Enhancement of Atmospheric Water Harvesting via Salt-Infused Sponges and Peltier Devices †
Abstract
:1. Introduction
2. Methods
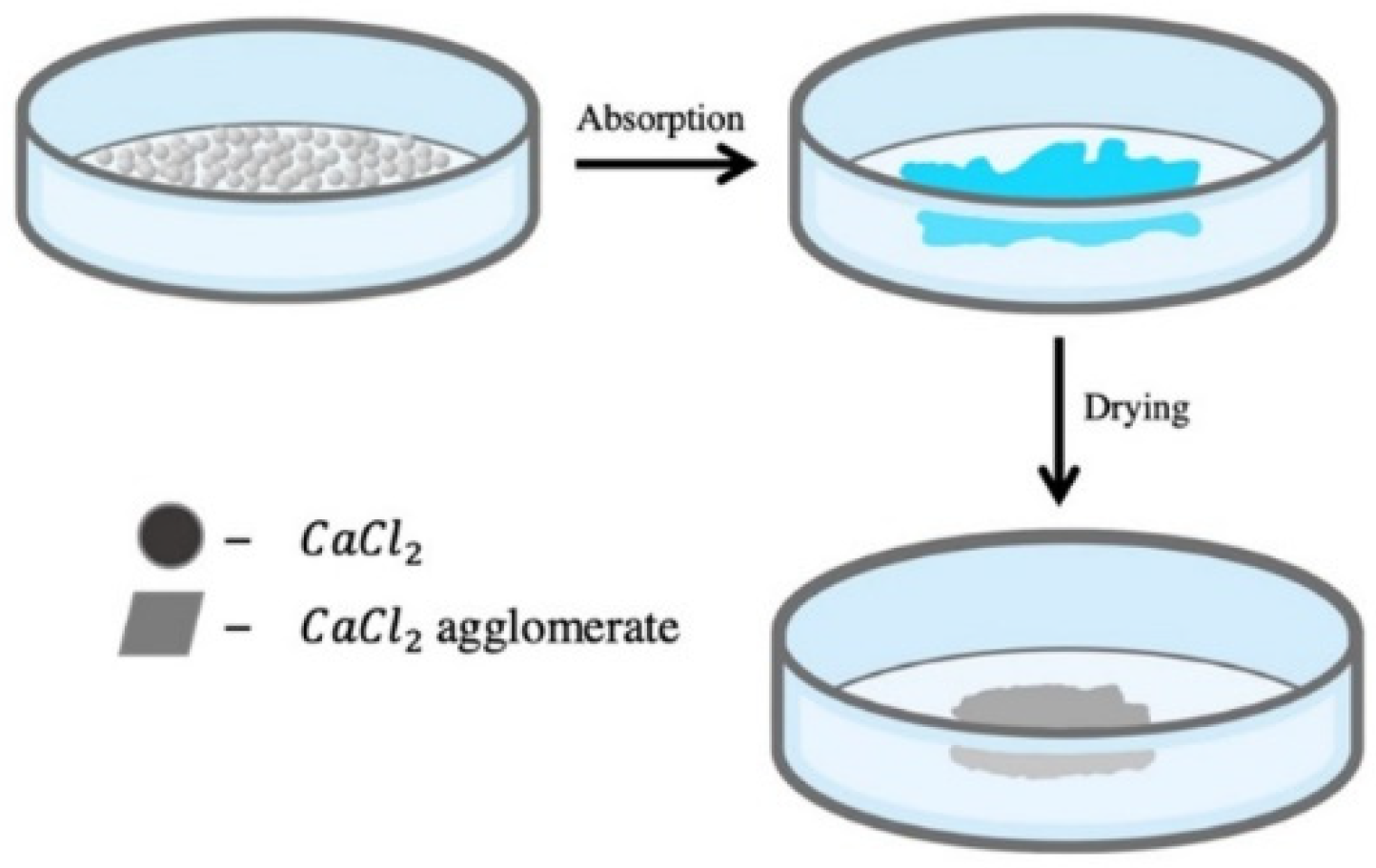
2.1. Sample Preparation
2.2. Moisture Absorption Time Dependence Measurement
3. Results and Discussion
3.1. Preliminary Experiment
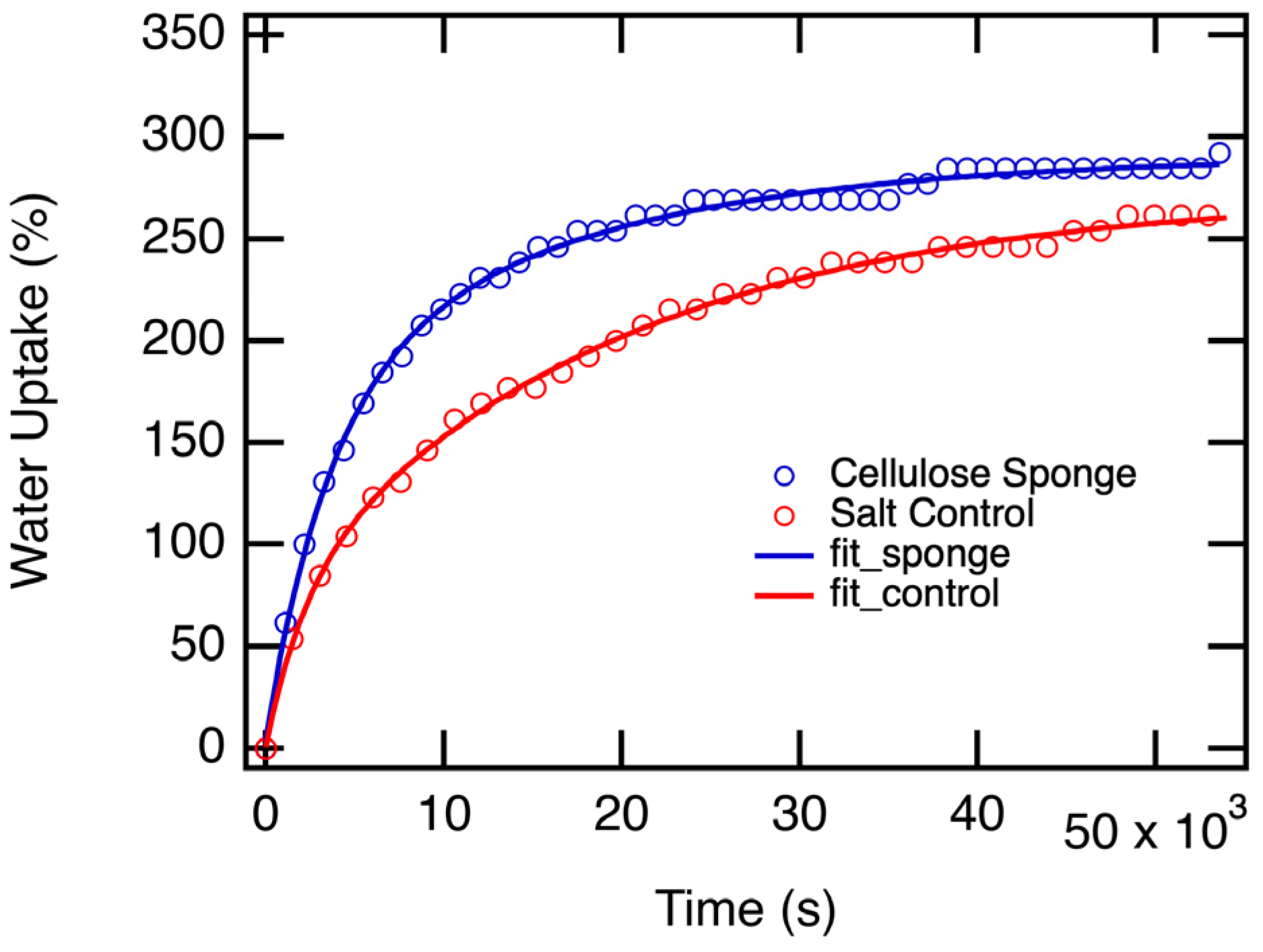
3.2. Salt-Cellulose Sponge Water Uptake Time Dependent Measurements
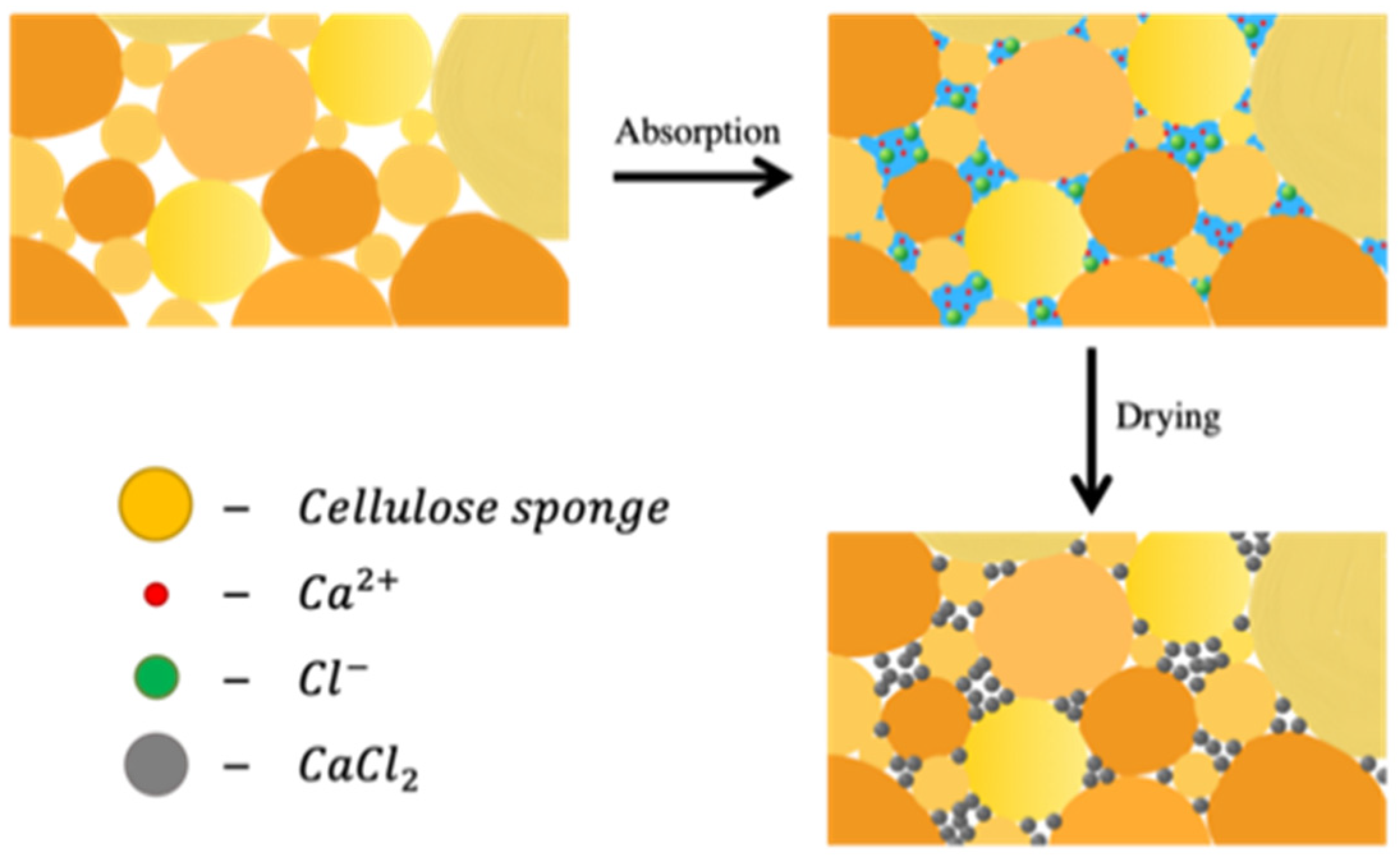
3.3. Proposed Model
3.4. Peltier Device Prototype
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofman-Caris, R.; Bertelkamp, C.; de Waal, L.; van den Brand, T.; Hofman, J.; van der Aa, R.; van der Hoek, J. Rainwater Harvesting for Drinking Water Production: A Sustainable and Cost-Effective Solution in The Netherlands? Water 2019, 11, 511. [Google Scholar] [CrossRef]
- Kallenberger, P.A.; Fröba, M. Water Harvesting from Air with a Hygroscopic Salt in a Hydrogel–Derived Matrix. Commun. Chem. 2018, 1, 28. [Google Scholar] [CrossRef]
