The GSK3-NRF2 Axis in Suicide
Abstract
1. Introduction
2. NRF2 Overview
3. Suicide Overview
4. GSK3 Activity in Suicide
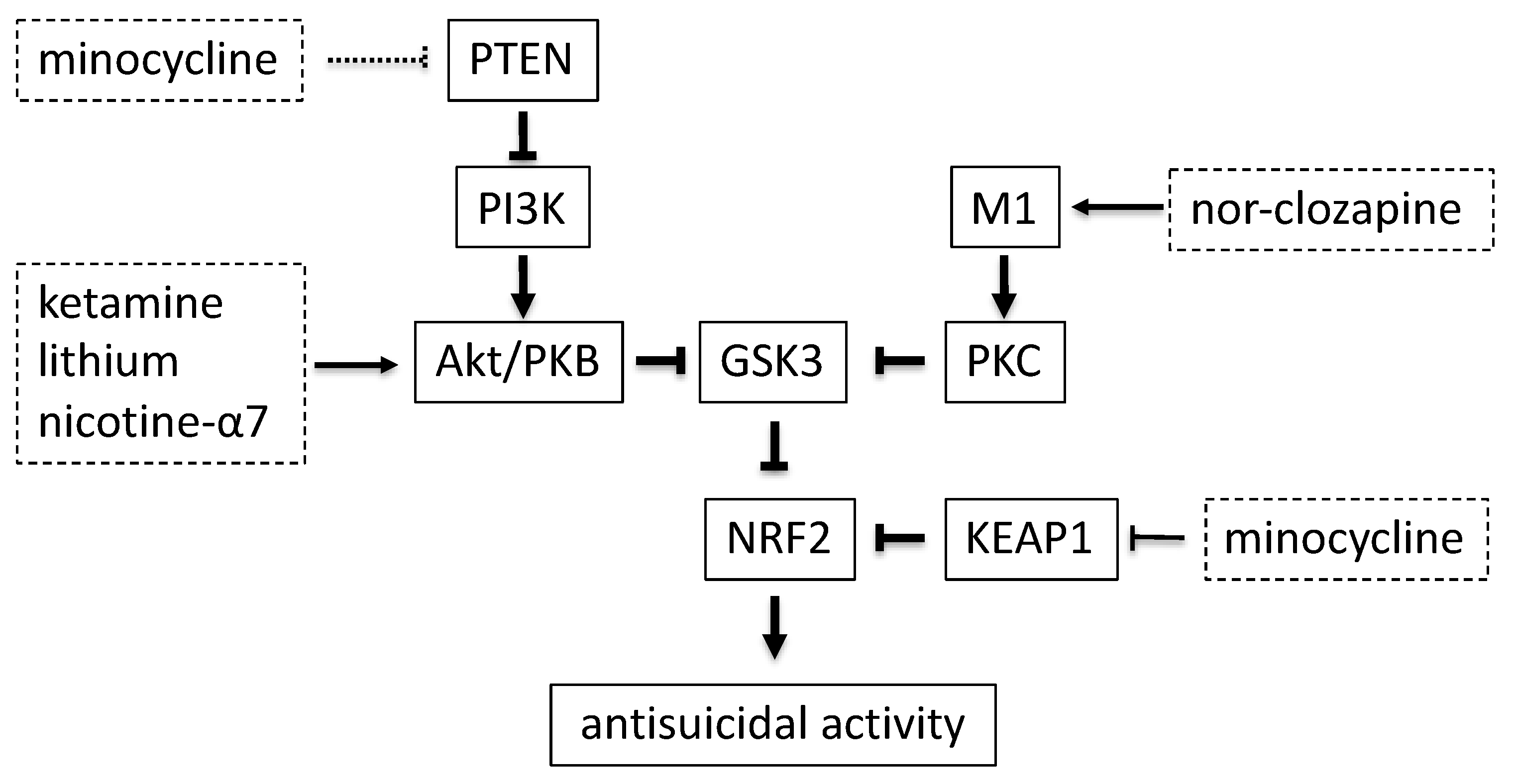
5. Therapeutic Effect of GSK3 Inhibition
6. KEAP1 Modulation by Minocycline
7. Discussion
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pandey, G.N.; Sharma, A.; Rizavi, H.S.; Ren, X. Dysregulation of protein kinase c in adult depression and suicide: Evidence from postmortem brain studies. Int. J. Neuropsychopharmacol. 2021. [Google Scholar] [CrossRef]
- Bentley, K.H.; Franklin, J.C.; Ribeiro, J.D.; Kleiman, E.M.; Fox, K.R.; Nock, M.K. Anxiety and its disorders as risk factors for suicidal thoughts and behaviors: A meta-analytic review. Clin. Psychol. Rev. 2016, 43, 30–46. [Google Scholar] [CrossRef]
- Ribeiro, J.D.; Franklin, J.C.; Fox, K.R.; Bentley, K.H.; Kleiman, E.M.; Chang, B.P.; Nock, M.K. Self-injurious thoughts and behaviors as risk factors for future suicide ideation, attempts, and death: A meta-analysis of longitudinal studies. Psychol. Med. 2016, 46, 225–236. [Google Scholar] [CrossRef]
- Garcia de la Garza, A.; Blanco, C.; Olfson, M.; Wall, M.M. Identification of suicide attempt risk factors in a national us survey using machine learning. JAMA Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Nock, M.K.; Charney, D.S.; Mathew, S.J. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry 2009, 66, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Pretty, H.; Hawton, K.; Geddes, J.R. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: A systematic review of randomized trials. Am. J. Psychiatry 2005, 162, 1805–1819. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y.; Alphs, L.; Green, A.I.; Altamura, A.C.; Anand, R.; Bertoldi, A.; Bourgeois, M.; Chouinard, G.; Islam, M.Z.; Kane, J.; et al. Clozapine treatment for suicidality in schizophrenia: International suicide prevention trial (intersept). Arch. Gen. Psychiatry 2003, 60, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; Leon, R.; Lopez, M.G.; Oliva, B.; et al. Transcription factor nrf2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the nrf2 and keap1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Withers, C.M.; Bartz, R.R.; MacGarvey, N.C.; Fu, P.; Sweeney, T.E.; Welty-Wolf, K.E.; Suliman, H.B. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J. Biol. Chem. 2011, 286, 16374–16385. [Google Scholar] [CrossRef]
- Egea, J.; Buendia, I.; Parada, E.; Navarro, E.; Leon, R.; Lopez, M.G. Anti-inflammatory role of microglial alpha7 nachrs and its role in neuroprotection. Biochem. Pharmacol. 2015, 97, 463–472. [Google Scholar] [CrossRef]
- Navarro, E.; Gonzalez-Lafuente, L.; Perez-Liebana, I.; Buendia, I.; Lopez-Bernardo, E.; Sanchez-Ramos, C.; Prieto, I.; Cuadrado, A.; Satrustegui, J.; Cadenas, S.; et al. Heme-oxygenase i and pcg-1alpha regulate mitochondrial biogenesis via microglial activation of alpha7 nicotinic acetylcholine receptors using pnu282987. Antioxid. Redox Signal. 2017, 27, 93–105. [Google Scholar] [CrossRef]
- Hashimoto, K. Essential role of keap1-nrf2 signaling in mood disorders: Overview and future perspective. Front. Pharmacol. 2018, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Rojo, A.I.; Chowdhry, S.; McMahon, M.; Hayes, J.D.; Cuadrado, A. Scf/{beta}-trcp promotes glycogen synthase kinase 3-dependent degradation of the nrf2 transcription factor in a keap1-independent manner. Mol. Cell. Biol. 2011, 31, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobon-Velasco, J.C.; Devijver, H.; Garcia-Mayoral, M.F.; Van Leuven, F.; et al. Structural and functional characterization of nrf2 degradation by the glycogen synthase kinase 3/beta-trcp axis. Mol. Cell. Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, H.K.; Shin, J.H.; Lee, J.K. Up-down regulation of ho-1 and inos gene expressions by ethyl pyruvate via recruiting p300 to nrf2 and depriving it from p65. Free Radic. Biol. Med. 2013, 65, 468–476. [Google Scholar] [CrossRef]
