Abstract
Organophosphate esters (OPEs), are used as flame retardants and plasticizers to protect or enhance the properties of plastics, textiles, and many other materials. Sampling was carried out in groundwater from the karst aquifer Bokanjac–Poličnik near the city of Zadar, Croatia. To determine their continuous presence, samples were taken once during each season for one year. In the collected samples, nine OPEs were identified: tris(2-butoxyethyl) phosphate-TBEP, tricresyl phosphate-TCP, triphenyl phosphate-TPPA, tris(1-chloro-2-propyl) phosphate-TCPP, tris(2-chloroethyl) phosphate-TCEP, tris(1,3-dichloroisopropyl) phosphate-TDCPP, diethyl phthalate-DEP, tri-n-butyl phosphate-TBP, and di(2-ethylhexyl) adipate-DEHA.
1. Introduction and Background
Groundwater is an essential water supply for settlements in many countries across the world, especially in Croatia, where 90% of water withdrawal is from underground aquifers. In Croatia, the quality of groundwater must be in line with the guidelines of the EU Commission Water Framework Directive (WFD) [1], as well as the Groundwater Directive (GWD) [2]. Emerging pollutants, particularly organic pollutants in groundwater, are under-researched due to the absence of monitoring regulations. Nevertheless, monitoring is carried out on a voluntary basis [3].
Organophosphate esters (OPEs), which are triesters, are used as flame retardants and plasticizers to protect or enhance the properties of plastics, textiles, and many other materials. They are high-production-volume chemicals with large variations in their physical–chemical properties that are widely used in many human activities and can be detected in groundwater due to their insolubility in water, especially wastewater. In [4], it was demonstrated that OPEs have been used for decades. Thus, their occurrence in the environment is not new, because since the 1980s, reports have described their detection in surface waters, groundwaters influenced by wastewater, and drinking water. OPEs can be a health hazard for humans due to their toxicity, and some of them have shown carcinogenic properties, e.g., chlorinated OPEs such as TCEP, TCPP, and TDCPP, which can accumulate in the liver and testis, thereby inducing tumors [5].
Various analytical methods for the determination of OPEs in water, i.e., various extraction techniques, have been developed, followed by gas chromatography with mass spectrometry (GC-MS) and liquid chromatography with mass spectrometry (LC-MS) [6].
Sampling was carried out in groundwater from the karst aquifer Bokanjac–Poličnik near the city of Zadar, Croatia, in order to detect OPEs. To determine their continuous presence in the case study aquifer, samples were taken throughout the entire hydrological year, that is, once during each season for one year. The preparation of samples was performed with the Solid Phase Extraction (SPE) method, and they were analyzed with liquid chromatography with mass spectrometry and quadrupole time-of-flight spectrometry (LC-MS/QTOF). In the collected samples, nine OPEs were identified with the Water Screening Personal Compound Database and Library (PCDL): tris(2-butoxyethyl) phosphate-TBEP, tricresyl phosphate-TCP, triphenyl phosphate-TPPA, tris(1-chloro-2-propyl) phosphate-TCPP, tris(2-chloroethyl) phosphate-TCEP, tris(1,3-dichloroisopropyl) phosphate-TDCPP, diethyl phthalate-DEP, tri-n-butyl phosphate-TBP, and di(2-ethylhexyl) adipate-DEHA. Their properties and usages are listed in Table 1.

Table 1.
OPEs properties detected in the case study aquifer and their usages.
