Abstract
Banned pesticides are continuously preferred by the planters of the Idukki District irrespective of their toxicity. Among the banned pesticides, acephate is preferred because of its high solubility in water and persistent character. Unfortunately, it detriments the biota, leading to neurogenic, carcinogenic, and physiological disorders in fish. The plantation near the Periyar River basin is contaminated with residues of pesticides, which eventually drain into the river. There is an urgent need for the removal of acephate. Therefore, we have focused on the removal of acephate into the lab scale. Batch adsorption studies were carried out for the removal of acephate. We selected a material Fe-MMT (Fe3O4-montmorillonite), which is benign and possesses a high adsorption capacity towards acephate. Adsorbent properties were examined by various analytical tools XRD, SEM, FTIR, and a Surface area analyzer. Adsorption followed Langmuir with first-order kinetic. Kinetic plots exhibited multistage adsorption, indicating film diffusion and pore diffusion during the adsorption or the mechanism of adsorption is chemisorption, physisorption, and Lewis’s acid-base interaction. Response surface methodology involving CCD (central composite design) was extracted to maximize the adsorption of acephate onto Fe-MMT. Dosage and concentration seem to be the major parameters that influenced the adsorption. Adsorption achieved a peak (83.18%) at optimum conditions corresponding to pH 6, initial acephate concentration of 2 mg/L, and adsorbent dosage corresponding to 0.5 g/L.
1. Introduction
Acephate, (O, S dimethyl acetyl phosphoroamidothioate) an organophosphorous pesticide, and its metabolite have immense potential to harm the biota. Acephate residues are present in the cardamom plantations of the Periyar River basin and are eventually found in the river in ppb ranges [1]. Additionally, residues are present in blood and breast milk, thereby transferring to the newborn [2]. Immediate removal is mandatory, as it affects the biodata and causes death. Among several techniques, adsorption is preferred due to its easy and cost-effective nature. Then, the selection of adsorbent paves another important role in the adsorption process. Fe-MMT was selected as the adsorbent for the removal of acephate, as it is environmentally benign in nature [3]. The paper focuses on the properties of the adsorbent characterized by SEM, FTIR, and the surface area analyzer. Additionally, the adsorbent performance for the removal of acephate was evaluated.
2. Materials and Methods
FTIR spectra of the samples were obtained using a spectrometer (500–4000 cm−1). XRD experiments were carried out using a diffractometer (Make: PAN Analytical Philips: Almelo, Netherland, and Model: XPERT-PRO). Morphology was studied by SEM (Make: TESCAN: Brno, Czech Republic, and Model: VEGA 3 LMU) and chemical composition was evaluated (EDX). Surface area by surface area analyzer (Make: Micrometrics: Norcross, GA, USA, and Model: Tristar II 3020 Version 3.02). The concentration of acephate was measured with the help of a UV-Vis spectrophotometer (Make: Shimadzu, Japan, Model: UV-1800). Montmorillonite was purchased from Sigma-Aldrich Company Response Model and Central Composite Design were employed to investigate the effect of adsorption efficiency of acephate onto Fe-MMT.
3. Results and Discussions
Montmorillonite exhibits typical diffraction peaks at 2θ = 19.5, 26.7, and 40.04, and additional peaks at 2θ = 26.5 and 47 corresponding to silica and aluminum. Diffraction peaks at 2θ of 36 and 42 indicate the presence of magnetite, suggesting that the modified phase is magnetite (Figure 1a), which leads to a dramatic increase in the adsorption efficiency, up to 87%, rather than the bare MMT possessing 60% adsorption capacity. FTIR interpretation revealed that Fe-MMT exhibited strong characteristic peaks at 536 cm−1, 1438 cm−1, and 3377 cm−1. Bands at 520–570 cm−1 represent Fe-O stretching vibration (symmetrical). Bands at 3377 cm−1 are due to Fe-OH (Figure 1b). SEM images indicate flower-like morphology with EDX spectrum, indicating a rise in the content of Fe (Figure 1c), which improved the material adsorption capacity. Figure 1d pictures the adsorption–desorption isotherms of Fe-MMT. The BET surface area of MMT (256 m2/g) is higher than that of the modified material (28 m2/g), highlighting chemical adsorption onto Fe-MMT rather than physisorption.
