Abstract
Progressive pulmonary fibrosis (PPF) is a clinical syndrome associated with worsening quality of life and increased mortality among patients with various interstitial lung diseases. This review aims to update the concepts and criteria that adequately define PPF, aiming to facilitate earlier recognition and optimize clinical management. Fibrosing interstitial lung disease (ILD-f) can progress over time despite optimal management of the underlying conditions. Current criteria for defining PPF include worsening respiratory symptoms, decline in pulmonary function tests (particularly forced vital capacity and diffusing capacity), and radiographic progression over a 1-year follow-up period. However, implementation of these criteria in clinical practice poses challenges. This review discusses the limitations of current evaluation methods and proposes future directions, including the need for validated symptom assessment tools, standardization of pulmonary function testing, and improvements in quantitative radiological evaluation methods.
1. Introduction
Interstitial lung diseases (ILDs) are a group of diseases with wide heterogeneity [1]. Idiopathic pulmonary fibrosis (IPF) is the most common and widely studied progressive ILD; other non-IPF ILDs can also exhibit a progressive fibrosing phenotype associated with a similarly poor prognosis, particularly when they develop progressive pulmonary fibrosis (PPF) [1,2].
PPF is characterized by worsening respiratory symptoms, declining lung function, and radiographic progression despite appropriate treatment of the underlying ILD [3]. Recognition of PPF is essential, as it can inform treatment decisions, alter prognosis, and guide the use of antifibrotic therapies [2]. This article provides a clinical perspective on PPF, focusing on the evolution of diagnostic criteria and their implications for clinical management and research. The increasing recognition of progressive pulmonary fibrosis (PPF) as a unifying clinical phenotype across different interstitial lung diseases (ILDs) has redefined the approach to diagnosis and management in recent years. Despite updated guidelines, heterogeneity in disease behavior and assessment tools continues to generate uncertainty in clinical decision-making. A deeper understanding of the clinical, functional, and radiological dimensions of PPF is necessary to optimize early detection and improve patient outcomes. Moreover, emerging evidence highlights the need to adopt more integrative models that reflect the complexity of disease progression beyond traditional classifications.
We hypothesize that an integrative approach to progressive pulmonary fibrosis (PPF)—encompassing standardized symptom assessment, optimized functional thresholds, and advanced imaging techniques—may enhance early diagnosis and therapeutic decision-making across diverse interstitial lung diseases. This perspective aims to propose a conceptual framework that supports the development of future clinical tools and research strategies.
Furthermore, this clinical perspective addresses current gaps in the application of PPF definitions in real-world settings, particularly in non-specialized centers where guideline implementation remains inconsistent. By integrating recent evidence with practical insights, this work aims to support clinicians facing uncertainties in diagnosis, follow-up timing, and therapeutic decisions.
2. Progressive Pulmonary Fibrosis (PPF): A New Concept
PPF is a term that describes pulmonary fibrosis that continues to progress—despite optimal therapy—regardless of the underlying cause [2].
Fibrosing ILDs may exhibit a progressive fibrosing phenotype with several features shared with IPF, including the potential for rapid pulmonary functional decline and poor prognosis [4]. A precise and early diagnosis is essential to ensure that each patient receives optimal treatment in a timely manner [5]. Although all ILDs have the potential to progress over time due to shared pathogenic mechanisms [5], the rate and pattern of progression can vary significantly [3].
PPF can develop in ILDs associated with connective tissue diseases (CTDs), including rheumatoid arthritis (RA), systemic sclerosis (SSc), inflammatory myopathies, Sjogren’s syndrome (SS), systemic lupus erythematosus (SEL), and mixed CTDs, as well as in hypersensitivity pneumonitis and pneumoconiosis (exposure to organic and inorganic substances respectively) [4,5]. Approximately 10% of progressive fibrosing lung diseases are classified as unclassifiable uILDs, in which clinical, radiological, or histopathological findings are inconclusive [4,5].
