Abstract
Dermatofibroma (DF) is a mesenchymal tumor of the dermis, but its exact differentiation lineage is still uncertain. A progenitor cell that may be able to differentiate into fibroblastic, myofibroblastic, or fibrohistiocytic cells has been hypothesized. Some authors have also proposed the possibility of a monocytic-histiocytic origin. We stained 47 consecutive dermatofibromas with CD64, CD34, CD14, CD163, and CD68 to test which marker is more reliable for the diagnosis and to gain insight into their histogenesis. From the 35 cases stained with the whole immunohistochemical panel, all were positive for CD64, mostly showing a strong and diffuse pattern. Regarding all the other staining, CD14 was strongly positive in 77% of the lesions and CD163 in 20%. The CD68 stain was intense and diffuse only in 20% of the cases. All lesions were negative for CD34, but two of them showed patchy and weak staining. DFs were immunohistochemically stained positively with a set of macrophage/monocyte/histiocyte lineage markers such as CD14, CD68, CD163, and CD64. This finding favors an active pro-inflammatory immature monocyte-lineage cell as the more suitable origin for DF. CD64 seems to be more sensitive than other markers to confirm the diagnosis.
1. Introduction
Dermatofibroma (DF) is a common skin tumor, predominantly occurring on the trunk or the extremities of young adults, that is histopathologically characterized by the presence of different cell types in varying proportions, including fibroblastic, histiocytes, and multinucleated giant cells [1]. Although its true differentiation lineage is still uncertain, a progenitor cell that may be able to differentiate into fibroblastic, myofibroblastic, or fibrohistocytic cells has been suggested.
Some authors have also proposed the possibility of a monocyte-histiocytic origin [2,3]. As in many examples, the lesion arises secondary to trauma, a reactive inflammatory process could be the cause [4], although most authors now consider DF as a neoplastic process [5,6].
FXIIIa, which was thought to stain dermal dendrocytes, is a classic immunohistochemical marker for the diagnosis of DF [1].
Fibrocytes, dermal dendritic cells, and histiocytes are all known to be derived from CD14 monocytes. Therefore, some authors have suggested that these cells could be the origin of DF [2]. Finally, histiocytic markers like HAM-56, C68, and alpha-1-antitrypsin positively stain DF [7,8].
In our study, we attempt to show the expression of CD64 in DFs. Additionally, we point out the usefulness of CD64 by comparing this staining with previously used markers for DF that are helpful to delineate macrophage-monocyte lineages, such as CD68, CD163, and CD14.
2. Materials and Methods
We stained 47 cases of DF with CD64, CD34, CD14, CD163, and CD68 antibodies. They included 7 cases of cellular dermatofibroma and one each of xanthomatous, subcutaneous, and hemorrhagic DFs, respectively. Cases were consecutively retrieved from the database of one of the authors (HK) throughout several weeks in 2016. Tissue sections from each case were stained with hematoxylin and eosin.
Immunohistochemistry
Four-μm sections were mounted on positively charged slides. Sections were later dried overnight at 45 °C. Slides were deparaffinized in xylene for 30 min, rehydrated using graded ethanol concentrations, and incubated for 30 min at 95 °C in EDTA buffer (pH = 9.0). They were cooled down to room temperature for 20 min. Following quenching with alkaline phosphatase and biotin blocking using avidin, sections were incubated with CD163, CD34, CD64, CD34, and CD14. A list of the antibodies used in this study can be found in Table 1. Automatic staining was performed by a TechMate 500 (Biotech Solutions, Dako, Glostrup, Denmark) as a detection system and labeled with streptavidin-biotin (LSAB).

Table 1.
List of antibodies used in this study.
3. Results
Staining results in the 35 cases where all stains were performed (Table 2).

Table 2.
Staining of 35 dermatofibroma cases with the complete set of biomarkers.
All cases were stained positively with CD64. Most of them in a diffuse way, with intense staining (91.5%). Regarding the staining with all the other markers, CD14 was intensively positive in 77.1% of the cases and CD163 in 62.9%, most of them with a focal dispersed staining mostly located in the deeper part or the periphery of the tumor. In five cases CD163 was similar to CD64 in intensity and distribution. CD68 stained positively and intensely only in 17% of the cases. No cases were positive for CD34, but two of them showed focal and weak staining.