- Placidi, G.P.; Oquendo, M.A.; Malone, K.M.; Huang, Y.Y.; Ellis, S.P.; Mann, J.J. Aggressivity, suicide attempts, and depression: Relationship to cerebrospinal fluid monoamine metabolite levels. Biol. Psychiatry 2001, 50, 783–791. [Google Scholar] [CrossRef]
- Brezo, J.; Paris, J.; Vitaro, F.; Hebert, M.; Tremblay, R.E.; Turecki, G. Predicting suicide attempts in young adults with histories of childhood abuse. Br. J. Psychiatry 2008, 193, 134–139. [Google Scholar] [CrossRef]
- Ballard, E.D.; Ionescu, D.F.; Vande Voort, J.L.; Niciu, M.J.; Richards, E.M.; Luckenbaugh, D.A.; Brutsche, N.E.; Ameli, R.; Furey, M.L.; Zarate, C.A., Jr. Improvement in suicidal ideation after ketamine infusion: Relationship to reductions in depression and anxiety. J. Psychiatr. Res. 2014, 58, 161–166. [Google Scholar] [CrossRef]
- Wiebenga, J.X.; Eikelenboom, M.; Heering, H.D.; van Oppen, P.; Penninx, B.W. Suicide ideation versus suicide attempt: Examining overlapping and differential determinants in a large cohort of patients with depression and/or anxiety. Aust. N. Z. J. Psychiatry 2021, 55, 167–179. [Google Scholar] [CrossRef]
- Nock, M.K.; Hwang, I.; Sampson, N.A.; Kessler, R.C. Mental disorders, comorbidity and suicidal behavior: Results from the national comorbidity survey replication. Mol. Psychiatry 2010, 15, 868–876. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.S.; Kim, D.J.; Hong, S.C.; Choi, K.H.; Oh, Y.; Wang, S.M.; Lee, H.K.; Kweon, Y.S.; Lee, C.T.; et al. Characteristic risk factors associated with planned versus impulsive suicide attempters. Clin. Psychopharmacol. Neurosci. 2015, 13, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Gradus, J.L.; Qin, P.; Lincoln, A.K.; Miller, M.; Lawler, E.; Sorensen, H.T.; Lash, T.L. Posttraumatic stress disorder and completed suicide. Am. J. Epidemiol. 2010, 171, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Fox, V.; Dalman, C.; Dal, H.; Hollander, A.C.; Kirkbride, J.B.; Pitman, A. Suicide risk in people with post-traumatic stress disorder: A cohort study of 3.1 million people in sweden. J. Affect. Disord. 2021, 279, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Oswald, P.; Souery, D.; Kasper, S.; Lecrubier, Y.; Montgomery, S.; Wyckaert, S.; Zohar, J.; Mendlewicz, J. Current issues in bipolar disorder: A critical review. Eur. Neuropsychopharmacol. 2007, 17, 687–695. [Google Scholar] [CrossRef]
- Sharma, T.; Guski, L.S.; Freund, N.; Gotzsche, P.C. Suicidality and aggression during antidepressant treatment: Systematic review and meta-analyses based on clinical study reports. BMJ 2016, 352, i65. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Bielau, H.; Brisch, R.; Danos, P.; Ullrich, O.; Mawrin, C.; Bernstein, H.G.; Bogerts, B. Immunological aspects in the neurobiology of suicide: Elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 2008, 42, 151–157. [Google Scholar] [CrossRef]
- Falcone, T.; Fazio, V.; Lee, C.; Simon, B.; Franco, K.; Marchi, N.; Janigro, D. Serum s100b: A potential biomarker for suicidality in adolescents? PLoS ONE 2010, 5, e11089. [Google Scholar] [CrossRef]
- Webb, R.T.; Kontopantelis, E.; Doran, T.; Qin, P.; Creed, F.; Kapur, N. Suicide risk in primary care patients with major physical diseases: A case-control study. Arch. Gen. Psychiatry 2012, 69, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Lim, C.K.; Linderholm, K.R.; Janelidze, S.; Lindqvist, D.; Samuelsson, M.; Lundberg, K.; Postolache, T.T.; Traskman-Bendz, L.; Guillemin, G.J.; et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 2013, 38, 743–752. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, A.; Rush, G.; Hoatam, G.; Hughes, B.M.; McCrohan, A.; Kelleher, C.; O’Farrelly, C.; Malone, K.M. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anxiety 2013, 30, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Black, C.; Miller, B.J. Meta-analysis of cytokines and chemokines in suicidality: Distinguishing suicidal versus nonsuicidal patients. Biol. Psychiatry 2015, 78, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, R.S.; Kelly, K.M.; Mezuk, B. Inflammation as a unique marker of suicide ideation distinct from depression syndrome among U.S. adults. J. Affect. Disord. 2019, 245, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Brundin, L.; Erhardt, S.; Bryleva, E.Y.; Achtyes, E.D.; Postolache, T.T. The role of inflammation in suicidal behaviour. Acta Psychiatr. Scand. 2015, 132, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Jope, R.S. Inflammation and lithium: Clues to mechanisms contributing to suicide-linked traits. Transl. Psychiatry 2014, 4, e488. [Google Scholar] [CrossRef]