The concept of PPF is particularly important because it shifts the focus from the underlying etiology to the shared progressive behavior, potentially enabling more targeted therapeutic strategies across different ILDs [6]. This paradigm shift has led to recent clinical trials evaluating antifibrotic therapies in non-IPF ILDs, with significant slowing of disease progression [3].
3. Criteria for Defining Progressive Pulmonary Fibrosis
The current criteria for defining PPF include worsening respiratory symptoms, decreased lung function tests (specifically focusing on forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DLCO)), and observed radiological progression, manifest within 12 months [2], after excluding alternatives causes of decline [4].
In the latest clinical practice guidelines, PPF is defined using three basic criteria [2], which will discuss in detail below.
3.1. Worsening of Symptoms
The most common presenting symptoms in interstitial lung diseases are exertional dyspnea and cough [7]. However, there are currently no standardized, validated protocols to monitor symptom change. The assessment relies heavily on the clinician’s subjective evaluation and how patients report their symptoms during visits. The relationship between pulmonary function and Health-Related Quality of Life (HRQoL) is not linear—it may be influenced by other factors, such as diminished physiologic reserve and development of pulmonary hypertension [8].
Although symptom worsening is one of the three criteria defining PPF in recent guidelines, this criterion remains difficult to assess in a standardized manner. Symptom changes are given the same weight as functional and radiological progression [2], yet this approach may not fully capture the complexity of symptom trajectory. Among symptoms, dyspnea stands out as a critical prognostic indicator: higher dyspnea scores correlate with increased mortality and reduced survival time [7]. As ILD progresses, the development of comorbidities, increased symptom burden, and long-term oxygen therapy further con-tribute to declines in Health-Related Quality of Life [5,7].
The development and validation of symptom-tracking tools in ILD are urgently needed. These would enhance the precision and reproducibility of symptom progression monitoring, thereby reinforcing the diagnostic value of symptoms in PPF.
3.2. Functional Progression of the Disease
The current PPF definition specifies an absolute decrease in FVC > 5% and/or DLCO > 10%, based on extrapolating from IPF outcomes [2]. Studies have shown that a 5% absolute decline in FVC over 12 months is associated with increased risk of death or transplantation at two years [9].
The absolute decline in FVC is calculated by subtracting the final FVC measurement from the initial FVC measurement, while the relative decrease in FVC is calculated as the difference between the initial and final FVC divided by the initial FVC measurement [2] (Table 1).

Table 1.
An example of the comparative calculation of the absolute vs. relative decline in 1 year of FVC follow-up: a change from 60% predicted to 55% predicted represents a 5% absolute decline, but an 8.3% relative decline.
It is crucial to clarify which values, methods (relative or absolute), and time intervals are optimal for early identification of PPF using functional criteria [10]. The chosen method influences clinical decision-making. Using a relative decline greater than 10% in FVC increases the sensitivity and identifies more cases than an absolute measure. However, previous studies have shown that a 5% decrease in FVC over 12 months predicts transplant-free survival, emphasizing the importance of evaluating initial disease severity. Consequently, the absolute method is preferred for identifying decline under <10%, as it may better reflect clinically significant changes [11] (Table 1).
The choice of method for calculating FVC change significantly affects the frequency of detected decreases. This is of particular importance as FVC change is used as a measure of disease progression in clinical practice. The goal is to maximize the chances of identifying clinically significant changes without sacrificing prognostic accuracy [2].
The trajectory of lung function is influenced by several factors, such as the type of ILD, HRCT pattern, and baseline lung function. Short-term changes are heterogeneous and are strongly influenced by the method used [12]. From previous work in IPF we know that significant decreases in FVC carry a higher risk of death [13].
Richeldi et al., based on a study conducted in patients with IPF, demonstrated that the method used to calculate the change in FVC has a significant impact on the decrease at 12-month follow-up, showing that a relative decrease greater than 10% is preferable to calculating a 10% absolute decrease to evaluate the progression of the disease. The absolute method for calculating a greater than 10% decrease in FVC fails to identify almost half of the patients who are identified using the relative method as having clinically significant decline, which could lead to delays in important clinical decisions. It should be considered that the assessment of patients with preserved lung function or with mild alteration could result in underestimation using this method [11].
