J. Mol. Neurosci. 2004, 24, 9–14. [Google Scholar] [CrossRef]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Raznahan, A.; Toro, R.; Daly, E.; Robertson, D.; Murphy, C.; Deeley, Q.; Bolton, P.F.; Paus, T.; Murphy, D.G. Cortical anatomy in autism spectrum disorder: An in vivo MRI study on the effect of age. Cereb. Cortex. 2010, 20, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, H.; Omori, M.; Munesue, T.; Ishitobi, M.; Matsumura, Y.; Takahashi, T.; Narita, K.; Murata, T.; Saito, D.N.; Uchiyama, H.; et al. Smaller insula and inferior frontal volumes in young adults with pervasive developmental disorders. Neuroimage 2010, 50, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Barnea-Goraly, N.; Kwon, H.; Menon, V.; Eliez, S.; Lotspeich, L.; Reiss, A.L. White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biol. Psychiatry 2004, 55, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Deeley, Q.; Murphy, D. Pathophysiology of autism: Evidence from brain imaging. Br. J. Hosp. Med. 2009, 70, 138–142. [Google Scholar] [CrossRef]
- Dapretto, M.; Davies, M.S.; Pfeifer, J.H.; Scott, A.A.; Sigman, M.; Bookheimer, S.Y.; Iacoboni, M. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006, 9, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Fick, K.M. The influence of an animal on social interactions of nursing home residents in a group setting. Am. J. Occup. Ther. 1993, 47, 529–534. [Google Scholar] [CrossRef]
- Macauley, B.L. Animal-assisted therapy for persons with aphasia: A pilot study. J. Rehabil. Res. Dev. 2006, 43, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Cuaya, L.V.; Hernández-Pérez, R.; Concha, L. Our faces in the dog’s brain: Functional imaging reveals temporal cortex activation during perception of human faces. PLoS ONE 2016, 11, e0149431. [Google Scholar] [CrossRef] [PubMed]




| No. | Apathy Scale Questions |
|---|---|
| 1 | Are you interested in learning new things? |
| 2 | Do you have any interests? |
| 3 | Are you concerned about your condition? |
| 4 | Do you put much effort into things? |
| 5 | Are you always looking for something to do? |
| 6 | Do you have plans or goals for the future? |
| 7 | Do you have motivation? |
| 8 | Do you have the energy for daily activities? |
| 9 | Does someone have to tell you what to do each day? |
| 10 | Are you indifferent to everything? |
| 11 | Are you unconcerned with many things? |
| 12 | Do you need a push to get started on things? |
| 13 | Are you neither happy nor sad, just in between? |
| 14 | Would you consider yourself apathetic? |
| Facility | Sex | Age | Disease | Medication | |
|---|---|---|---|---|---|
| 1 | A | Male | 75 | Schizophrenia | Risperidone |
| 2 | A | Female | 51 | Schizophrenia | Risperidone |
| 3 | A | Female | 34 | Extensive Developmental Disorder | Bronanserin, Sulpiride |
| 4 | A | Female | 37 | Schizophrenia | Zepreon, Abilify |
| 5 | A | Male | 50 | Schizophrenia | Aripiprazole |
| 6 | A | Male | 51 | Bipolar Mood Disorder | Risperidone, Lithium |
| 7 | A | Female | 73 | Schizophrenia | Risperidone |
| 8 | A | Female | 73 | Schizophrenia | Blonanserin |
| 9 | A | Female | 34 | Extensive Developmental Disorder | Bronanserin, Sulpiride |
| 10 | A | Male | 30 | Developmental Disorder | Strattera |
| 11 | A | Female | 49 | Schizophrenia | Risperidone, Serenace, Quetiapine |
| 12 | B | Male | 65 | Bipolar Disorder | Tegretol, Duloxetine capsules, Tsumura |