- Lund-Sorensen, H.; Benros, M.E.; Madsen, T.; Sorensen, H.J.; Eaton, W.W.; Postolache, T.T.; Nordentoft, M.; Erlangsen, A. A nationwide cohort study of the association between hospitalization with infection and risk of death by suicide. JAMA Psychiatry 2016, 73, 912–919. [Google Scholar] [CrossRef]
- Arling, T.A.; Yolken, R.H.; Lapidus, M.; Langenberg, P.; Dickerson, F.B.; Zimmerman, S.A.; Balis, T.; Cabassa, J.A.; Scrandis, D.A.; Tonelli, L.H.; et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J. Nerv. Ment. Dis. 2009, 197, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Okusaga, O.; Langenberg, P.; Sleemi, A.; Vaswani, D.; Giegling, I.; Hartmann, A.M.; Konte, B.; Friedl, M.; Groer, M.W.; Yolken, R.H.; et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia. Schizophr. Res. 2011, 133, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Groer, M.; Beckie, T. New findings: Depression, suicide, and toxoplasma gondii infection. J. Am. Assoc. Nurse Pract. 2014, 26, 629–637. [Google Scholar] [CrossRef]
- Calati, R.; Courtet, P.; Norton, J.; Ritchie, K.; Artero, S. Association between lifetime headache and history of suicide attempts in the elderly. Eur. Psychiatry 2017, 41, 132–139. [Google Scholar] [CrossRef]
- Messias, E.; Clarke, D.E.; Goodwin, R.D. Seasonal allergies and suicidality: Results from the national comorbidity survey replication. Acta Psychiatr. Scand. 2010, 122, 139–142. [Google Scholar] [CrossRef]
- Jeon-Slaughter, H.; Claassen, C.A.; Khan, D.A.; Mihalakos, P.; Lee, K.B.; Brown, E.S. Temporal association between nonfatal self-directed violence and tree and grass pollen counts. J. Clin. Psychiatry 2016, 77, 1160–1167. [Google Scholar] [CrossRef]
- Chung, J.H.; Kim, S.H.; Lee, Y.W. Suicidal ideation and suicide attempts among asthma. Ann. Gen. Psychiatry 2016, 15, 35. [Google Scholar] [CrossRef]
- Tonelli, L.H.; Stiller, J.; Rujescu, D.; Giegling, I.; Schneider, B.; Maurer, K.; Schnabel, A.; Moller, H.J.; Chen, H.H.; Postolache, T.T. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr. Scand. 2008, 117, 198–206. [Google Scholar] [CrossRef]
- Fegg, M.; Kraus, S.; Graw, M.; Bausewein, C. Physical compared to mental diseases as reasons for committing suicide: A retrospective study. BMC Palliat. Care 2016, 15, 14. [Google Scholar] [CrossRef]
- Torres, M.E.; Lowe, B.; Schmitz, S.; Pienta, J.N.; Van Der Feltz-Cornelis, C.; Fiedorowicz, J.G. Suicide and suicidality in somatic symptom and related disorders: A systematic review. J. Psychosom. Res. 2021, 140, 110290. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Klinitzke, G.; Brahler, E.; Kersting, A. Extreme obesity is associated with suicidal behavior and suicide attempts in adults: Results of a population-based representative sample. Depress. Anxiety 2013, 30, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Glader, E.L.; Norrving, B.; Asplund, K. Poststroke suicide attempts and completed suicides: A socioeconomic and nationwide perspective. Neurology 2015, 84, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Fralick, M.; Thiruchelvam, D.; Tien, H.C.; Redelmeier, D.A. Risk of suicide after a concussion. CMAJ 2016, 188, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, L.; Siebert, S.; McInnes, I.B.; Cavanagh, J. Rheumatoid arthritis and depression: An inflammatory perspective. Lancet Psychiatry 2019, 6, 164–173. [Google Scholar] [CrossRef]