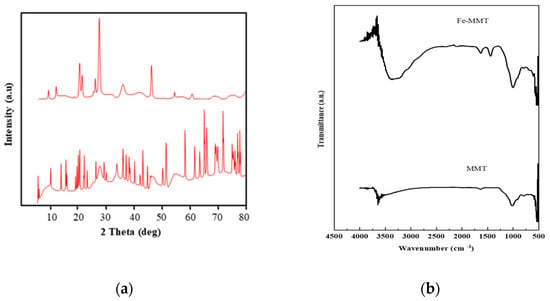
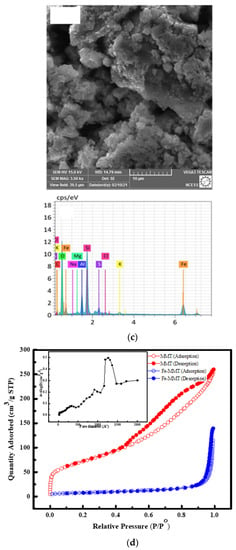
Figure 1.
Images of (a) XRD (b) FTIR (c) SEM-EDAX (d) N2 adsorption-desorption isotherms of Fe-MMT.
4. Batch Adsorption Studies
Optimization of Parameters for Acephate Adsorption
RSM (Response surface methodology), based on the central composite design (CCD), was employed to examine the performance of adsorption. CCD was opted to achieve accurate predictions around extremes of the factors. Concentration, pH, and dosage were the factors that were identified as key parameters contributing to adsorption. Response surface plots were generated using a design expert for the determination of the effect of the input factors on the responses. pH parameter was optimized for the batch adsorption of acephate onto Fe-MMT by testing with pH (1–10) [4]. Figure 2a shows that the acidic medium favored the adsorption and the peak of adsorption was achieved at 5. As the pH increases from 5 to 8, the adsorption of acephate decreases. The reduced adsorption of acephate onto clay may be due to the electrostatic repulsion between the adsorbent and the adsorbate. Adsorption increased with an increase in the initial acephate. The amount of adsorption increased with an increase in the initial acephate concentration and was found to be 0.9, 2.175, 4.2, 5.925, and 7.4 mg/g for the initial acephate concentrations of 2, 5, 10, 15, and 20 mg/L, respectively. Dosage was another vital factor that influenced the adsorption of acephate onto Fe-MMT. As dosage increases (0.1–0.5), adsorption increases, and the peak of adsorption is reached at 0.5g/L, with an efficiency of 90%. Temperature [5] is another parameter that affects the adsorption. As the temperature increases, adsorption efficiency is also enhanced (endothermic nature) [5].
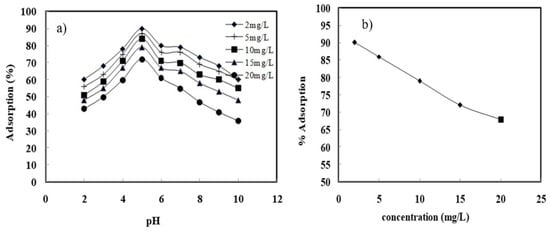
Figure 2.
Effect of (a) pH and (b) initial acephate concentration on the adsorption of acephate onto Fe-MMT.
5. 3D Response Surface Plots
pH, dosage, and concentration significantly influenced the adsorption of acephate by Fe-MMT, summarized in Figure 3. Figure 3 reveals the synergistic effect of two parameters on the adsorption capacity of acephate onto Fe-MMT. The other parameter was retained at the zero level. According to Figure 3, the concentration and adsorbent dose are the most influential factors in the adsorption capacity. Meanwhile, pH partly contributes to the adsorption results. It was found that higher dosage and lower concentration gave the highest adsorption capacity.
