The Relationship between Mutations in Gene-Specific Domains of Salivary Fibronectin (cFn) and Dynamin-2 (Dynm-2) and the Development of Porphyromonas gingivalis-Initiated Periodontitis
Abstract
:1. Introduction
2. Material and Methods of Study
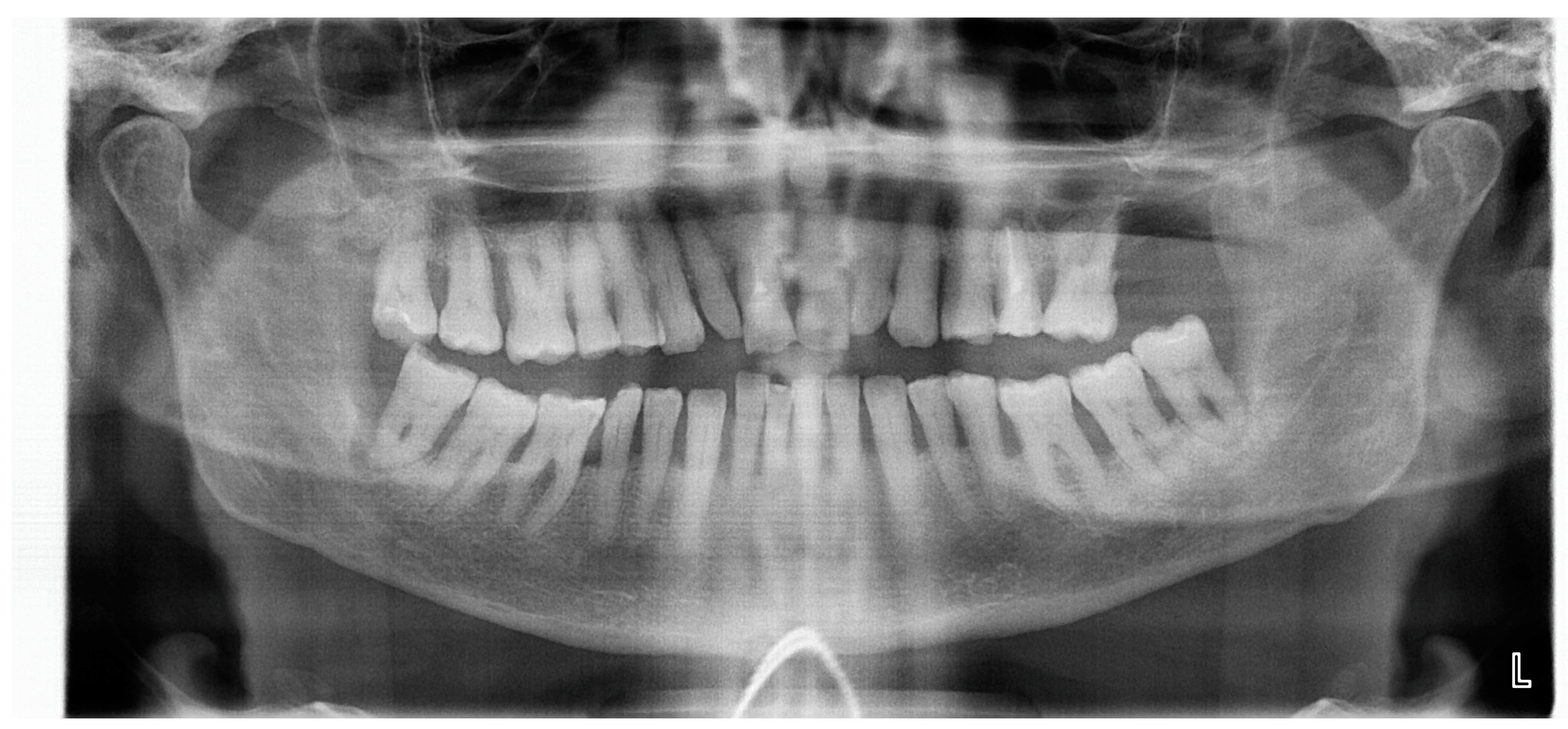
- Group 1 (12 people)—patients with periodontal disease of severity II (periodontal pockets 3–4 mm, coronal third 15–33%);
- Group 2 (20 people)—patients with healthy periodontal disease.