- Finkelsztejn, A.; Fragoso, Y.D.; Ferreira, M.L.; Lana-Peixoto, M.A.; Alves-Leon, S.V.; Gomes, S.; Damasceno, B.P.; Mendes, M.F.; Salgado, P.R.; Correa, E.C.; et al. The brazilian database on pregnancy in multiple sclerosis. Clin. Neurol. Neurosurg. 2011, 113, 277–280. [Google Scholar] [CrossRef]
- Lindqvist, D.; Janelidze, S.; Hagell, P.; Erhardt, S.; Samuelsson, M.; Minthon, L.; Hansson, O.; Bjorkqvist, M.; Traskman-Bendz, L.; Brundin, L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry 2009, 66, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Isung, J.; Aeinehband, S.; Mobarrez, F.; Nordstrom, P.; Runeson, B.; Asberg, M.; Piehl, F.; Jokinen, J. High interleukin-6 and impulsivity: Determining the role of endophenotypes in attempted suicide. Transl. Psychiatry 2014, 4, e470. [Google Scholar] [CrossRef] [PubMed]
- Gananca, L.; Oquendo, M.A.; Tyrka, A.R.; Cisneros-Trujillo, S.; Mann, J.J.; Sublette, M.E. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology 2016, 63, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Sublette, M.E.; Galfalvy, H.C.; Fuchs, D.; Lapidus, M.; Grunebaum, M.F.; Oquendo, M.A.; Mann, J.J.; Postolache, T.T. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav. Immun. 2011, 25, 1272–1278. [Google Scholar] [CrossRef]
- Parrott, J.M.; O’Connor, J.C. Kynurenine 3-monooxygenase: An influential mediator of neuropathology. Front. Psychiatry 2015, 6, 116. [Google Scholar] [CrossRef]
- Carlborg, A.; Jokinen, J.; Jonsson, E.G.; Erhardt, S.; Nordstrom, P. Csf kynurenic acid and suicide risk in schizophrenia spectrum psychosis. Psychiatry Res. 2013, 205, 165–167. [Google Scholar] [CrossRef]
- Courtet, P.; Jaussent, I.; Genty, C.; Dupuy, A.M.; Guillaume, S.; Ducasse, D.; Olie, E. Increased crp levels may be a trait marker of suicidal attempt. Eur. Neuropsychopharmacol. 2015, 25, 1824–1831. [Google Scholar] [CrossRef]
- Gibbs, H.M.; Davis, L.; Han, X.; Clothier, J.; Eads, L.A.; Caceda, R. Association between c-reactive protein and suicidal behavior in an adult inpatient population. J. Psychiatr. Res. 2016, 79, 28–33. [Google Scholar] [CrossRef]
- Fiori, L.M.; Bureau, A.; Labbe, A.; Croteau, J.; Noel, S.; Merette, C.; Turecki, G. Global gene expression profiling of the polyamine system in suicide completers. Int. J. Neuropsychopharmacol. 2011, 14, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Le-Niculescu, H.; Levey, D.F.; Ayalew, M.; Palmer, L.; Gavrin, L.M.; Jain, N.; Winiger, E.; Bhosrekar, S.; Shankar, G.; Radel, M.; et al. Discovery and validation of blood biomarkers for suicidality. Mol. Psychiatry 2013, 18, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Schnieder, T.P.; Trencevska, I.; Rosoklija, G.; Stankov, A.; Mann, J.J.; Smiley, J.; Dwork, A.J. Microglia of prefrontal white matter in suicide. J. Neuropathol. Exp. Neurol. 2014, 73, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.E.; Mars, B.; Wen, C.P.; Chang, S.S.; Gunnell, D. Evidence for an association between inflammatory markers and suicide: A cohort study based on 359,849 to 462,747 taiwanese adults. J. Affect. Disord. 2021, 281, 967–971. [Google Scholar] [CrossRef]
- Wu, S.; Ding, Y.; Wu, F.; Xie, G.; Hou, J.; Mao, P. Serum lipid levels and suicidality: A meta-analysis of 65 epidemiological studies. J. Psychiatry Neurosci. 2016, 41, 56–69. [Google Scholar] [CrossRef]
- Suneson, K.; Asp, M.; Traskman-Bendz, L.; Westrin, A.; Ambrus, L.; Lindqvist, D. Low total cholesterol and low-density lipoprotein associated with aggression and hostility in recent suicide attempters. Psychiatry Res. 2019, 273, 430–434. [Google Scholar] [CrossRef]
- Kulak-Bejda, A.; Bejda, G.; Lech, M.; Waszkiewicz, N. Are lipids possible markers of suicide behaviors? J. Clin. Med. 2021, 10, 333. [Google Scholar] [CrossRef]
- Tatro, E.T.; Everall, I.P.; Masliah, E.; Hult, B.J.; Lucero, G.; Chana, G.; Soontornniyomkij, V.; Achim, C.L.; Center, H.I.V.N.R. Differential expression of immunophilins fkbp51 and fkbp52 in the frontal cortex of hiv-infected patients with major depressive disorder. J. Neuroimmune Pharmacol. 2009, 4, 218–226. [Google Scholar] [CrossRef]
