Potential Hazard to Human and Animal Health from Bacterial and Fungal Contaminants in Small Freshwater Reservoirs †
Abstract
1. Introduction
2. Materials and Methods
2.1. Localization of Water Tanks
2.2. Culture Media
2.3. Water Sampling and Microbiological Analyses
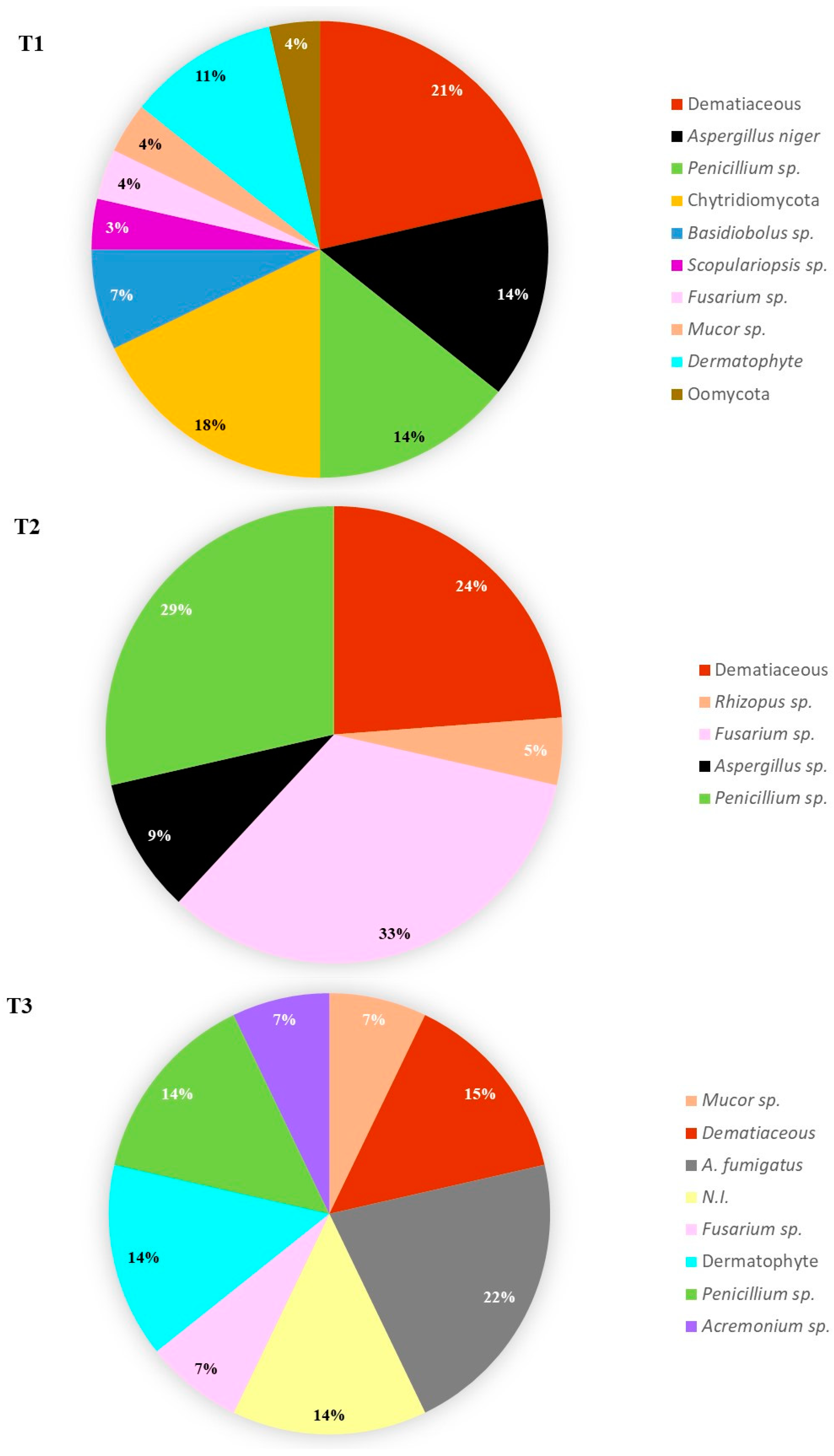
3. Results and Discussion
3.1. Water Samples Bacterial Load
3.2. Water Samples Fungal Load
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, H. Surface waters. In Handbook of Water and Wastewater Microbiology; Mara, D., Horan, N., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 611–626. [Google Scholar]
- Ashbolt, N.J.; Grabow, W.O.; Snozzi, M. Indicators of microbial water quality. In Water Quality: Guidelines, Standards and Health: Assessment of Risk and Risk Management for Water-Related Infectious Disease; Fewtrell, L., Bartram, J., Eds.; IWA Publishing: London, UK, 2001; pp. 289–316. [Google Scholar]
- Kator, H.; Rhodes, M. 8—Detection, enumeration, and identification of environmental microorganisms of public health significance. In Handbook of Water and Wastewater Microbiology; Mara, D., Horan, N., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 113–144. [Google Scholar]
- Hageskal, G.; Knutsen, A.K.; Gaustad, P.; de Hoog, G.S.; Skaar, I. Diversity and significance of mold species in Norwegian drinking water. Appl. Environ. Microbiol. 2006, 72, 7586–7593. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.J.; Basílio, M.C.; Fernandes, D.; Domingues, M.; Paiva, J.M.; Benoliel, M.J.; San Romão, M.V. Occurrence of filamentous fungi and yeasts in three different drinking water sources. Water Res. 2009, 43, 3813–3819. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.J.; Burton, M.J.; Leck, A. Mycotic keratitis—A global threat from the filamentous fungi. J. Fungi 2021, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Brandão, J.; Gangneux, J.P.; Arikan-Akdagli, S.E.V.T.A.P.; Barac, A.; Bostanaru, A.C.; Brito, S.; Segal, E. Mycosands: Fungal diversity and abundance in beach sand and recreational waters—Relevance to human health. Sci. Total Environ. 2021, 781, 146598. [Google Scholar] [CrossRef] [PubMed]
- Shearer, C.A.; Descals, E.; Kohlmeyer, B.; Kohlmeyer, J.; Marvanová, L.; Padgett, D.; Voglymayr, H. Fungal biodiversity in aquatic habitats. Biodivers. Conserv. 2007, 16, 49–67. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Shivaprakash, M.R. Microbiology of systemic fungal infections. J. Postgrad Med. 2005, 51, 16. [Google Scholar]
- WHO. Guidelines on Recreational Water Quality. Volume 1: Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2021; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Ameen, M.; Arenas, R. Developments in the management of mycetomas. Clin. Exp. Dermatol. 2009, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Revankar, S.G.; Sutton, D.A. Melanized Fungi in Human Disease. Clin. Microbiol. Rev. 2012, 25, 720. [Google Scholar] [CrossRef]