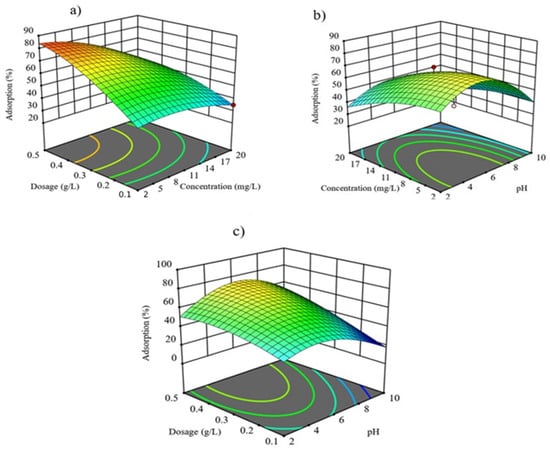
Figure 3.
Effect of (a) pH and (b) initial acephate concentration and (c) dosage on the adsorption of acephate onto Fe-MMT.
6. Adsorption Kinetics, Isotherm, and Thermodynamic Parameters
Lagergren’s pseudo-first-order, pseudo-second-order, Elovich, and Weber and Morris’s Intraparticle diffusion models were scrutinized to study the kinetics of adsorption. Kinetics of adsorption and the model of isotherm obeyed pseudo-first-order (R2 > 0.99) and the Langmuir model (adsorbed homogeneously on a monolayer surface of Fe-MMT). The maximum Langmuir capacity at pH 5 and 303 K is 13.66 mg/g. Thermodynamic parameters revealed that the adsorption is endothermic ∆H0 = 22.855 kJ/mol, ∆S0 = 105.920 J/K) and spontaneous in nature (∆G0 = −7.99, −8.087, 9.245, and −10.357 kJ/mol).
7. Mechanism of Adsorption
The mechanism of adsorption corresponds to chemisorption (positive group of phosphorous in acephate and deprotonated silanol group), physisorption (hydrogen-bearing amino group of acephate and the oxygen atoms of MMT), and Lewis’s acid–base interaction nitrogen atom of the amino group of acephate and Al3+ montmorillonite.
Author Contributions
R.S.R.: methodology, investigation, writing–original draft, resources, K.A.K.: conceptualization, writing-review & editing, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
University Grants Commission, Awardee: R. Shiny Raj, Sr No: 2121410217, Ref No: 21/12/2014(ii) EU-V.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Gayathri, S.; Dev, V.V.; Shiny Raj, R.; Krishnakumar, A.; Vishnu Maya, T.M.; Anoop Krishnan, K. Spatiotemporal evaluation of hydrochemical facies and pesticide residues in the cardamom plantations of Southern Western Ghats, India. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100599. [Google Scholar] [CrossRef]
- Lin, Z.; Pang, S.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Degradation of Acephate and Its Intermediate Methamidophos: Mechanisms and Biochemical Pathways. Front. Microbiol. 2020, 11, 02045. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, M.; Naeimi, A.; Komeili, M. Magnetite nanoparticles coated with methoxy polyethylene glycol as an efficient adsorbent of diazinon pesticide from water. Adv. Environ. Technol. 2016, 2, 25–31. [Google Scholar] [CrossRef]
- Mahadevan, H.; Nayana, A.R.; Viswadas, V.; Antony, S.; Dev, V.V.; Sudhakaran, S.; Priya Pious, H.; Anoop Krishnan, K. A pilot level approach to remove anionic species from industrial effluents using a novel carbonate-steam pyrolysed activated charcoal system. Adv. Powder Technol. 2019, 30, 98–110. [Google Scholar] [CrossRef]
- Dev, V.V.; Baburaj, G.; Antony, S.; Arun, V.; Krishnan, K.A. Zwitterion-chitosan bed for the simultaneous immobilization of Zn(II), Cd(II), Pb(II) and Cu(II) from multi-metal aqueous systems. J. Clean. Prod. 2020, 255, 120309. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).