- Lekman, M.; Laje, G.; Charney, D.; Rush, A.J.; Wilson, A.F.; Sorant, A.J.; Lipsky, R.; Wisniewski, S.R.; Manji, H.; McMahon, F.J.; et al. The fkbp5-gene in depression and treatment response--an association study in the sequenced treatment alternatives to relieve depression (star*d) cohort. Biol. Psychiatry 2008, 63, 1103–1110. [Google Scholar] [CrossRef]
- Willour, V.L.; Chen, H.; Toolan, J.; Belmonte, P.; Cutler, D.J.; Goes, F.S.; Zandi, P.P.; Lee, R.S.; MacKinnon, D.F.; Mondimore, F.M.; et al. Family-based association of fkbp5 in bipolar disorder. Mol. Psychiatry 2009, 14, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Brent, D.; Melhem, N.; Ferrell, R.; Emslie, G.; Wagner, K.D.; Ryan, N.; Vitiello, B.; Birmaher, B.; Mayes, T.; Zelazny, J.; et al. Association of fkbp5 polymorphisms with suicidal events in the treatment of resistant depression in adolescents (tordia) study. Am. J. Psychiatry 2010, 167, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Gorodetsky, E.; Yuan, Q.; Goldman, D.; Enoch, M.A. Interaction of fkbp5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology 2010, 35, 1674–1683. [Google Scholar] [CrossRef]
- Supriyanto, I.; Sasada, T.; Fukutake, M.; Asano, M.; Ueno, Y.; Nagasaki, Y.; Shirakawa, O.; Hishimoto, A. Association of fkbp5 gene haplotypes with completed suicide in the japanese population. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Diaz, Y.; Gonzalez-Castro, T.B.; Tovilla-Zarate, C.A.; Juarez-Rojop, I.E.; Lopez-Narvaez, M.L.; Perez-Hernandez, N.; Rodriguez-Perez, J.M.; Genis-Mendoza, A.D. Association between polymorphisms of fkbp5 gene and suicide attempt in a mexican population: A case-control study. Brain Res. Bull. 2021, 166, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.C.; Verrier, J.D.; Semple-Rowland, S.; Kumar, A.; Foster, T.C. Phlpp1 splice variants differentially regulate akt and pkcalpha signaling in hippocampal neurons: Characterization of phlpp proteins in the adult hippocampus. J. Neurochem. 2010, 115, 941–955. [Google Scholar] [CrossRef]
- Mistafa, O.; Ghalali, A.; Kadekar, S.; Hogberg, J.; Stenius, U. Purinergic receptor-mediated rapid depletion of nuclear phosphorylated akt depends on pleckstrin homology domain leucine-rich repeat phosphatase, calcineurin, protein phosphatase 2a, and pten phosphatases. J. Biol. Chem. 2010, 285, 27900–27910. [Google Scholar] [CrossRef]
- O’Neill, A.K.; Niederst, M.J.; Newton, A.C. Suppression of survival signalling pathways by the phosphatase phlpp. FEBS J. 2013, 280, 572–583. [Google Scholar] [CrossRef]
- Hsiung, S.C.; Adlersberg, M.; Arango, V.; Mann, J.J.; Tamir, H.; Liu, K.P. Attenuated 5-ht1a receptor signaling in brains of suicide victims: Involvement of adenylyl cyclase, phosphatidylinositol 3-kinase, akt and mitogen-activated protein kinase. J. Neurochem. 2003, 87, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Perroud, N.; Burkhardt, S.; Schwald, M.; Ballmann, E.; La Harpe, R.; Malafosse, A. Alteration in kinase activity but not in protein levels of protein kinase b and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol. Psychiatry 2007, 61, 240–245. [Google Scholar] [CrossRef]
- Pandey, G.N.; Dwivedi, Y.; Rizavi, H.S.; Ren, X.; Conley, R.R. Decreased catalytic activity and expression of protein kinase c isozymes in teenage suicide victims: A postmortem brain study. Arch. Gen. Psychiatry 2004, 61, 685–693. [Google Scholar] [CrossRef][Green Version]
- Ren, X.; Rizavi, H.S.; Khan, M.A.; Dwivedi, Y.; Pandey, G.N. Altered wnt signalling in the teenage suicide brain: Focus on glycogen synthase kinase-3beta and beta-catenin. Int. J. Neuropsychopharmacol. 2013, 16, 945–955. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zill, P.; Buttner, A.; Eisenmenger, W.; Moller, H.J.; Bondy, B.; Ackenheil, M. Single nucleotide polymorphism and haplotype analysis of a novel tryptophan hydroxylase isoform (tph2) gene in suicide victims. Biol. Psychiatry 2004, 56, 581–586. [Google Scholar] [CrossRef]
