Exploring the Association between Cannabis Use and Testosterone Levels in Men Receiving Methadone Maintenance Treatment
Abstract
1. Introduction
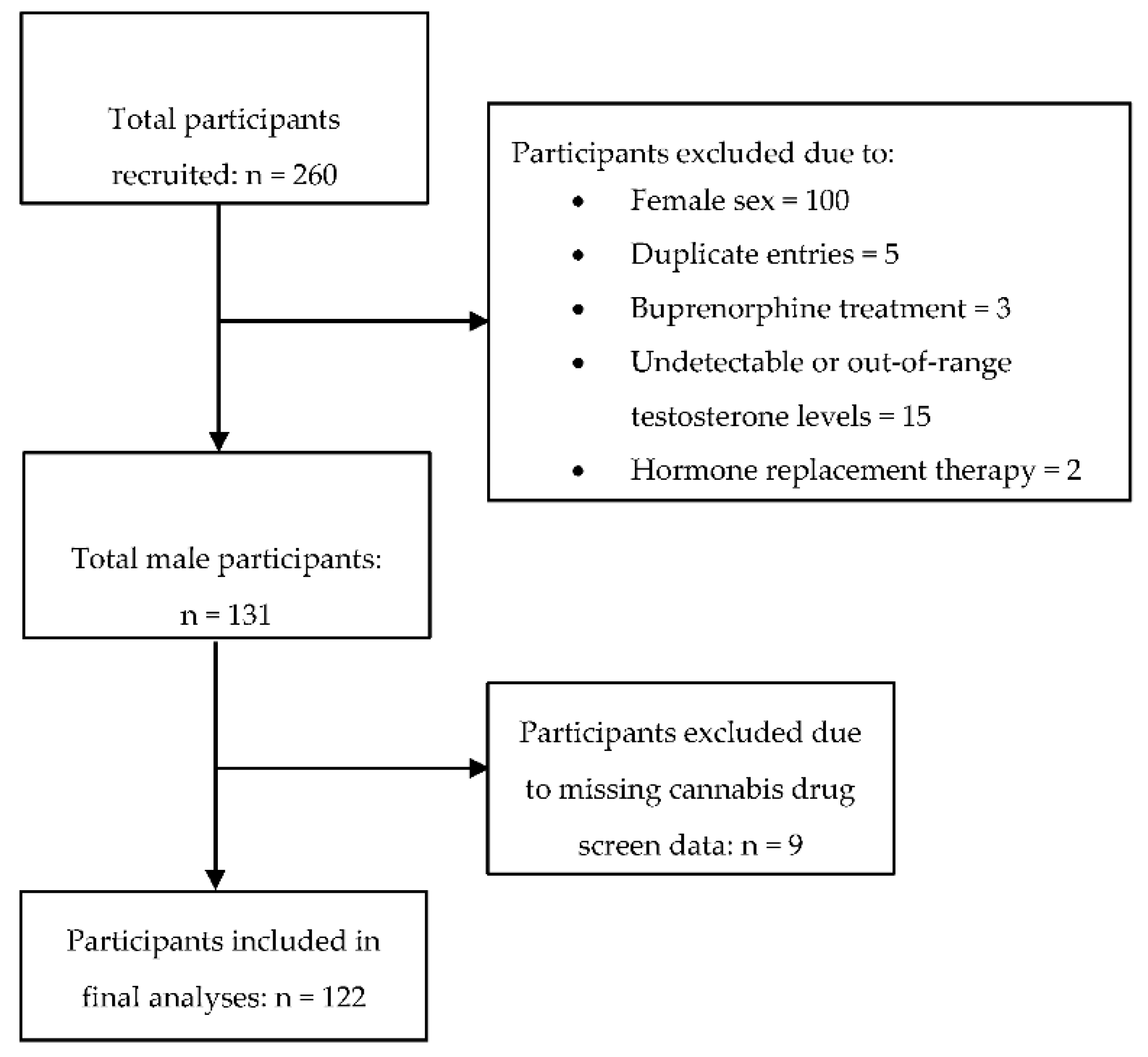
2. Materials and Methods
2.1. Data and Outcome Measures
2.2. Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Factors Associated with Serum Testosterone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vineet, T.; Michael, S.; Richard, S.Y.; Frank, A.L.; Loren, W.G. Revisiting the role of testosterone: Are we missing something? Rev. Urol. 2017, 19, 16–24. [Google Scholar]
- O’Rourke, T.K.; Wosnitzer, M.S. Opioid-Induced Androgen Deficiency (OPIAD): Diagnosis, management, and literature review. Curr. Urol. Rep. 2016, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Bawor, M.; Dennis, B.B.; Samaan, M.C.; Plater, C.; Worster, A.; Varenbut, M.; Daiter, J.; Marsh, D.C.; Desai, D.; Steiner, M.; et al. Methadone induces testosterone suppression in patients with opioid addiction. Sci. Rep. 2014, 4, 6189. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, T.D.; Jørgensen, N.; Andersson, A.; Bang, A.K.; Nordkap, L.; Skakkebæk, N.E.; Priskorn, L.; Juul, A.; Jensen, T.K. Association between use of marijuana and male reproductive hormones and semen quality: A study among 1,215 healthy young men. Am. J. Epidemiol. 2015, 182, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Thistle, J.E.; Graubard, B.I.; Braunlin, M.; Vesper, H.; Trabert, B.; Cook, M.B.; McGlynn, K.A. Marijuana use and serum testosterone concentrations among U.S. males. Andrology 2017, 5, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Nassan, F.L.; Arvizu, M.; Mínguez-Alarcón, L.; Williams, P.L.; Attaman, J.; Petrozza, J.; Hauser, R.; Chavarro, J.; EARTH Study Team. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum. Reprod. 2019, 34, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.; Clavijo, R.I. Adverse effects of cannabis on male reproduction. Eur. Urol. Focus 2018, 4, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Reisfield, G.M.; Wasan, A.D.; Jamison, R.N. The prevalence and significance of cannabis use in patients prescribed chronic opioid therapy: A Review of the Extant Literature. Pain Med. 2009, 10, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, L.; Bhatt, M.; Sanger, N.; Plater, C.; Worster, A.; Varenbut, M.; Daiter, J.; Pare, G.; Marsh, D.C.; Desai, D.; et al. Association between cannabis use and methadone maintenance treatment outcomes: An investigation into sex differences. Biol. Sex Differ. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Rosic, T.; Naji, L.; Bawor, M.; Dennis, B.B.; Plater, C.; Marsh, D.C.; Thabane, L.; Samaan, Z. The impact of comorbid psychiatric disorders on methadone maintenance treatment in opioid use disorder: A prospective cohort study. Neuropsychiatr. Dis. Treat. 2017, 13, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Analysis of Trends in the Prevalence of Cannabis Use and Related Metrics in Canada. Government of Canada, Statistics Canada. Available online: https://www150.statcan.gc.ca/n1/pub/82-003-x/2019006/article/00001-eng.htm (accessed on 16 August 2020).