| 13 | B | Female | 49 | Bipolar Disorder | Olanzapine, Abilify |
| 14 | B | Female | 45 | Schizophrenia | Sobotril, Abilify |
| 15 | B | Female | 31 | Anxiety Disorders, Bipolar Disorder | Iron tablets, vitamins, zinc |
| 16 | B | Female | 34 | Autism Spectrum | Invega, Tegrelor, Lorazepam |
| 17 | B | Female | 64 | Depression | Sertraline, Abilify |
| 18 | B | male | 55 | Depression | Quetiapine, Rhythmic, Sodium valproate tablets |
| Facility A n = 11 | Facility B n = 7 | p Value *1 | ||||
|---|---|---|---|---|---|---|
| Unit | Mean Value | Standard Deviation | Mean Value | Standard Deviation | ||
| Age (years) | 50.64 | 16.62 | 49.00 | 13.43 | 0.785 | |
| Pre-intervention Oxytocin | (μg/dL) | 0.12 | 0.07 | 0.15 | 0.07 | 0.495 |
| Pre-intervention Cortisol | (pg/mL) | 315.88 | 252.72 | 276.78 | 349.55 | 0.051 |
| Pre-intervention Apathy Scale Score | 25.36 | 8.64 | 28.00 | 11.39 | 0.554 | |
| Pre-control Oxytocin | (μg/dL) | 0.16 | 0.11 | 0.18 | 0.07 | 0.315 |
| Pre-control Cortisol | (pg/mL) | 337.44 | 270.99 | 425.10 | 407.23 | 0.891 |
| Pre-control Apathy Scale Score | 25.60 | 8.45 | 26.86 | 7.82 | 0.768 | |
| Before Intervention | After Intervention | p Value *1 | ||||
|---|---|---|---|---|---|---|
| Item | Mean Value | Standard Deviation | Mean Value | Standard Deviation | ||
| Intervention Day Apathy Scale Score | 26.389 | 9.562 | 29.167 | 8.952 | 0.003 | |
| Intervention Day Cortisol | (μg/dL) | 0.133 | 0.070 | 0.129 | 0.140 | 0.435 |
| Intervention Day Oxytocin | (pg/mL) | 299.781 | 286.596 | 368.258 | 385.218 | 0.062 |
| Control Day Apathy Scale Score | 26.118 | 7.968 | 30.000 | 7.802 | 0.003 | |
| Control Day Cortisol | (μg/dL) | 0.164 | 0.097 | 0.126 | 0.093 | 0.055 |
| Control Day Oxytocin | (pg/mL) | 371.531 | 321.965 | 356.488 | 416.676 | 0.523 |
| Intervention Day | Control Day | p Value *1 | ||||
|---|---|---|---|---|---|---|
| Unit | Mean Value | Standard Deviation | Mean Value | Standard Deviation | ||
| Apathy Scale Score change | 2.778 | 3.439 | 3.882 | 4.498 | 0.531 | |
| Cortisol change | (μg/dL) | 0.001 | 0.103 | −0.039 | 0.097 | 0.379 |
| Oxytocin change | (pg/mL) | 80.475 | 155.626 | −15.043 | 265.990 | 0.093 |
| Subject | Sequence | Carryover Effect | Treatment Effect | ||||
|---|---|---|---|---|---|---|---|
| Apathy Scale | Cortisol Change | Oxytocin Change | Apathy Scale | Cortisol Change | Oxytocin Change | ||
| 1 | 1 (AB) | 12 | −0.24 | −760.85 | 2 | 0.04 | −500.45 |
| 2 | 1 (AB) | 10 | 0.14 | −41.25 | 0 | 0.18 | −53.25 |
| 3 | 1 (AB) | 1 | −0.1 | 28.81 | 1 | 0 | 106.95 |
| 4 | 1 (AB) | 4 | −0.01 | −84.6 | 0 | −0.03 | 89.38 |
| 5 | 1 (AB) | 2 | 0.09 | −63.58 | −2 | −0.01 | 63.58 |
| 6 | 1 (AB) | 0 | −0.04 | 186.45 | 2 | −0.04 | −291.37 |
| 7 | 1 (AB) | 3 | 0.02 | 171.67 | −1 | 0 | −102.47 |
| 8 | 1 (AB) | 6 | −0.21 | 69.26 | 4 | −0.25 | −146.8 |
| 9 | 1 (AB) | 5 | −0.21 | 49.89 | −5 | −0.21 | 49.89 |
| 10 | 1 (AB) | 8 | −0.06 | 102.66 | 8 | −0.04 | −203.12 |
| 11 | 1 (AB) | 12 | 0.1 | 51.35 | −4 | −0.2 | −58.55 |
| 12 | 2 (BA) | 1 | −0.11 | 12.1 | 1 | −0.03 | −32.08 |
| 13 | 2 (BA) | 2 | −0.07 | −8.81 | 4 | −0.07 | 17.09 |
| 14 | 2 (BA) | 18 | 0.03 | 699.79 | −14 | 0.03 | −35.21 |
| 15 | 2 (BA) | 6 | −0.12 | −162.45 | −6 | 0.02 | 223.59 |
| 16 | 2 (BA) | 21 | −0.12 | 16.64 | 3 | 0.00 | 84.00 |
| 17 | 2 (BA) | 3 | −0.17 | −313.41 | 7 | −0.01 | 522.65 |
| 18 | 2 (BA) | 2 | 0.45 | 1143.67 | −6 | 0.17 | −187.41 |