- Bach-Mizrachi, H.; Underwood, M.D.; Kassir, S.A.; Bakalian, M.J.; Sibille, E.; Tamir, H.; Mann, J.J.; Arango, V. Neuronal tryptophan hydroxylase mrna expression in the human dorsal and median raphe nuclei: Major depression and suicide. Neuropsychopharmacology 2006, 31, 814–824. [Google Scholar] [CrossRef]
- Lopez de Lara, C.; Brezo, J.; Rouleau, G.; Lesage, A.; Dumont, M.; Alda, M.; Benkelfat, C.; Turecki, G. Effect of tryptophan hydroxylase-2 gene variants on suicide risk in major depression. Biol. Psychiatry 2007, 62, 72–80. [Google Scholar] [CrossRef]
- Lopez, V.A.; Detera-Wadleigh, S.; Cardona, I.; Kassem, L.; McMahon, F.J.; National Institute of Mental Health Genetics Initiative Bipolar Disorder Consortium. Nested association between genetic variation in tryptophan hydroxylase ii, bipolar affective disorder, and suicide attempts. Biol. Psychiatry 2007, 61, 181–186. [Google Scholar] [CrossRef]
- Yoon, H.K.; Kim, Y.K. Tph2 -703g/t snp may have important effect on susceptibility to suicidal behavior in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 403–409. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Yuan, G.; Yao, J.; Cheng, Z.; Liu, C.; Liu, Q.; Wan, G.; Shi, G.; Cheng, Y.; et al. Effect of tryptophan hydroxylase-2 rs7305115 snp on suicide attempts risk in major depression. Behav. Brain Funct. 2010, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, T.; Pregelj, P.; Tomori, M.; Komel, R.; Paska, A.V. Tph2 polymorphisms and alcohol-related suicide. Neurosci. Lett. 2011, 490, 78–81. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Zhang, X.; Rodriguiz, R.M.; Sotnikova, T.D.; Cools, M.J.; Wetsel, W.C.; Gainetdinov, R.R.; Caron, M.G. Role of gsk3 beta in behavioral abnormalities induced by serotonin deficiency. Proc. Natl. Acad. Sci. USA 2008, 105, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Chalecka-Franaszek, E.; Chuang, D.M. Lithium activates the serine/threonine kinase akt-1 and suppresses glutamate-induced inhibition of akt-1 activity in neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 8745–8750. [Google Scholar] [CrossRef]
- Zhang, F.; Phiel, C.J.; Spece, L.; Gurvich, N.; Klein, P.S. Inhibitory phosphorylation of glycogen synthase kinase-3 (gsk-3) in response to lithium. Evidence for autoregulation of gsk-3. J. Biol. Chem. 2003, 278, 33067–33077. [Google Scholar] [CrossRef]
- Freland, L.; Beaulieu, J.M. Inhibition of gsk3 by lithium, from single molecules to signaling networks. Front. Mol. Neurosci. 2012, 5, 14. [Google Scholar] [CrossRef]
- Salazar, M.; Rojo, A.I.; Velasco, D.; de Sagarra, R.M.; Cuadrado, A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor nrf2. J. Biol. Chem. 2006, 281, 14841–14851. [Google Scholar] [CrossRef]
- Rojo, A.I.; Rada, P.; Egea, J.; Rosa, A.O.; Lopez, M.G.; Cuadrado, A. Functional interference between glycogen synthase kinase-3 beta and the transcription factor nrf2 in protection against kainate-induced hippocampal cell death. Mol. Cell. Neurosci. 2008, 39, 125–132. [Google Scholar] [CrossRef]
- Rizak, J.; Tan, H.; Zhu, H.; Wang, J.F. Chronic treatment with the mood-stabilizing drug lithium up-regulates nuclear factor e2-related factor 2 in rat pheochromocytoma pc12 cells in vitro. Neuroscience 2014, 256, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Espada, S.; Rojo, A.I.; Salinas, M.; Cuadrado, A. The muscarinic m1 receptor activates nrf2 through a signaling cascade that involves protein kinase c and inhibition of gsk-3beta: Connecting neurotransmission with neuroprotection. J. Neurochem. 2009, 110, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Kang, U.G.; Seo, M.S.; Roh, M.S.; Kim, Y.; Yoon, S.C.; Kim, Y.S. The effects of clozapine on the gsk-3-mediated signaling pathway. FEBS Lett. 2004, 560, 115–119. [Google Scholar] [CrossRef]
- Sur, C.; Mallorga, P.J.; Wittmann, M.; Jacobson, M.A.; Pascarella, D.; Williams, J.B.; Brandish, P.E.; Pettibone, D.J.; Scolnick, E.M.; Conn, P.J. N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates n-methyl-d-aspartate receptor activity. Proc. Natl. Acad. Sci. USA 2003, 100, 13674–13679. [Google Scholar] [CrossRef]