- United Nations. World Drug Report 2019 (Booklet 5); United Nations Publication: Vienna, Austria, 2019. [Google Scholar]
- Cannabis. World Health Organization. Available online: https://www.who.int/substance_abuse/facts/cannabis/en/ (accessed on 16 August 2020).
- Bawor, M.; Bami, H.; Dennis, B.B.; Plater, C.; Worster, A.; Varenbut, M.; Daiter, J.; Marsh, D.C.; Steiner, M.; Anglin, R.; et al. Testosterone suppression in opioid users: A systematic review and meta-analysis. Drug Alcohol Depend. 2015, 149, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Samaan, Z.; Bawor, M.; Dennis, B.B.; Plater, C.; Varenbut, M.; Daiter, J.; Worster, A.; Marsh, D.C.; Tan, C.; Desai, D.; et al. Genetic influence on methadone treatment outcomes in patients undergoing methadone maintenance treatment for opioid addiction: A pilot study. Neuropsychiatr. Dis. Treat. 2014, 10, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, A.; Stridsberg, M.; Gordh, T. Opioid Endocrinopathy: A clinical problem in patients with chronic pain and long-term oral opioid treatment. Clin. J. Pain 2010, 26, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Leung, J.Y.Y.; Lin, S.L.; Schooling, C.M. Cigarette smoking and testosterone in men and women: A systematic review and meta-analysis of observational studies. Prev. Med. 2016, 85, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Beattie, M.C.; Adekola, L.; Papadopoulos, V.; Chen, H.; Zirkin, B.R. Leydig cell aging and hypogonadism. Exp. Gerontol. 2015, 68, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.; Popa, F.I.; Ferigo, M.; Clari, G.; Foresta, C. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J. Clin. Endocrinol. Metab. 2005, 90, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Rottermann, M.; Langlois, K. Health Reports Prevalence and correlates of marijuana use in Canada, 2012. Health Rep. 2015, 26, 10–15. [Google Scholar]
| Characteristics | Cannabis Non-User (n = 58, 47.5%) | Cannabis User a (n = 64, 52.5%) | p-Value |
|---|---|---|---|
| Age, years; mean (SD) | 40.1 (11.0) | 37.6 (11.0) | 0.212 |
| BMI, mean (SD) | 27.3 (4.2) | 27.0 (5.6) | 0.757 |
| Ethnicity | |||
| European, n (%) | 47 (81.0%) | 58 (90.6%) | 0.127 |
| Indigenous, n (%) | 5 (8.6%) | 3 (4.7%) | 0.381 |
| Other b, n (%) | 6 (10.3%) | 3 (4.7%) | 0.233 |
| Socioeconomic Factors | |||
| Married/common law; n (%) | 24 (41.4%) | 26 (40.6%) | 0.933 * |
| Completed post-secondary education; n (%) | 18 (31.0%) | 23 (35.9%) | 0.170 |
| Employment; n (%) | 18 (31.0%) | 23 (35.9%) | 0.567 |
| Opioid Use History | |||