2. Methodology
The sampling of the groundwater was caried out at three piezometer wells in the Bokanjac–Poličnik basin near the city of Zadar in Croatia. Samples was taken four times in one year in order to over all the seasons, i.e., to obtain samples from one hydrological year. For the sampling, sterile glass bottles were used, and they were refrigerated during transport and up to the time when sample preparation began. The three sites where the samples were taken are marked as J, B, and B4. Due to the complexity of the groundwater samples, which were relatively clear and of high quality according to the regulation parameters for human consumption, the small concentrations of the expected organic pollution could not be directly measured, and there was a need to concentrate the samples for the analysis. The analyte prepared for analysis was concentrated using the Solid Phase Extraction (SPE) method to increase the selectivity of the method. The SPE method was used to eluate the concentrated analyte in order to simplify the identification and quantification of the expected compounds in extremely low concentrations. The cartridges used for SPE were Bond elut plexa C18, and the samples were eluted with methanol (MeOH) and filtered through a KX syringe filter (PET 25 mm, 0.22). The filtered samples were analyzed using an Agilent 6530 LC/MS QToF device that detects the ion mass (M/z) and relative abundance of isotopes. With this device, we determined which ions were in the samples based on the masses that were detected [14].
LC/MS QToF analysis was performed with an InfinityLab Poroshell 120 EC–C18 (3.0 × 100 mm, 2.7 μm, Agilent Technologies, Inc.) column. We injected 0,4 mL/min of analyte into the following mobile phases: (A) deionized water with 0.1 % formic acid, (B) MeOH with 0.1 % formic acid, and (C) acetonitrile (ACN) with 0.1 % formic acid. These mobile phases were used in 20 min runs in the positive ionization mode, with a column temperature of 35 °C.
3. Results
The OPEs that were detected with the above-mentioned methodology were identified with the Agilent Water Screening Personal Compound Database and Library (PCDL), and the results are shown in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6.
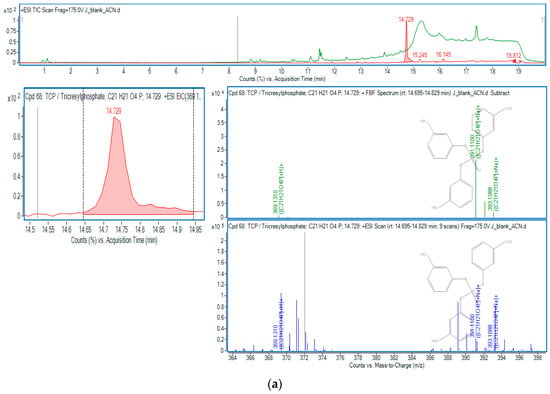
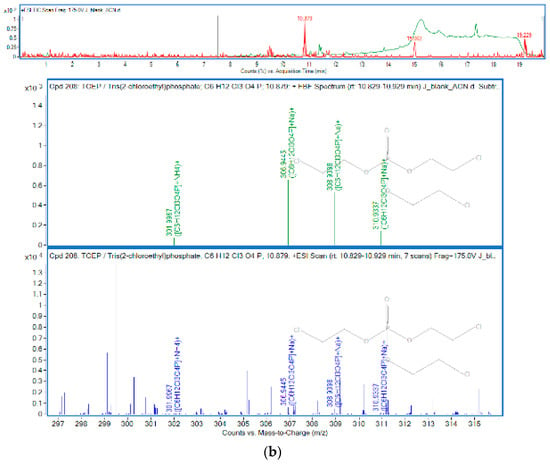
Figure 1.
OPEs in sample J: (a) TCP; (b) TCEP.
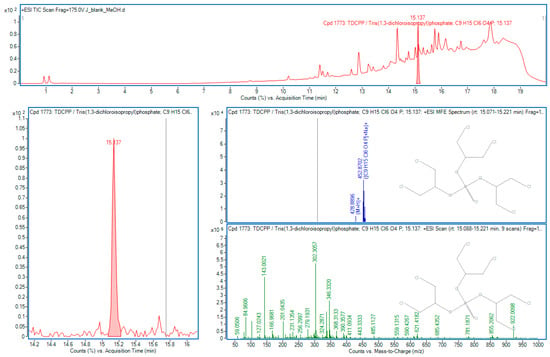
Figure 2.
TDCPP in sample J.