- Zanos, P.; Gould, T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Sanacora, G.; Duman, R.S.; Krystal, J.H. The neurobiology of depression, ketamine and rapid-acting antidepressants: Is it glutamate inhibition or activation? Pharmacol. Ther. 2018, 190, 148–158. [Google Scholar] [CrossRef]
- Beurel, E.; Song, L.; Jope, R.S. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol. Psychiatry 2011, 16, 1068–1070. [Google Scholar] [CrossRef]
- Zhou, W.; Dong, L.; Wang, N.; Shi, J.Y.; Yang, J.J.; Zuo, Z.Y.; Zhou, Z.Q. Akt mediates gsk-3beta phosphorylation in the rat prefrontal cortex during the process of ketamine exerting rapid antidepressant actions. Neuroimmunomodulation 2014, 21, 183–188. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, Z.Q.; Gao, Z.Q.; Shi, J.Y.; Yang, J.J. Acute increases in plasma mammalian target of rapamycin, glycogen synthase kinase-3beta, and eukaryotic elongation factor 2 phosphorylation after ketamine treatment in three depressed patients. Biol.Psychiatry 2013, 73, e35–e36. [Google Scholar] [CrossRef]
- Hoetzel, A.; Schmidt, R. Regulatory role of anesthetics on heme oxygenase-1. Curr. Drug Targets 2010, 11, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Zunszain, P.A.; Horowitz, M.A.; Cattaneo, A.; Lupi, M.M.; Pariante, C.M. Ketamine: Synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol. Psychiatry 2013, 18, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Parada, E.; Egea, J.; Buendia, I.; Negredo, P.; Cunha, A.C.; Cardoso, S.; Soares, M.P.; Lopez, M.G. The microglial alpha7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid. Redox Signal. 2013, 19, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Rosito, M.; Testi, C.; Parisi, G.; Cortese, B.; Baiocco, P.; Di Angelantonio, S. Exploring the use of dimethyl fumarate as microglia modulator for neurodegenerative diseases treatment. Antioxidants 2020, 9, 700. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, X.; Guo, S.; Ma, L.; Huang, W.; Zhao, X. Minocycline attenuates sevoflurane-induced cell injury via activation of nrf2. Int. J. Mol. Med. 2017, 39, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Scofield, V.L.; Yan, M.; Stoica, G.; Liu, N.; Wong, P.K. Attenuation of oxidative stress, inflammation and apoptosis by minocycline prevents retrovirus-induced neurodegeneration in mice. Brain Res. 2009, 1286, 174–184. [Google Scholar] [CrossRef]
- Shahzad, K.; Bock, F.; Al-Dabet, M.M.; Gadi, I.; Nazir, S.; Wang, H.; Kohli, S.; Ranjan, S.; Mertens, P.R.; Nawroth, P.P.; et al. Stabilization of endogenous nrf2 by minocycline protects against nlrp3-inflammasome induced diabetic nephropathy. Sci. Rep. 2016, 6, 34228. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Zarzuelo, A.; Galvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Reis, D.J.; Casteen, E.J.; Ilardi, S.S. The antidepressant impact of minocycline in rodents: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox regulation by nrf2 in aging and disease. Free Radic. Biol. Med. 2019, 134, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, P.N.; Anish, P.K.; George, B. Risk factors for suicide in elderly in comparison to younger age groups. Indian J. Psychiatry 2015, 57, 249–254. [Google Scholar] [CrossRef]
- Shah, A. The relationship between suicide rates and age: An analysis of multinational data from the world health organization. Int. Psychogeriatr. 2007, 19, 1141–1152. [Google Scholar] [CrossRef]
- Xu, J.; Donepudi, A.C.; More, V.R.; Kulkarni, S.R.; Li, L.; Guo, L.; Yan, B.; Chatterjee, T.; Weintraub, N.; Slitt, A.L. Deficiency in nrf2 transcription factor decreases adipose tissue mass and hepatic lipid accumulation in leptin-deficient mice. Obesity 2015, 23, 335–344. [Google Scholar] [CrossRef]
- Rowe, A.R.; Mercer, L.; Casetti, V.; Sendt, K.V.; Giaroli, G.; Shergill, S.S.; Tracy, D.K. Dementia praecox redux: A systematic review of the nicotinic receptor as a target for cognitive symptoms of schizophrenia. J. Psychopharmacol. 2015, 29, 197–211. [Google Scholar] [CrossRef]
