Genetic Diversity of Silver Fir (Abies alba) and European Beech (Fagus sylvatica) Populations from the South-Eastern Limits of Their Natural Distribution †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling, DNA Extraction, SSR Genotyping and Data Analysis
2.2. Comparing Populations
3. Results and Discussion
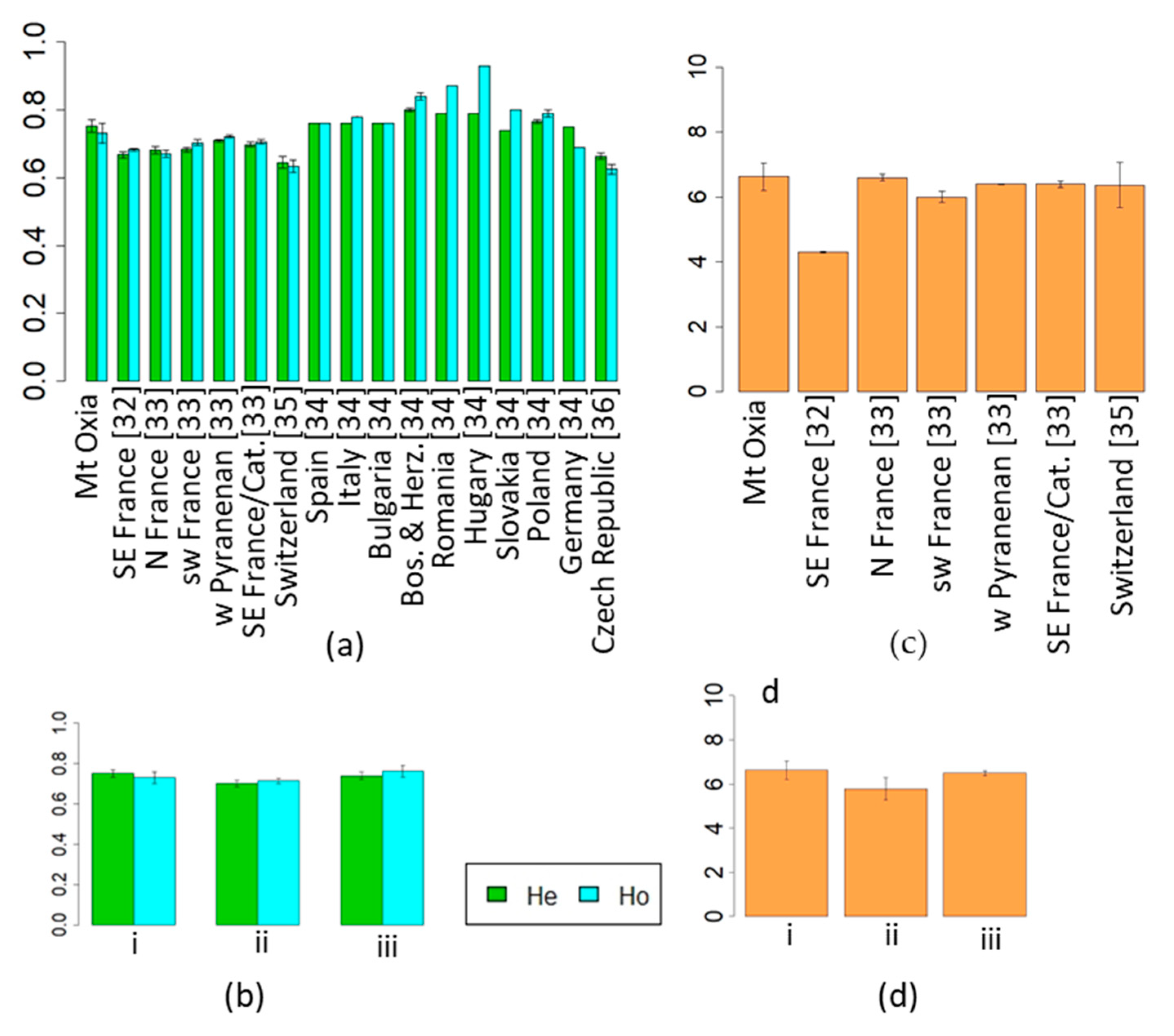
3.1. Comparison of the Abies alba Marginal Population with Populations from Others Parts of Their Distribution
3.2. Comparison of the Fagus sylvatica Marginal Population with Populations from Others Parts of Their Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Aravanopoulos, F.A.; Alizoti, P.G.; Farsakoglou, A.-M.; Malliarou, E.; Avramidou, E.V.; Tourvas, N. State of Biodiversity and Forest Genetic Resources in Greece in Relation to Conservation. In Forests of Southeast Europe Under a Changing Climate: Conservation of Genetic Resources; Šijačić-Nikolić, M., Milovanović, J., Nonić, M., Eds.; Springer International Publishing: Cham, The Netherlands, 2019; pp. 73–83. [Google Scholar]
- European Commision. Green Paper from the Commission to the Council, the European Parliament, the European Economic and Social Committee and the Committee of the Regions—Adapting to climate change in Europe–options for EU action {SEC(2007) 849}/* COM/2007/0354 final */. 2007. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52007DC0354 (accessed on 23 November 2022).