| Brain Coordinate Measurement Position | Oxygen Hemoglobin Concentration (Integral) | Mann–Whitney U p-Value | |
|---|---|---|---|
| Channel 1 | Intervention | −57,010.491 | 0.458 |
| Control | −28,992.879 | ||
| Channel 2 | Intervention | −29,273.356 | 0.652 |
| Control | −8469.174 | ||
| Channel 3 | Intervention | −14,776.647 | 0.852 |
| Control | −18,856.741 | ||
| Channel 4 | Intervention | 30,126.428 | 0.032 |
| Control | −58,048.605 | ||
| Channel 5 | Intervention | −2770.151 | 0.942 |
| Control | 1090.629 | ||
| Channel 6 | Intervention | 15,648.397 | 0.270 |
| Control | −8267.619 | ||
| Channel 7 | Intervention | 6226.593 | 0.553 |
| Control | −19,568.200 | ||
| Channel 8 | Intervention | −27,745.876 | 0.691 |
| Control | −5306.442 | ||
| Channel 9 | Intervention | −83,291.332 | 0.151 |
| Control | −8779.836 | ||
| Channel 10 | Intervention | −53,775.030 | 0.568 |
| Control | −23,492.277 | ||
| Channel 11 | Intervention | 37,053.522 | 0.452 |
| Control | 14,198.655 | ||
| Channel 12 | Intervention | −51,526.818 | 0.280 |
| Control | 2286.230 | ||
| Channel 13 | Intervention | 9789.809 | 0.808 |
| Control | 1629.656 | ||
| Channel 14 | Intervention | 18,595.649 | 0.978 |
| Control | 17,685.441 | ||
| Channel 15 | Intervention | −1839.110 | 0.443 |
| Control | −18,314.892 | ||
| Channel 16 | Intervention | 33,583.087 | 0.045 |
| Control | −20,404.002 | ||
| Channel 17 | Intervention | 117.952 | 0.733 |
| Control | −17,721.914 | ||
| Channel 18 | Intervention | 3192.267 | 0.577 |
| Control | −8242.235 | ||
| Channel 19 | Intervention | −3593.690 | 0.715 |
| Control | −8797.640 | ||
| Channel 20 | Intervention | 16,986.230 | 0.330 |
| Control | 1771.079 | ||
| Channel 21 | Intervention | −21,777.784 | 0.467 |
| Control | 787.291 | ||
| Channel 22 | Intervention | −25,666.015 | 0.745 |
| Control | −12,234.070 | ||
| Channel 23 | Intervention | 31,754.014 | 0.098 |
| Control | −13,519.307 | ||
| Channel 24 | Intervention | 17,887.825 | 0.645 |
| Control | −170.636 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, N.; Ohe, S.; Asano, K.; Ishida, M. Effects of a One-Day Experiential Sheep-Rearing Experience on Motivation, Anxiety, and Frontal Lobe Brain Activity in Patients with Chronic Psychiatric Disorders: A Crossover Pilot Study. Psychiatry Int. 2024, 5, 134-153. https://doi.org/10.3390/psychiatryint5020010
Shimizu N, Ohe S, Asano K, Ishida M. Effects of a One-Day Experiential Sheep-Rearing Experience on Motivation, Anxiety, and Frontal Lobe Brain Activity in Patients with Chronic Psychiatric Disorders: A Crossover Pilot Study. Psychiatry International. 2024; 5(2):134-153. https://doi.org/10.3390/psychiatryint5020010
Chicago/Turabian StyleShimizu, Nobuko, Shingo Ohe, Keigo Asano, and Motohiko Ishida. 2024. "Effects of a One-Day Experiential Sheep-Rearing Experience on Motivation, Anxiety, and Frontal Lobe Brain Activity in Patients with Chronic Psychiatric Disorders: A Crossover Pilot Study" Psychiatry International 5, no. 2: 134-153. https://doi.org/10.3390/psychiatryint5020010
APA StyleShimizu, N., Ohe, S., Asano, K., & Ishida, M. (2024). Effects of a One-Day Experiential Sheep-Rearing Experience on Motivation, Anxiety, and Frontal Lobe Brain Activity in Patients with Chronic Psychiatric Disorders: A Crossover Pilot Study. Psychiatry International, 5(2), 134-153. https://doi.org/10.3390/psychiatryint5020010