In addition to evaluating changes in FVC, DLCO values must be considered since the risk of mortality increases when we start from low values. It is mandatory to exclude alternative causes of worsening DLCO such as anemia, pulmonary embolism, pulmonary hypertension, and pulmonary edema, among others, before attributing any decrease to progressive fibrosis. Further evaluation, usually including HRCT, should be performed to rule out alternative causes of worsening DLCO [2].
3.3. Radiological Progression of the Disease
Current guidelines include radiographic progression as one of the diagnostic criteria for PPF, specifically the presence of one or more of the following radiological findings (Figure 1):
- Increased extent or severity of traction bronchiectasis and bronchiolectasis;
- New ground-glass opacity with traction bronchiectasis;
- New fine reticulation;
- Increased extent or increased coarseness of reticular abnormality;
- New or increased honeycombing;
- Increased lobar volume loss.
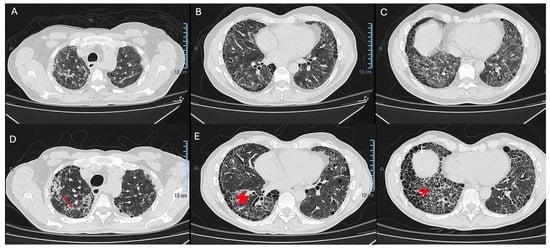
Figure 1.
Definitive UIP fibrotic pattern (A–C). Architectural distortion of the lung parenchyma with a heterogeneous temporal and spatial distribution, with subpleural reticulation, thickening of the interlobular septa, and apical–basal gradient. (D) Definitive UIP fibrotic pattern, with signs of progressive pulmonary fibrosis during one year of follow-up. (Red arrow) Increased extensive architectural distortion of the lung parenchyma, apex–basal gradient. (E) (Red star) Traction bronchiectasis–bronchiolectasis. (F) Volume loss and (red asterisk) exuberant honeycombing.
It is well established that pulmonary volume loss, the presence of a UIP pattern, traction bronchiectasis, and widespread fibrotic extension indicate a diffuse fibrosing process with a progressive course—independent of the underlying ILD diagnosis [14,15]. Therefore, a comprehensive radiological assessment is essential. However, this evaluation is significantly limited by inter-observer variability in objective quantification [4].
Implementing these criteria in practice presents a significant challenge in standardizing the quantification of new findings. Moreover, guidelines do not specify fixed radiological follow-up. Current guidelines recommend imaging when clinical or functional decline is suspected, after excluding other causes [2].
To address these limitations, computed tomography (CT) quantification has been combined with mathematical models to automatically stratify patients. This approach independently predicts mortality [2].
The visual determination of the progression of pulmonary fibrosis is based on the percentage of lung volume that contains fibrotic characteristics, where the extent of these is evaluated. It is important to consider that in non-IPF ILDs, the pattern of progression is variable [2].
Despite these advances, there remains a need for more standardized and objective methods of radiological assessment in PPF. Current research is focused on developing and validating quantitative CT analysis techniques that leverage artificial intelligence focusing on the analysis of lung textures to produce more precise, reproducible measures of disease progression [14].
Despite the central role of HRCT in evaluating disease progression, its use in clinical practice faces several limitations. Interobserver variability remains high, especially in differentiating early fibrotic changes or subtle increases in reticulation or ground-glass opacities. Moreover, the degree of progression needed to qualify as significant is not well standardized, leading to variability in clinical decisions across centers.
The prognostic implications of certain radiologic features, such as the presence and extent of honeycombing, traction bronchiectasis, and lobar volume loss, are well established. For instance, a definite UIP pattern is strongly associated with worse outcomes. However, in non-IPF ILDs, radiologic patterns are more heterogeneous (e.g., fibrotic NSIP, chronic HP), and progression may be more subtle or patchy, complicating detection.