- Falk, W.; Hempelmann, N. Species Favourability Shift in Europe due to Climate Change: A Case Study for Fagus sylvatica L. and Picea abies (L.) Karst. Based on an Ensemble of Climate Models. J. Climatol. 2013, 2013, 18. [Google Scholar] [CrossRef]
- Fréjaville, T.; Fady, B.; Kremer, A.; Ducousso, A.; Benito Garzón, M. Inferring phenotypic plasticity and population responses to climate across tree species ranges using forest inventory data. Glob. Ecol. Biogeogr. 2019, 28, 1259–1271. [Google Scholar] [CrossRef]
- Diaci, J.; Rozenbergar, D.; Anic, I.; Mikac, S.; Saniga, M.; Kucbel, S.; Visnjic, C.; Ballian, D. Structural dynamics and synchronous silver fir decline in mixed old-growth mountain forests in Eastern and Southeastern Europe. Forestry 2011, 84, 479–491. [Google Scholar] [CrossRef]
- Gárate-Escamilla, H.; Hampe, A.; Vizcaíno-Palomar, N.; Robson, T.M.; Benito Garzón, M. Range-wide variation in local adaptation and phenotypic plasticity of fitness-related traits in Fagus sylvatica and their implications under climate change. Glob. Ecol. Biogeogr. 2019, 28, 1336–1350. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Gutiérrez, E.; Popa, I.; Andreu-Hayles, L.; Motta, R.; Nola, P.; Ribas, M.; Sangüesa-Barreda, G.; Urbinati, C.; et al. Distinct effects of climate warming on populations of silver fir (Abies alba) across Europe. J. Biogeogr. 2015, 42, 1150–1162. [Google Scholar] [CrossRef]
- Korakis, G. Forest Botany. Available online: https://repository.kallipos.gr/handle/11419/742 (accessed on 23 November 2022).
- Fady, B.; Aravanopoulos, F.A.; Alizoti, P.; Mátyás, C.; von Wühlisch, G.; Westergren, M.; Belletti, P.; Cvjetkovic, B.; Ducci, F.; Huber, G.; et al. Evolution-based approach needed for the conservation and silviculture of peripheral forest tree populations. For. Ecol. Manag. 2016, 375, 66–75. [Google Scholar] [CrossRef]
- Kramer, K.; Degen, B.; Buschbom, J.; Hickler, T.; Thuiller, W.; Sykes, M.T.; de Winter, W. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change—Range, abundance, genetic diversity and adaptive response. For. Ecol. Manag. 2010, 259, 2213–2222. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Koufaki, T.; Sazeides, C.I.; Spyroglou, G.; Theodorou, K. Potential impacts of climate change on the habitat suitability of the dominant tree species in Greece. Plants 2022, 11, 1616. [Google Scholar] [CrossRef]
- Vella, E.; Kyriakopoulou, E.; Tsiaoussi, V.; Doulgeris, C.; Kemitzoglou, D.; Xepapadeas, A.; Papadimos, D.; Seferlis, M.; Chrysopolitou, V. Climate Change Impacts on Biodiversity and Ecosystems. 2011. Available online: https://www.bankofgreece.gr/Publications/ClimateChange_FullReport_bm.pdf (accessed on 23 November 2022).