An example of CD14 and CD64 staining can be seen in Figure 1.
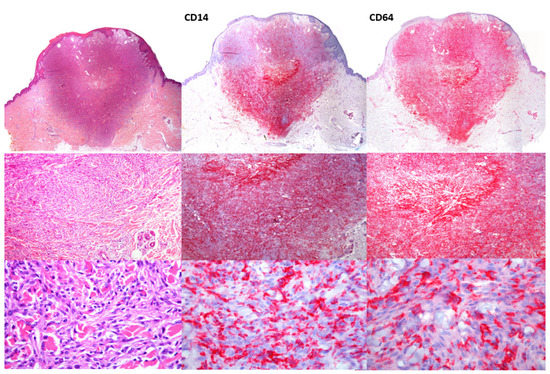
Figure 1.
HE staining of dermatofibroma. Panoramic image (20× magnification, upper panel) and details of fibrohistiocytic cells (100× middle panels) and (200×, lower panels). CD14 shows a diffuse pattern, although more intense in the deeper areas of the lesion. CD64 shows a similar staining pattern but with a stronger and more diffuse reactivity.
CD14 was negative in 5 of the cases stained with CD64. Twenty-six cases had a quite similar pattern with CD14 and CD64, but the intensity of stain and clear delimitation of DF tumor cells with CD64 was slightly better in 17 cases.
Finally, CD68 usually showed a focal, dispersed cytoplasmic staining while it stained some cells in the periphery of the tumor and highlighted multinucleated giant cells when present.
4. Discussion
Although the histopathologic diagnosis of a classic DF is generally straightforward, immunohistochemistry is an additional help in distinguishing some variants, such as cellular DF, from other tumors [9]. In the differential diagnosis between DF and dermatofibrosarcoma protuberans (DFSP), many immunohistochemical markers have been used. A classic one is CD34, which is mostly negative in DF, although it could be expressed in 6 to 20% of the cases [2,9]. Other markers have also been used, such as the extracellular matrix metalloproteinase stromelysin3 (usually positive in DF), high mobility group A proteins (HMGA2) (usually positive in DF), apolipoprotein D (negative in DF), nestin (negative in DF), and CD117 (negative in DF) [2,9].
In our cases, we included typical DFs as well as several variants of DF to test the usefulness of different histiocytic/monocytic markers.
CD163 is a 175 KDa glycoprotein belonging to the family of scavenger transmembrane receptors. It functions as a hemoglobin scavenger receptor expressed by monocytes and macrophages. In eyelid-located dermatofibromas, a group of authors reported a case that stained with CD163, which is a lineage-specific biomarker for bone marrow-derived monocytic/histiocytic cells, and they concluded that FXIIIa (typically found in monocytes) and CD163 were the benchmark biomarkers for dendritic cells (in a cytoplasmic staining) [3].
As dermal dendritic cells form DF express factor XIIIa+ and/or CD163, those cells have been postulated to arise in the bone marrow as monocytes and to settle after circulating in the blood. The authors of [3] describe how CD163 is usually expressed in DFs (89%), cellular fibrous histiocytoma (100%), and rarely in DFSP (17%) [10], but it is not expressed in atypical fibroxanthoma (AFX) [11]. In our study, we found similar results for CD163, which was expressed in 80% of cases and strongly and diffusely positive in 14.3% of cases.
CD68 is a 110 kDa glycoprotein associated with lysosomes and used for the identification of monocytes and macrophages. It is comparatively less specific because it positively stains myeloid cells, dendritic cells, fibroblasts, and Schwann cells [3]. In our study, 88.6% of the cases were positive, albeit with moderate and patchy staining.
Remarkably, CD64 was positive in all but two cases. This marker is expressed in macrophages and, as CD64-positive cells are a target for chronic inflammation, its positivity in DF explains why these lesions frequently appear after an inflammatory process secondary to an unknown stimulus.
All our cases were negative for CD34 even when we used the QBend10 antibody due to its greater sensitivity.