Quantitative imaging techniques, including texture-based analysis and artificial intelligence (AI)-driven volumetric tools, are emerging as promising methods to provide objective and reproducible measures of disease progression. These tools allow for the automated calculation of fibrotic volume, lung density, and vascular metrics, and in some studies, have been shown to outperform visual assessment in predicting mortality. Nevertheless, their integration into routine practice remains limited by accessibility and validation in non-IPF cohorts [16].
Current guidelines recommend imaging follow-up when clinical or functional deterioration is suspected, but do not specify the optimal timing or frequency of imaging. This leads to inconsistent monitoring and may delay recognition of progression. Future efforts should aim to develop standardized protocols for imaging intervals and incorporate quantitative methods into clinical workflows.
4. An Integrative Perspective on PPF Evaluation and Future Directions
Based on the current evidence and the challenges discussed, we hypothesize that a multidimensional scoring system combining symptom trajectory, standardized functional thresholds (preferably absolute FVC changes), and AI-assisted quantitative imaging may offer a more robust framework for diagnosing and monitoring PPF. Such an integrative model could be the foundation for a prospective validation tool to guide earlier treatment decisions and optimize resource use (Table 2).

Table 2.
Evolution of diagnostic criteria for progressive pulmonary fibrosis (PPF): before and after 2022 guidelines [2,8].
5. Discussion
The concept of progressive pulmonary fibrosis (PPF) represents a paradigm shift in the evaluation and management of fibrosing interstitial lung diseases (ILDs). While recent guidelines have provided clearer diagnostic criteria, significant challenges remain in their implementation across diverse clinical settings (Table 2).
Symptom progression remains a subjective and under-standardized domain, often underestimated in its prognostic significance. Functional decline, especially changes in forced vital capacity (FVC), offers a more objective metric, yet debate continues over the superiority of relative versus absolute thresholds. Radiological progression, while critical, is affected by inter-observer variability and the absence of standardized imaging follow-up protocols.
In this context, we propose that future clinical tools incorporate a multidimensional model integrating patient-reported symptom outcomes, validated pulmonary function cutoffs, and AI-assisted imaging analysis. This integrative framework may improve early detection of PPF, facilitate more timely initiation of antifibrotic therapies, and standardize research outcomes across studies.
Additionally, differences in PPF behavior across ILD subtypes, such as connective tissue disease-associated ILD versus hypersensitivity pneumonitis, suggest that a “one-size-fits-all” approach may be insufficient. More research is needed to tailor diagnostic and therapeutic strategies to disease-specific trajectories.
This review emphasizes not only the current definitions and challenges of PPF but also outlines future directions aimed at refining clinical criteria and supporting personalized care approaches.
Despite growing consensus around the criteria for defining progressive pulmonary fibrosis (PPF), the implementation of these guidelines varies considerably between academic centers and community-based practices. Academic centers often have access to multidisciplinary teams, high-resolution imaging interpretation, pulmonary function laboratories, and experience in antifibrotic therapies. In contrast, community settings may face delays in diagnosis due to limited access to HRCT or lack of standardized follow-up protocols. Additionally, physicians may feel less confident applying subjective criteria such as symptom progression, and treatment decisions may be influenced by restricted availability of antifibrotic agents or reimbursement issues.
These disparities contribute to delayed recognition of PPF and suboptimal care in real-world settings. Therefore, our perspective highlights the urgent need to develop simplified, reproducible, and scalable tools that can be implemented beyond tertiary care hospitals. Furthermore, fostering collaborative networks between referral centers and community clinicians may enhance guideline adherence and improve patient outcomes.
Our clinical experience suggests that the current criteria for PPF, while conceptually clear, are difficult to operationalize in practice due to variability in symptom reporting, access to lung function testing, and non-standardized imaging protocols. These limitations often delay recognition of disease progression and timely initiation of antifibrotic therapy. This perspective seeks to bridge that gap by proposing practical considerations to refine implementation.