- Permyakova, A.; Wang, S.; Courbon, E.; Nouar, F.; Heymans, N.; D’Ans, P.; Barrier, N.; Billemont, P.; Weireld, G.D.; Steunou, N.; et al. Design of Salt–Metal Organic Framework Composites for Seasonal Heat Storage Applications. J. Mater. Chem. A 2017, 5, 12889–12898. [Google Scholar] [CrossRef]
- Towsif Abtab, S.M.; Alezi, D.; Bhatt, P.M.; Shkurenko, A.; Belmabkhout, Y.; Aggarwal, H.; Weselinski, L.J.; Alsadun, N.S.; Samin, U.; Hedhili, M.N.; et al. Reticular Chemistry in Action: A Hydrolytically Stable MOF Capturing Twice Its Weight in Adsorbed. Water Chem. 2018, 4, 94–105. [Google Scholar] [CrossRef]
- Rieth, A.J.; Yang, S.; Wang, E.N.; Dincă, M. Record Atmospheric Fresh Water Capture and Heat Transfer with a Material Operating at the Water Uptake Reversibility Limit. ACS Cent. Sci. 2017, 3, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Tso, C.Y.; Chao, C.Y.H. Activated Carbon, Silica-Gel and Calcium Chloride Composite Adsorbents for Energy Efficient Solar Adsorption Cooling and Dehumidification Systems. Int. J. Refrig. 2012, 35, 1626–1638. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Gough, R.V.; Chevrier, V.F.; Tolbert, M.A. Formation of Liquid Water at Low Temperatures via the Deliquescence of Calcium Chloride: Implications for Antarctica and Mars. Planet. Space Sci. 2016, 131, 79–87. [Google Scholar] [CrossRef]
- Britannica, The Editors of Encyclopaedia. “Deliquescence”. Available online: https://www.britannica.com/science/deliquescence (accessed on 13 April 2022).
- Hostomsky, J.; Jones, A.G. Calcium Carbonate Crystallization, Agglomeration and Form during Continuous Precipitation from Solution. J. Phys. Appl. Phys. 1991, 24, 165–170. [Google Scholar] [CrossRef]


| Name | Medium | Water Uptake (%) | Conditions (Temperature, Dewpoint [°C], Water Vapor Pressure [mbar], RH [%]) | Reference |
|---|---|---|---|---|
| Alg-CaCl2 | Alginate based | 288 | 28, 24.1, 30, 79% | [2] |
| AC07 | 39 | 27, 7.9, 10.7, 30% | [6] | |
| MIL-101 (Cr) | MOF | 88 | 30, 10.6, 12.8, 30% | [3] |
| Cr-soc-MOF-1 | MOF | 200 | 25, 19.1, 22.2, 70% | [4] |
| MOF-801 | MOF | 30 | 25, 6,2, 9.5, 30% | [7] |