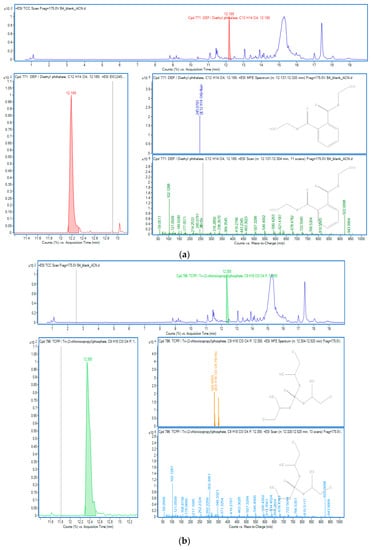
Figure 3.
OPEs in sample B4: (a) DEP; (b) TCPP.
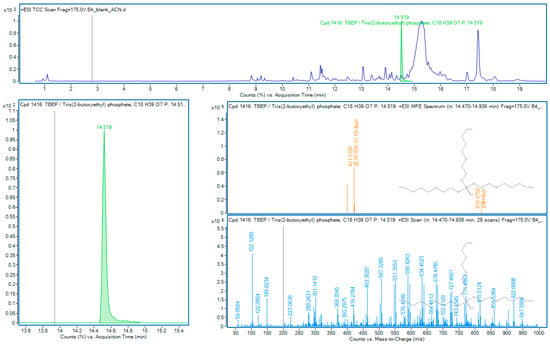
Figure 4.
TBEP in sample B4.
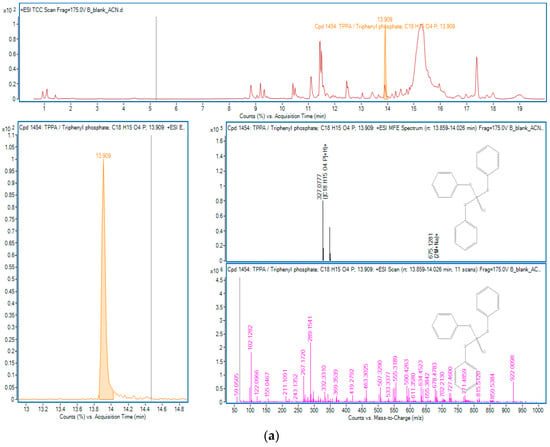
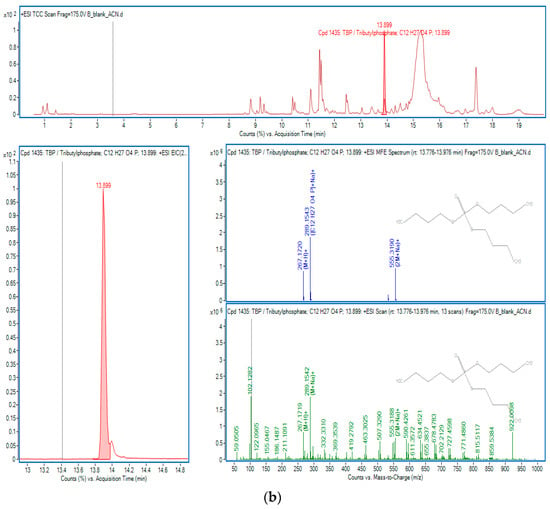
Figure 5.
OPEs in sample B: (a) TPPA; (b) TBP.
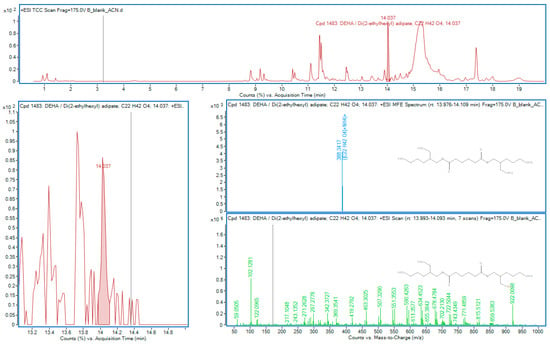
Figure 6.
DEHA in sample B.