6. Conclusions
PPF represents more than just an isolated deterioration in patients with pre-existing ILD. It is a distinct disease trajectory associated with significantly higher mortality that requires early recognition and strategic adjustments in patient management. The ability to accurately and promptly diagnose PPF could significantly alter the disease course and patient outcomes. Further refinement of PPF criteria through future studies is crucial for improving prognosis in ILD management.
Current challenges in PPF diagnosis and management include
- The need for validated tools to objectively assess symptom progression;
- Standardization of methods for measuring and interpreting pulmonary function;
- Development of more reliable and reproducible quantitative CT analysis techniques;
- The establishment of optimal follow-up intervals for radiological evaluation.
Despite these challenges, the concept of PPF has already improved our understanding of fibrotic ILDs and opened new avenues for treatment. The use of antifibrotic therapies in non-IPF ILDs with a PPF criteria is a prime example of how this concept is changing clinical practice.
Beyond the individual assessment of clinical, functional, and radiologic parameters, we propose that future approaches to PPF should incorporate a multidimensional index that integrates symptom burden, validated FVC thresholds, and AI-assisted quantitative imaging. This would facilitate a more sensitive and specific identification of progression across ILD subtypes. Such an integrative model may serve not only to standardize PPF diagnosis but also to support earlier and more personalized therapeutic strategies.
Moving forward, a multidisciplinary approach combining clinical, functional, and radiological assessments remains crucial for accurate PPF diagnosis and management. Ongoing research into biomarkers and genetic factors may further enhance our ability to predict and monitor disease progression.
By highlighting the limitations of current criteria and proposing integrative assessment approaches, this perspective contributes to closing the gap between expert guidelines and daily clinical realities. It emphasizes the need for flexible, yet standardized tools that reflect the heterogeneity of ILDs and support timely, individualized care.
The current conceptual framework of progressive pulmonary fibrosis has directly influenced therapeutic decisions by expanding the use of antifibrotic agents beyond idiopathic pulmonary fibrosis, particularly in patients with chronic fibrosing interstitial lung diseases that demonstrate progression despite immunosuppressive treatment. Moreover, the identification of a progressive phenotype prompts earlier intervention and tighter monitoring, potentially improving outcomes by avoiding further irreversible lung damage.
In conclusion, while significant progress has been made in defining and understanding PPF, continued research and refinement of diagnostic criteria and their implementation in clinical workflows are essential. These efforts will improve outcomes for patients with fibrotic ILD.
Author Contributions
M.B.N.-S. wrote the manuscript, arranged the meetings with the reviewers. F.H.-G., manuscript reviewer. S.C.-C., manuscript reviewer. X.A.-R., manuscript reviewer. N.P.-R., manuscript reviewer. A.F.-G., manuscript reviewer. M.A.-V. wrote the manuscript, E.M.-R. wrote the manuscript. J.S., manuscript reviewer, senior author. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Acknowledgments
We want to thank all the authors, especially the senior author Jacobo Sellarés, for their great contributions, for their valuable support in providing resources and technical assistance. Their contribution has been essential in the completion of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ILD | Interstitial lung diseases |
| IPF | Idiopathic pulmonary fibrosis |
| PPF | Progressive pulmonary fibrosis |
| CTD | Connective tissue diseases |
| AR | Rheumatoid arthritis |
| SSc | Systemic sclerosis |