- Boehm, A.B.; Sassoubre, L.M. Enterococci as Indicators of Environmental Fecal Contamination. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014; pp. 73–90. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- O’Hara, C.M.; Brenner, F.W.; Miller, J.M. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 2000, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Arvanitidou, M.; Kanellou, K.; Vagiona, D.G. Diversity of Salmonella spp. and fungi in northern Greek rivers and their correlation to fecal pollution indicators. Environ. Res. 2005, 99, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Hageskal, G.; Lima, N.; Skaar, I. The study of fungi in drinking water. Mycol. Res. 2009, 113, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.B.; Paterson, R.R.M.; Lima, N. Survey and significance of filamentous fungi from tap water. Int. J. Hyg. Environ. Health 2006, 209, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.; Paterson, R.; Kinsey, G.; Pitchers, R.; Rossmoore, H. Identification, significance and control of fungi in water distribution systems. In Proceedings of the Water Technology Conference, Denver, CO, USA, 9–12 November 1997. [Google Scholar]
- Webster, J.; Weber, R. Introduction to Fungi; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Raugi, G.; Nguyen, T.U. 22—Superficial Dermatophyte Infections of the Skin. In Netter’s Infectious Diseases; Jong, E.C., Stevens, D.L., Eds.; Saunders: Philadelphia, PA, USA, 2012; pp. 102–109. [Google Scholar]
- Araújo, R.; Pina-Vaz, C.; Rodrigues, A.G. Surveillance of airborne Aspergillus in a Portuguese University Hospital. Mycoses 2005, 48, 45. [Google Scholar]

| Presumptive Bacteria | Tank 1 | Tank 2 | Tank 3 |
|---|---|---|---|
| E. coli (UFC/100 mL) 1 | 4 ± 2 | 43 ± 6 | 133 ± 58 |
| Fecal coliforms (UFC/100 mL) 2 | >300 | >300 | >300 |
| Fecal enterococci (UFC/100 mL) 1 | 8 ± 3 | 3 ± 1 | 167 ± 115 |
| S. aureus (UFC/mL) | 23 ± 15 | 3 ± 6 | 77 ± 45 |
| Proteus sp. (UFC/mL) | 2690 ± 279 | 260 ± 46 | 740 ± 426 |
| Other (UFC/mL) 3 | 0 | 0 | 27 ± 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourão, A.V.; Sampaio, A. Potential Hazard to Human and Animal Health from Bacterial and Fungal Contaminants in Small Freshwater Reservoirs. Environ. Sci. Proc. 2022, 24, 7. https://doi.org/10.3390/ECERPH-4-13071
Mourão AV, Sampaio A. Potential Hazard to Human and Animal Health from Bacterial and Fungal Contaminants in Small Freshwater Reservoirs. Environmental Sciences Proceedings. 2022; 24(1):7. https://doi.org/10.3390/ECERPH-4-13071
Chicago/Turabian StyleMourão, Ana V., and Ana Sampaio. 2022. "Potential Hazard to Human and Animal Health from Bacterial and Fungal Contaminants in Small Freshwater Reservoirs" Environmental Sciences Proceedings 24, no. 1: 7. https://doi.org/10.3390/ECERPH-4-13071
APA StyleMourão, A. V., & Sampaio, A. (2022). Potential Hazard to Human and Animal Health from Bacterial and Fungal Contaminants in Small Freshwater Reservoirs. Environmental Sciences Proceedings, 24(1), 7. https://doi.org/10.3390/ECERPH-4-13071