- 1—Mild periodontal ligament destruction: loss of attachment by less than 1/4 of the tooth root length;
- 2—Moderate destruction of periodontal ligaments: loss of attachment by 1/4 to 1/3 of the tooth root length;
- 3—Severe destruction of the periodontal ligaments: loss of compliance for more than 1/3 of the tooth root length;
- 4—Severe complicated destruction of periodontal ligaments: loss of compliance for more than 1/3 of the tooth root length, accompanied by intraosseous lesions, involvement in the pathological process of the root separation zone and/or increased mobility of I–II-degree teeth.
- The first digit is the age of the patient:
- The second digit—the number of lost teeth out of 28;
- The third digit—the number of teeth with mild periodontitis;
- The fourth digit—number of teeth with moderate periodontitis;
- The fifth figure—the number of teeth with severe periodontitis;
- The sixth digit—the number of teeth with severe complicated periodontitis.
2.1. DNA Extraction Technique
2.2. Statistical Research Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FN | Fibronectin |
| DYNM2 | Dynamin-2 |
| RGD | Arg-Gly-Asp motif |
| CCBD | central cell-binding domain |
| PRD—C | terminal proline-rich domain (DYNM2) |
| CAL | clinical attachment loss |
| RBL | radiological bone loss |
| SNP | Single Nucleotide Polymorphism |
References
- Amano, A. Disruption of epithelial barrier and impairment of cellular function by Porphyromonas gingivalis. Front. Biosci. 2007, 12, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Amano, A.I.; Nakagawa, N.; Okahashi, N. Hamada: Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J. Periodontal Res. 2004, 39, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Amaya Arbeláez, M.I.; de Paula ESilva, A.C.A.; Navegante, G.; Valente, V.; Barbugli, P.A.; Vergani, C.E. Proto-Oncogenes and Cell Cycle Gene Expression in Normal and Neoplastic Oral Epithelial Cells Stimulated With Soluble Factors From Single and Dual Biofilms of Candida albicans and Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2021, 11, 627043. [Google Scholar] [CrossRef] [PubMed]
- Cecil, J.D.; O’Brien-Simpson, N.M.; Lenzo, J.C.; Holden, J.A.; Singleton, W.; Perez-Gonzalez, A.; Mansell, A.; Reynolds, E.C. Outer Membrane Vesicles Prime and Activate Macrophage Inflammasomes and Cytokine Secretion In Vitro and In Vivo. Front. Immunol. 2017, 8, 1017. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.; Shibata, S.; Naito, M.; Nakayama, K. Transport and Polimerization of Porphyromonas Gingivalis Type V Pili. Periodontal Pathog. 2021, 2210, 61–73. [Google Scholar] [CrossRef]
- Tsuda, K.; Amano, A.; Umebayashi, K.; Inaba, H.; Nakagawa, I.; Nakanishi, Y.; Yoshimori, T. Molecular dissection of internalization of Porphyromonas gingivalis by cells using fluorescent beads coated with bacterial membrane vesicle. Cell Struct. Funct. 2005, 30, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Amano, A.H.; Inaba, S.K.; Hamada, S. Inhibitory effects of Porphyromonas gingivalis fimbriae on interactions between extracellular matrix proteins and cellular integrins. Microbes Infect. 2005, 7, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Veith, P.D.; Luong, C.; Tan, K.H.; Dashper, S.G.; Reynolds, E.C. Outer Membrane Vesicle Proteome of Porphyromonas gingivalis Is Differentially Modulated Relative to the Outer Membrane in Response to Heme Availability. J. Proteome Res. 2018, 17, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, D.; Liu, S.; Zhang, S.; Pan, Y. Porphyromonas gingivalis Outer Membrane Vesicles. Front. Cell. Infect. Microbiol. 2021, 10, 585917. [Google Scholar] [CrossRef] [PubMed]