4. Conclusions and Discussion
OPEs are potential pollutants of groundwaters that are not under regulations, and there are no defined maximum available concentrations for such emerging pollutants. OPEs can be found in nature in relatively small concentrations, and it is not easy to identify their presence in groundwater. In this research, nine OPEs were detected in groundwater samples from a karst basin in Croatia, which were taken during each season for one year. In the areas where the samples were taken, the water used for the water supply system is withdrawn from the karst aquifer, used here as a case study. Consequently, there is a need to raise public awareness of the usage and release of OPEs into the environment. Additionally, some of the OPEs found in the samples are toxic, i.e., dangerous for human health, due to their persistence in the water environment. This is why research on their identification, quantification, and behavior in groundwater is important.
Author Contributions
Conceptualization, L.P. and A.P.S.; methodology, L.P.; validation, I.G., A.P.S. and R.B.; formal analysis, L.P.; investigation, L.P.; writing—original draft preparation, L.P.; writing—review and editing, I.G., R.B. and A.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EU funded projects, grant numbers KK.01.1.1.04.0006 and KK.05.1.1.02.0022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000L0060 (accessed on 17 October 2022).
- Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the Protection of Groundwater against Pollution and Deterioration. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02006L0118-20140711 (accessed on 17 October 2022).
- Common Implementation Strategy for the Water Framework Directive and the Floods Directive, Voluntary Groundwater Watch List. June 2019. Available online: https://circabc.europa.eu/sd/a/e6882891-d4a2-4a64-9cf7-f04e13b0d17e/Voluntary%20Groundwater%20Watch%20List%20(Endorsed%20V3.1%20-%20June%202019).pdf (accessed on 17 October 2022).
- Reemtsma, T.; García-López, M.; Rodríguez, I.; Quintana, J.B.; Rodil, R. Organophosphorus Flame Retardants and Plasticizers in Water and Air I. Occurrence and Fate. TrAC-Trends Anal. Chem. 2008, 27, 727–737. [Google Scholar] [CrossRef]
- Chokwe, T.B.; Abafe, O.A.; Mbelu, S.P.; Okonkwo, J.O.; Sibali, L.L. A Review of Sources, Fate, Levels, Toxicity, Exposure and Transformations of Organophosphorus Flame-Retardants and Plasticizers in the Environment. Emerg. Contam. 2020, 6, 345–366. [Google Scholar] [CrossRef]
- Quintana, J.B.; Rodil, R.; Reemtsma, T.; García-López, M.; Rodríguez, I. Organophosphorus Flame Retardants and Plasticizers in Water and Air II. Analytical Methodology. TrA-Trends Anal. Chem. 2008, 27, 904–915. [Google Scholar] [CrossRef]
- EPA Chemicals under the TSCA. Available online: https://www.epa.gov/chemical-data-reporting (accessed on 20 November 2022).
- O’Neil, M.J. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals; Merck and Co., Inc.: Whitehouse Station, NJ, USA, 2006; p. 1676. [Google Scholar]
- Weil, E.D. Flame Retardants, Phosphorus. In Kirk-Othmer Encyclopedia of Chemical Technology (1999–2012); John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Documentation of the TLVs and BEIs with Other World Wide Occupational Exposure Values, 7th ed.; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2013.
- European Commission/European Chemical Substances Information System (ESIS); European Union Risk Assessment Report, Tris(2-Chloroethyl) Phosphate (115-96-8). July 2009, p. 17. Available online: https://esis.jrc.ec.europa.eu/ (accessed on 3 September 2014).
- Haz-Map, Information on Hazardous Chemicals and Occupational Diseases. Available online: https://haz-map.com/Agents/1943 (accessed on 20 November 2022).
- Crump, D.; Chiu, S.; Kennedy, S.W. Effects of Tris(1,3-dichloro-2-propyl) phosphate and Tris(1-chloropropyl) phosphate on Cytotoxicity and mRNA Expression in Primary Cultures of Avian Hepatocytes and Neuronal Cells. Toxicol. Sci. 2012, 126, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Moschet, C.; Lew, B.M.; Hasenbein, S.; Anumol, T.; Young, T.M. LC- and GC-QTOF-MS as Complementary Tools for a Comprehensive Micropollutant Analysis in Aquatic Systems. Environ. Sci. Technol. 2017, 51, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).