- Rojo, A.I.; Rada, P.; Mendiola, M.; Ortega-Molina, A.; Wojdyla, K.; Rogowska-Wrzesinska, A.; Hardisson, D.; Serrano, M.; Cuadrado, A. The pten/nrf2 axis promotes human carcinogenesis. Antioxid. Redox Signal. 2014, 21, 2498–2514. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Singh, R.; Parhar, I.; Kuhad, A.; Soga, T. Quinolinic acid and nuclear factor erythroid 2-related factor 2 in depression: Role in neuroprogression. Front. Pharmacol. 2019, 10, 452. [Google Scholar] [CrossRef]
- Bjork, J.M.; Dougherty, D.M.; Moeller, F.G.; Swann, A.C. Differential behavioral effects of plasma tryptophan depletion and loading in aggressive and nonaggressive men. Neuropsychopharmacology 2000, 22, 357–369. [Google Scholar] [CrossRef]
- Mann, J.J.; Currier, D.; Stanley, B.; Oquendo, M.A.; Amsel, L.V.; Ellis, S.P. Can biological tests assist prediction of suicide in mood disorders? Int. J. Neuropsychopharmacol. 2006, 9, 465–474. [Google Scholar] [CrossRef]
- Hood, S.; Amir, S. Biological clocks and rhythms of anger and aggression. Front. Behav. Neurosci. 2018, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, J.; Meerlo, P.; Koolhaas, J.M.; Lancel, M. Poor sleep as a potential causal factor in aggression and violence. Sleep Med. 2012, 13, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, R.C.; Barnes, J.C.; Hay, C. Sleep deprivation, low self-control, and delinquency: A test of the strength model of self-control. J. Youth Adolesc. 2015, 44, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Stanley, M.; Brown, G.M. Melatonin levels are reduced in the pineal glands of suicide victims. Psychopharmacol. Bull. 1988, 24, 484–488. [Google Scholar]
- Sandyk, R.; Awerbuch, G.I. Nocturnal melatonin secretion in suicidal patients with multiple sclerosis. Int. J. Neurosci. 1993, 71, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Levey, D.F.; Niculescu, E.M.; Le-Niculescu, H.; Dainton, H.L.; Phalen, P.L.; Ladd, T.B.; Weber, H.; Belanger, E.; Graham, D.L.; Khan, F.N.; et al. Towards understanding and predicting suicidality in women: Biomarkers and clinical risk assessment. Mol. Psychiatry 2016, 21, 768–785. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kern, J.T.; Walker, J.R.; Johnson, J.A.; Schultz, P.G.; Luesch, H. A genomic screen for activators of the antioxidant response element. Proc. Natl. Acad. Sci. USA 2007, 104, 5205–5210. [Google Scholar] [CrossRef] [PubMed]
| Inflammatory Process | Citation for Role in Suicide |
|---|---|
| Hospitalization for infection (HIV, hepatitis, respiratory tract, sepsis) | [40] |
| Seropositivity to Toxoplasma gondii | [41,42,43] |
| Physical and sexual abuse | [22] |
| Life time headache | [44] |
| Allergy to tree- and grass pollen | [45,46] |
| Asthma | [47] |
| Brain IL-4 and IL-13 mRNA | [48] |
| Lung disease, cancer, chronic pain | [33,49] |
| Coronary artery disease, osteoporosis | [33] |
| Somatic disorders | [50] |
| Extreme obesity | [51] |
| Stroke | [52] |
| Concussion | [53] |
| Rheumatoid arthritis | [54] |
| IFNβ treatment in multiple sclerosis | [55] |
| IL-6 in CSF and serum (dose-dependent) | [35,56,57,58] |
| Elevated Kynurenine and Quinolinic acid in plasma and CSF | [34,59,60,61] |
| CRP levels in MDD (dose-dependent) | [62,63] |
| High SAT1 mRNA in brain and PBMCs | [64,65] |
| Juxtavascular microglia activation (IBA1) | [31,66] |
| High serum S100β | [32] |
| High white blood cell count | [67] |
| Lipid abnormalities (low total-cholesterol, low LDL, low HDL) | [68,69,70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalkman, H.O. The GSK3-NRF2 Axis in Suicide. Psychiatry Int. 2021, 2, 108-119. https://doi.org/10.3390/psychiatryint2010008
Kalkman HO. The GSK3-NRF2 Axis in Suicide. Psychiatry International. 2021; 2(1):108-119. https://doi.org/10.3390/psychiatryint2010008
Chicago/Turabian StyleKalkman, Hans O. 2021. "The GSK3-NRF2 Axis in Suicide" Psychiatry International 2, no. 1: 108-119. https://doi.org/10.3390/psychiatryint2010008
APA StyleKalkman, H. O. (2021). The GSK3-NRF2 Axis in Suicide. Psychiatry International, 2(1), 108-119. https://doi.org/10.3390/psychiatryint2010008