| SEL | Systemic lupus erythematosus |
| SS | Sjogren’s syndrome |
| UILDs | Unclassifiable interstitial lung diseases |
| CVF | Forced vital capacity |
| DLCO | Diffusing capacity of the lung for carbon monoxide |
| HRQoL | Health-Related Quality of Life |
| UIP | Usual interstitial pneumonia |
| HRCT | High-resolution chest tomography |
| CT | Computed tomography |
References
- Wijsenbeek, M.; Cottin, V. Spectrum of Fibrotic Lung Diseases. N. Engl. J. Med. 2020, 383, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Hirani, N.A.; Hotchkin, D.L.; Nambiar, A.M.; Ogura, T.; Otaola, M.; Skowasch, D.; Park, J.S.; Poonyagariyagorn, H.K.; Wuyts, W.; et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur. Respir. Rev. 2018, 27, 180076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cerro Chiang, G.; Parimon, T. Understanding Interstitial Lung Diseases Associated with Connective Tissue Disease (CTD-ILD): Genetics, Cellular Pathophysiology, and Biologic Drivers. Int. J. Mol. Sci. 2023, 24, 2405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajan, S.K.; Cottin, V.; Dhar, R.; Danoff, S.; Flaherty, K.R.; Brown, K.K.; Mohan, A.; Renzoni, E.; Mohan, M.; Udwadia, Z.; et al. Progressive pulmonary fibrosis: An expert group consensus statement. Eur. Respir. J. 2023, 61, 2103187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maher, T.M. Interstitial Lung Disease: A Review. JAMA 2024, 331, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.U.; Flaherty, K.R.; Brown, K.K.; Inoue, Y.; Devaraj, A.; Richeldi, L.; Moua, T.; Crestani, B.; Wuyts, W.A.; Stowasser, S.; et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: A randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir. Med. 2020, 8, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.Y.; Park, Y.S.; Choi, S.M.; Lee, J.H.; Park, J. Prognostic implication of 1-year decline in diffusing capacity in newly diagnosed idiopathic pulmonary fibrosis. Sci. Rep. 2024, 14, 8857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noboa-Sevilla, M.; Hernández-González, F.; Alsina-Restoy, X.; Pérez-Rodas, N.; Sellarés, J. Functional Criteria to Define Progressive Pulmonary Fibrosis: Searching for the Holy Grail. Am. J. Respir. Crit. Care Med. 2023, 207, 368–369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richeldi, L.; Ryerson, C.J.; Lee, J.S.; Wolters, P.J.; Koth, L.L.; Ley, B.; Elicker, B.M.; Jones, K.D.; King, T.E., Jr.; Ryu, J.H.; et al. Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax 2012, 67, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Oldham, J.M.; Lee, C.T.; Wu, Z.; Bowman, W.S.; Pugashetti, J.V.; Dao, N.; Tonkin, J.; Seede, H.; Echt, G.; Adegunsoye, A.; et al. Lung function trajectory in progressive fibrosing interstitial lung disease. Eur. Respir. J. 2022, 59, 2101396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reichmann, W.M.; Yu, Y.F.; Macaulay, D.; Wu, E.Q.; Nathan, S.D. Change in forced vital capacity and associated subsequent outcomes in patients with newly diagnosed idiopathic pulmonary fibrosis. BMC Pulm. Med. 2015, 15, 167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldin, J.G.; Kim, G.H.J.; Tseng, C.H.; Volkmann, E.; Furst, D.; Clements, P.; Brown, M.; Roth, M.; Khanna, D.; Tashkin, D.P. Longitudinal Changes in Quantitative Interstitial Lung Disease on Computed Tomography after Immunosuppression in the Scleroderma Lung Study II. Ann. Am. Thorac. Soc. 2018, 15, 1286–1295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maldonado, F.; Moua, T.; Rajagopalan, S.; Karwoski, R.A.; Raghunath, S.; Decker, P.A.; Hartman, T.E.; Bartholmai, B.J.; Robb, R.A.; Ryu, J.H. Automated quantification of radiological patterns predicts survival in idiopathic pulmonary fibrosis. Eur. Respir. J. 2014, 43, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Chelala, L.; Brixey, A.G.; Hobbs, S.B.; Kanne, J.P.; Kligerman, S.J.; Lynch, D.A.; Chung, J.H. Current state of fibrotic interstitial lung disease imaging. Radiology 2025, 316, e242531. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).