| Age of initial opioid use in years; mean (SD) | 24.9 (10.6) | 22.1 (9.4) | 0.117 |
| Methadone dose (mg); mean (SD) | 89.8 (60.4) | 92.5 (73.6) | 0.828 |
| Duration on MMT (months); mean (SD) | 44.2 (41.1) | 37.7 (38.0) | 0.377 |
| Percentage of opioid-positive urine drug screens; mean (SD) | 17.9 (22.2) | 14.3 (18.4) | 0.341 |
| Substance use history | |||
| Number of cigarettes smoked/day; mean (SD) | 15.4 (10.0) | 19.5 (13.7) | 0.062 |
| Polysubstance use; n (%) | 20 (35.1%) | 33 (51.6%) | 0.068 |
| Current cigarette smokers; n (%) | 50 (86.2%) | 58 (90.6%) | 0.444 |
| Psychiatric comorbidity, self-reported; n (%) | 26 (44.8%) | 25 (39.1%) | 0.519 |
| Total drug screens; mean (SD) | 6.98 (3.71) | 7.08 (6.25) | 0.920 |
| Percentage of cannabis-positive urine drug screens; mean (SD) | 0.0 (0.0) | 79.5 (29.4) | <0.001 * |
| Serum Testosterone [ng/dL] (SD) | 88.2 (61.6) | 110.8 (83.9) | 0.096 |
| Unadjusted Analysis | Adjusted Analysis | |||||
|---|---|---|---|---|---|---|
| Covariate | Estimated Beta Coefficient [β] | 95% CI | p-Value | Estimated Beta Coefficient [β] | 95% CI | p-Value |
| Age | −0.008 | −0.020, 0.003 | 0.156 | −0.005 | −0.018, 0.008 | 0.423 |
| Age of initial opioid use | −0.004 | −0.017, 0.009 | 0.553 | - | - | - |
| Cigarettes per day | 0.008 | −0.003, 0.019 | 0.142 | 0.008 | −0.002, 0.018 | 0.133 |
| Methadone dose | −0.002 | −0.005, −0.001 | 0.004 * | −0.003 | −0.005, −0.001 | 0.003 * |
| Duration on MMT | >−0.001 | −0.004, 0.003 | 0.736 | 0.002 | −0.002, 0.005 | 0.270 |
| Illicit opioid use | 0.002 | −0.005, 0.008 | 0.611 | - | - | - |
| BMI | −0.025 | −0.051, 0.001 | 0.062 | −0.017 | −0.042, 0.009 | 0.199 |
| Percent of positive cannabis drug screens | 0.003 | <0.001, 0.006 | 0.035 * | 0.002 | >−0.001, 0.005 | 0.116 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, D.; Rosic, T.; Bawor, M.; Samaan, Z. Exploring the Association between Cannabis Use and Testosterone Levels in Men Receiving Methadone Maintenance Treatment. Psychiatry Int. 2020, 1, 67-74. https://doi.org/10.3390/psychiatryint1020008
Chai D, Rosic T, Bawor M, Samaan Z. Exploring the Association between Cannabis Use and Testosterone Levels in Men Receiving Methadone Maintenance Treatment. Psychiatry International. 2020; 1(2):67-74. https://doi.org/10.3390/psychiatryint1020008
Chicago/Turabian StyleChai, Darren, Tea Rosic, Monica Bawor, and Zainab Samaan. 2020. "Exploring the Association between Cannabis Use and Testosterone Levels in Men Receiving Methadone Maintenance Treatment" Psychiatry International 1, no. 2: 67-74. https://doi.org/10.3390/psychiatryint1020008
APA StyleChai, D., Rosic, T., Bawor, M., & Samaan, Z. (2020). Exploring the Association between Cannabis Use and Testosterone Levels in Men Receiving Methadone Maintenance Treatment. Psychiatry International, 1(2), 67-74. https://doi.org/10.3390/psychiatryint1020008