- Postolache, D.; Leonarduzzi, C.; Piotti, A.; Spanu, I.; Roig, A.; Fady, B.; Roschanski, A.; Liepelt, S.; Vendramin, G.G. Transcriptome versus Genomic Microsatellite Markers: Highly informative multiplexes for genotyping Abies alba Mill. and congeneric species. Plant Mol. Biol. Rep. 2014, 32, 750–760. [Google Scholar] [CrossRef]
- Cremer, E.; Liepelt, S.; Sebastiani, F.; Buonamici, A.; Michalczyk, I.M.; Ziegenhagen, B.; Vendramin, G.G. Identification and characterization of nuclear microsatellite loci in Abies alba Mill. Mol. Ecol. Notes 2006, 6, 374–376. [Google Scholar] [CrossRef]
- Hansen, O.K.; Vendramin, G.G.; Sebastiani, F.; Edwards, K.J. Development of microsatellite markers in Abies nordmanniana (Stev.) Spach and cross-species amplification in the Abies genus. Mol. Ecol. Notes 2005, 5, 784–787. [Google Scholar] [CrossRef]
- Lefevre, S.; Wagner, S.; Petit, R.J.; de Lafontaine, G. Multiplexed microsatellite markers for genetic studies of beech. Mol. Ecol. Resour. 2012, 12, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinf. (Oxf. Engl.) 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T. hp-rare 1.0: A computer program for performing rarefaction on measures of allelic richness. Mol. Ecol. Notes 2005, 5, 187–189. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Peguero-Pina, J.J.; Cremer, E.; Camarero, J.; Cancio, Á.; Ibáñez, N.; Konnert, M.; Pelegrín, E. Genetic and environmental characterization of Abies alba Mill. populations at its western rear edge. Pirineos 2014, 169, e007. [Google Scholar] [CrossRef]
- Dering, M.; Sękiewicz, K.; Boratyńska, K.; Litkowiec, M.; Iszkuło, G.; Romo, A.; Boratyński, A. Genetic diversity and inter-specific relations of western Mediterranean relic Abies taxa as compared to the Iberian A. alba. Flora Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 367–374. [Google Scholar] [CrossRef][Green Version]
- Belletti, P.; Ferrazzini, D.; Ducci, F.; De Rogatis, A.; Mucciarelli, M.; Mucciarelli, M. Genetic diversity of Italian populations of Abies alba. Dendrobiology 2017, 77, 147–159. [Google Scholar] [CrossRef]
- Ducci, F.; De Rogatis, A.; Proietti, R.; Curtu, A.L.; Marchi, M.; Benetti, P. Establishing a baseline to monitor future climate-change-effects on peripheral populations of Abies alba in central Apennines. Ann. For. Res. 2021, 64, 33–66. [Google Scholar] [CrossRef]
- De Luca, D.; Menale, B.; Caputo, P.; Cennamo, P. Population genetics analysis in a relic population of silver fir (Abies alba Mill.) in southern Italy: A comparison with microsatellites and reference data. Plant Biosyst. 2017, 151, 567–573. [Google Scholar] [CrossRef]
- Leonarduzzi, C.; Piotti, A.; Spanu, I.; Vendramin, G.G. Effective gene flow in a historically fragmented area at the southern edge of silver fir (Abies alba Mill.) distribution. Tree Genet. Genomes 2016, 12, 95. [Google Scholar] [CrossRef]
- Piotti, A.; Leonarduzzi, C.; Postolache, D.; Bagnoli, F.; Spanu, I.; Brousseau, L.; Urbinati, C.; Leonardi, S.; Vendramin, G.G. Unexpected scenarios from Mediterranean refugial areas: Disentangling complex demographic dynamics along the Apennine distribution of silver fir. J. Biogeogr. 2017, 44, 1547–1558. [Google Scholar] [CrossRef]
- Teodosiu, M.; Mihai, G.; Fussi, B.; Ciocîrlan, E. Genetic diversity and structure of Silver fir (Abies alba Mill.) at the south-eastern limit of its distribution range. Ann. For. Res. 2019, 62, 139–156. [Google Scholar] [CrossRef]