Moreover, although these markers favor a DF differentiation lineage mimicking monocytes, there are plenty of differences between monocyte-macrophage lineage cells in their transcriptional profiling and tissue homing, thus indicating that this differentiation is a very dynamic process.
Macrophages show wide plasticity and differentiation dynamics towards M1 (CD64, CD80) and M2 (CD163, CD209) profiles. The M1 cell type results in the release of proinflammatory cytokines such as IL-6, IL-12, and IL-23 that promote Th1 and Th17 polarization, whereas M2 cells are alternatively activated by IL-4, IL-10, or IL-13. A subpopulation with a less mature phenotype and characterized by high CD64 expression, along with a weak expression of CD163 and absence of CD34 was the most frequent finding in our DF series. This points to an M1 pro-inflammatory macrophage differentiation in most of the cases, although up to 14.3% of the cases presented M2 CD163+ cells. We postulate that this staining pattern could be related to the presence of mainly active pro-inflammatory immature M1-type monocyte-lineage cells in DF, as described in previous articles on macrophage-based inflammation [12,13]. Moreover, in the blood, IL-6 modestly increases CD64 and seems to stimulate M1 pro-inflammatory macrophage differentiation [14]. It is well known that a proper course of inflammation is strongly dependent on a correctly balanced dynamic ratio of M1 and M2 macrophages. The failure to switch from a predominance of M1 to M2 causes a perpetuation of chronic inflammation [15], which may lead later to the appearance of a clone selection and a neoplastic process in DF. In fact, a macrophage related chronic inflammation has been demonstrated in non-healing wounds, and the relevance of CD64+ cells in skin inflammation has been tested by using an antibody directed against CD64 that demonstrates histological clearance of M1 macrophages followed by other inflammatory cells [16,17]. Other experimental therapeutic approaches, such as the use of a modified nanoparticle, plyethylenimine grafted with a mannose receptor ligand to induce CD163 to reverse chronic wound, have not been tested to treat DF but may be useful for a non-surgical therapeutic approach to these lesions [17].
In our work, FXIIIa was not included in the antibody panel because we focused on markers to clarify DF lineage, not to diagnose DF [18]. Nevertheless, based on the few cases that were also stained with FXIIIa, the sensitivity of CD64 seemed to mirror that of FXIIIa. However, a more detailed study would be necessary to compare the diagnostic performance of both markers.
Finally, it is important to note that no cases of some infrequent variants, such as granular cell DF, were included in our series. Previous studies on this variant, caused by dysfunction of a lysosomal enzyme or a lysosomal-associated protein involved in enzyme activation, reported CD68 diffuse positive staining as well as lack of expression of CD34, Melan-A, and CD10 [19].
5. Conclusions
In conclusion, DF positively stained with a set of macrophage/monocyte/histiocyte lineage markers such as CD14, CD68, CD163, and CD64. In our experience, CD64 stains tumor cells more diffusely and intensely, making it a good marker for DF. Since all these markers can be found in immature pro-inflammatory monocytes, their presence in DF raises the question of a M1 monocyte-type differentiation lineage in DF, which should be further studied.