- Cecil, J.D.; Sirisaengtaksin, N.; O’Brien-Simpson, N.M.; Krachler, A.M. Outer Membrane Vesicle-Host Cell Interactions. Microbiol. Spectr. 2019, 7, 201–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inaba, H.S.; Kawai, T.; Kato, I.; Nakagawa, A. Amano: Association between epithelial cell death and invasion by microspheres conjugated to Porphyromonas gingivalis vesicles with different types of fimbriae. Infect. Immun. 2006, 74, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Nakamura, Y.; Tanabe, K.; Li, S.; Takei, K. Dynamin 2 associates with microtubules at mitosis and regulates cell cycle progression. Cell Struct. Funct. 2011, 36, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Khater, I.M.; Liu, Q.; Chou, K.C.; Hamarneh, G.; Nabi, I.R. Super-resolution modularity analysis shows polyhedral caveolin-1 oligomers combine to form scaffolds and caveolae. Sci. Rep. 2019, 9, 9888. [Google Scholar] [CrossRef] [PubMed]
- Kraehling, J.R.; Hao, Z.; Lee, M.Y.; Vinyard, D.J.; Velazquez, H.; Liu, X.; Stan, R.V.; Brudvig, G.W.; Sessa, W.C. Uncoupling Caveolae from intracellular signaling in vivo. Circ. Res. 2016, 118, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lafont, F.; van der Goot, F.G. Bacterial invasion via lipid rafts. Cell Microbiol. 2005, 7, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Nichols, B.J.; Sandin, S. Architecture of the caveolar coat complex. J. Cell Sci. 2016, 129, 3077–3083. [Google Scholar] [CrossRef] [PubMed]
- Manabe, R.; Ohe, N.; Maeda, T.; Fukuda, T.; Sekiguchi, K. Modulation of Fibronectin by EDA Segment. J. Cell Biol. 1997, 139, 295–306. [Google Scholar] [CrossRef] [PubMed]
- González-Jamett, A.M.; Momboisse, F.; Haro-Acuña, V.; Bevilacqua, J.A.; Caviedes, P.; Cárdenas, A.M. Dynamin-2 in the secretory pathway. Front. Endocrinol. 2013, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- Kierdaszuk, B.; Berdynski, M.; Karolczak, J.; Redowicz, M.J.; Zekanowski, C.; Kaminska, A.M. A novel mutation in the DNM2 gene impairs dynamin 2 localization in skeletal muscle of a patient with late onset centronuclear myopathy. Neuromuscul. Disord. 2013, 23, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Lukiyanchuk, V.; Schmid, S.L. Common membrane trafficking defects of disease-associated dynamin 2 mutations. Traffic 2011, 12, 1620–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleinik, E.A.; Goncharenko, A.V. The Relationship between Mutations in Gene-Specific Domains of Salivary Fibronectin (cFn) and Dynamin-2 (Dynm-2) and the Development of Porphyromonas gingivalis-Initiated Periodontitis. J. Mol. Pathol. 2022, 3, 182-189. https://doi.org/10.3390/jmp3030015
Oleinik EA, Goncharenko AV. The Relationship between Mutations in Gene-Specific Domains of Salivary Fibronectin (cFn) and Dynamin-2 (Dynm-2) and the Development of Porphyromonas gingivalis-Initiated Periodontitis. Journal of Molecular Pathology. 2022; 3(3):182-189. https://doi.org/10.3390/jmp3030015
Chicago/Turabian StyleOleinik, Elena A., and Anna V. Goncharenko. 2022. "The Relationship between Mutations in Gene-Specific Domains of Salivary Fibronectin (cFn) and Dynamin-2 (Dynm-2) and the Development of Porphyromonas gingivalis-Initiated Periodontitis" Journal of Molecular Pathology 3, no. 3: 182-189. https://doi.org/10.3390/jmp3030015
APA StyleOleinik, E. A., & Goncharenko, A. V. (2022). The Relationship between Mutations in Gene-Specific Domains of Salivary Fibronectin (cFn) and Dynamin-2 (Dynm-2) and the Development of Porphyromonas gingivalis-Initiated Periodontitis. Journal of Molecular Pathology, 3(3), 182-189. https://doi.org/10.3390/jmp3030015