- Todea Morar, I.M.; Rensen, S.; Vilanova, S.; Boscaiu, M.; Holonec, L.; Sestras, A.F.; Vicente, O.; Prohens, J.; Sestras, R.E.; Plazas, M. Genetic relationships and reproductive traits of Romanian populations of silver ir (Abies alba): Implications for the sustainable management of local populations. Sustainability 2020, 12, 4199. [Google Scholar] [CrossRef]
- Gömöry, D.; Paule, L.; Krajmerová, D.; Romšáková, I.; Longauer, R. Admixture of genetic lineages of different glacial origin: A case study of Abies alba Mill. in the Carpathians. Plant Syst. Evol. 2012, 298, 703–712. [Google Scholar] [CrossRef]
- Cvrčková, H.; Máchová, P.M.; Malá, J. Use of nuclear microsatellite loci for evaluating genetic diversity among selected populations of Abies alba Mill. in the Czech Republic. J. For. Sci. 2015, 61, 345–351. [Google Scholar] [CrossRef]
- Paluch, J.; Zarek, M.; Kempf, M. The effect of population density on gene flow between adult trees and the seedling bank in Abies alba Mill. Eur. J. For. Res. 2019, 138, 203–217. [Google Scholar] [CrossRef]
- Lander, T.A.; Klein, E.K.; Roig, A.; Oddou-Muratorio, S. Weak founder effects but significant spatial genetic imprint of recent contraction and expansion of European beech populations. Heredity 2021, 126, 491–504. [Google Scholar] [CrossRef]
- de Lafontaine, G.; Ducousso, A.; Lefèvre, S.; Magnanou, E.; Petit, R.J. Stronger spatial genetic structure in recolonized areas than in refugia in the European beech. Mol. Ecol. 2013, 22, 4397–4412. [Google Scholar] [CrossRef]
- Höhn, M.; Major, E.; Avdagic, A.; Bielak, K.; Bosela, M.; Coll, L.; Dinca, L.; Giammarchi, F.; Ibrahimspahic, A.; Mataruga, M.; et al. Local characteristics of the standing genetic diversity of european beech with high within-region differentiation at the eastern part of the range. Can. J. For. Res. 2021, 51, 1791–1798. [Google Scholar] [CrossRef]
- Cuervo-Alarcon, L.; Arend, M.; Müller, M.; Sperisen, C.; Finkeldey, R.; Krutovsky, K.V. Genetic variation and signatures of natural selection in populations of European beech (Fagus sylvatica L.) along precipitation gradients. Tree Genet. Genomes 2018, 14, 84. [Google Scholar] [CrossRef]
- Zádrapová, D. Microsatellite analysis of genetic diversity in Czech populations of European beech (Fagus sylvatica L.). Metsanduslikud Uurim. 2021, 73, 64–76. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalmaris, E.; Tourvas, N.; Aravanopoulos, F.A. Genetic Diversity of Silver Fir (Abies alba) and European Beech (Fagus sylvatica) Populations from the South-Eastern Limits of Their Natural Distribution. Environ. Sci. Proc. 2022, 22, 37. https://doi.org/10.3390/IECF2022-13129
Dalmaris E, Tourvas N, Aravanopoulos FA. Genetic Diversity of Silver Fir (Abies alba) and European Beech (Fagus sylvatica) Populations from the South-Eastern Limits of Their Natural Distribution. Environmental Sciences Proceedings. 2022; 22(1):37. https://doi.org/10.3390/IECF2022-13129
Chicago/Turabian StyleDalmaris, Eleftheria, Nikolaos Tourvas, and Filippos A. Aravanopoulos. 2022. "Genetic Diversity of Silver Fir (Abies alba) and European Beech (Fagus sylvatica) Populations from the South-Eastern Limits of Their Natural Distribution" Environmental Sciences Proceedings 22, no. 1: 37. https://doi.org/10.3390/IECF2022-13129
APA StyleDalmaris, E., Tourvas, N., & Aravanopoulos, F. A. (2022). Genetic Diversity of Silver Fir (Abies alba) and European Beech (Fagus sylvatica) Populations from the South-Eastern Limits of Their Natural Distribution. Environmental Sciences Proceedings, 22(1), 37. https://doi.org/10.3390/IECF2022-13129