Author Contributions
M.L.-V., T.M., M.T.F.-F., E.O.-M. and H.K. authors have worked in conceptualization, methodology, data curation, writing, and editing. M.L.-V., T.M. and H.K. have worked in resources. M.L.-V., T.M., M.T.F.-F., E.O.-M. and H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the absence of identifiable information in the clinical data used.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, Y.; Sakamoto, F.; Ito, M. Characterization of factor XIIIa+ dendritic cells in dermatofibroma: Immunohistochemical, electron and immunoelectron microscopical observations. J. Dermatol. Sci. 2005, 39, 89–96. [Google Scholar] [CrossRef]
- Jin, S.Y.; Choi, J.S.; La Choi, Y.; Kim, D.H.; Lee, S.H. Identification of Leukocyte-Specific Protein 1-Positive Cells: A Clue to the Cell of Origin and a Marker for the Diagnosis of Dermatofibroma. Ann. Dermatol. 2015, 27, 157–162. [Google Scholar] [CrossRef]
- Jakobiec, F.A.; Zakka, F.R.; Tu, Y.; Freitag, S.K. Dermatofibroma of the Eyelid: Immunohistochemical Diagnosis. Ophthalmic Plast. Reconstr. Surg. 2017, 33, e134–e138. [Google Scholar] [CrossRef] [PubMed]
- Zelger, B.G.; Zelger, B. Dermatofibroma (fibrous histiocytoma): An inflammatory or neoplastic disorder? Histopathology 2001, 38, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Calonje, E. Is cutaneous benign fibrous histiocytoma (dermatofibroma) a reactive inflammatory process or a neoplasm? Histopathology 2000, 37, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-C.; Kuo, T.-T.; Chan, H.-L. Dermatofibroma is a clonal proliferative disease. J. Cutan. Pathol. 2000, 27, 36–39. [Google Scholar] [CrossRef] [PubMed]
- du Boulay, C.E. Demonstration of alpha-1-antitrypsin and alpha-1-antichymotrypsin in fibrous histiocytomas using the immunoperoxidase technique. Am. J. Surg. Pathol. 1982, 6, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y. Cell Differentiation in Benign Cutaneous Fibrous Histiocytomas an Immunohistochemical Study with Antibodies to Histiomonocytic Cells and Intermediate Filament Proteins. Am. J. Dermatopathol. 1990, 12, 134–140. [Google Scholar] [CrossRef] [PubMed]
- West, K.L.; Cardona, D.M.; Su, Z.; Puri, P.K. Immunohistochemical markers in fibrohistiocytic lesions: Factor XIIIa, CD34, S-100 and p75. Am. J. Dermatopathol. 2014, 36, 414–419. [Google Scholar] [CrossRef]
- Sachdev, R.; Sundram, U. Expression of CD163 in dermatofibroma, cellular fibrous histiocytoma, and dermatofibrosarcoma protuberans: Comparison with CD68, CD34, and Factor XIIIa. J. Cutan. Pathol. 2006, 33, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, K.; Takahashi, K.; Maeda, F.; Oikawa, H.; Akasaka, T. A Case of Atypical Fibrous Histiocytoma with Positivity for CD163 and CD44. Acta Derm. Venereol. 2013, 93, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Clanchy, F.I.L.; Holloway, A.C.; Lari, R.; Cameron, P.U.; Hamilton, J.A. Detection and properties of the human proliferative monocyte subpopulation. J. Leukoc. Biol. 2006, 79, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Clanchy, F.I. High-Affinity FcReceptor Expression Indicates Relative Immaturity in Human Monocytes. J. Interferon Cytokine Res. 2016, 36, 279–290. [Google Scholar] [CrossRef]
- Swangphon, P.; Pientong, C.; Sunthamala, N.; Bumrungthai, S.; Azuma, M.; Kleebkaow, P.; Kongyingyoes, B.; Ekalaksananan, T. Correlation of Circulating CD64+/CD163+ Monocyte Ratio and stroma/peri-tumoral CD163+ Monocyte Density with Human Papillomavirus Infected Cervical Lesion Severity. Cancer Microenviron. 2017, 10, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hristodorov, D.; Mladenov, R.; Von Felbert, V.; Huhn, M.; Fischer, R.; Barth, S.; Thepen, T. Targeting CD64 mediates elimination of M1 but not M2 macrophages in vitro and in cutaneous inflammation in mice and patient biopsies. mAbs 2015, 7, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Thepen, T.; Van Vuuren, A.J.H.; Kiekens, R.C.M.; Damen, C.A.; Vooijs, W.C.; Van De Winkel, J.G.J. Resolution of cutaneous inflammation after local elimination of macrophages. Nat. Biotechnol. 2000, 18, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef]
- Nonaka, D. A study of monocytic and dendritic cell markers in benign cutaneous fibrous histiocytoma (dermatofibroma). Histopathology 2008, 52, 896–897. [Google Scholar] [CrossRef]
- Cazzato, G.; Colagrande, A.; Cimmino, A.; Marrone, M.; Stellacci, A.; Arezzo, F.; Lettini, T.; Resta, L.; Ingravallo, G. Granular Cell Dermatofibroma: When Morphology Still Matters. Dermatopathology 2021, 8, 371–375. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).