| Co2Cl2 (BTDD) | MOF | 90 | 25, 6,2, 9.5, 30% | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Jobiliong, E.; Bastiaan, T.; Manua, D.J.; Taniara, E.; Steven, E. Enhancement of Atmospheric Water Harvesting via Salt-Infused Sponges and Peltier Devices. Environ. Sci. Proc. 2023, 25, 67. https://doi.org/10.3390/ECWS-7-14177
Lee J, Jobiliong E, Bastiaan T, Manua DJ, Taniara E, Steven E. Enhancement of Atmospheric Water Harvesting via Salt-Infused Sponges and Peltier Devices. Environmental Sciences Proceedings. 2023; 25(1):67. https://doi.org/10.3390/ECWS-7-14177
Chicago/Turabian StyleLee, Jaewoong, Eric Jobiliong, Timothy Bastiaan, Darren Johanes Manua, Ezekhiel Taniara, and Eden Steven. 2023. "Enhancement of Atmospheric Water Harvesting via Salt-Infused Sponges and Peltier Devices" Environmental Sciences Proceedings 25, no. 1: 67. https://doi.org/10.3390/ECWS-7-14177
APA StyleLee, J., Jobiliong, E., Bastiaan, T., Manua, D. J., Taniara, E., & Steven, E. (2023). Enhancement of Atmospheric Water Harvesting via Salt-Infused Sponges and Peltier Devices. Environmental Sciences Proceedings, 25(1), 67. https://doi.org/10.3390/ECWS-7-14177





