Calcite Mineral Catalyst Capable of Enhancing Micropollutant Degradation during the Ozonation Process at pH7 †
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Adsorption of Micropollutants by Calcite
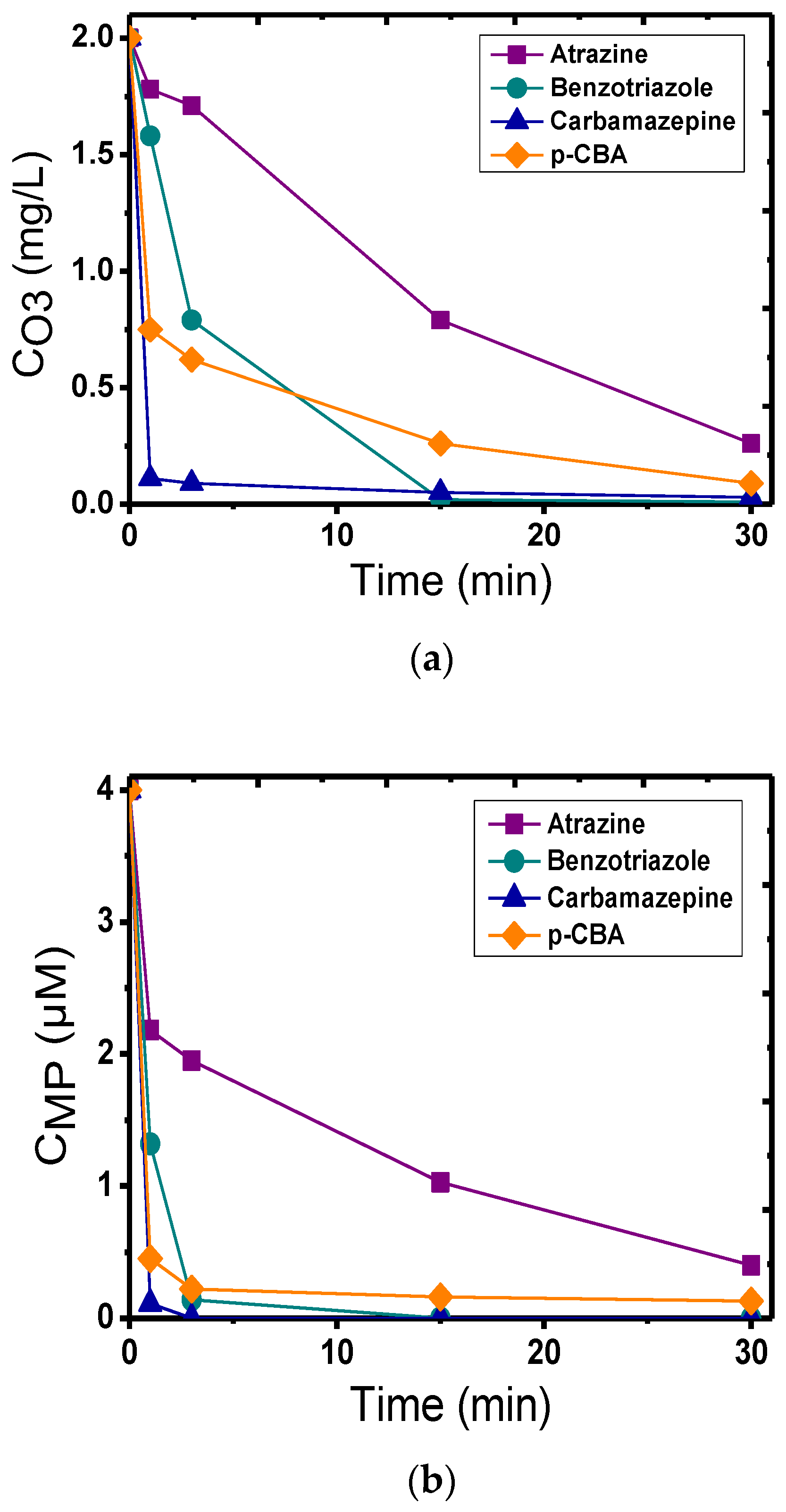
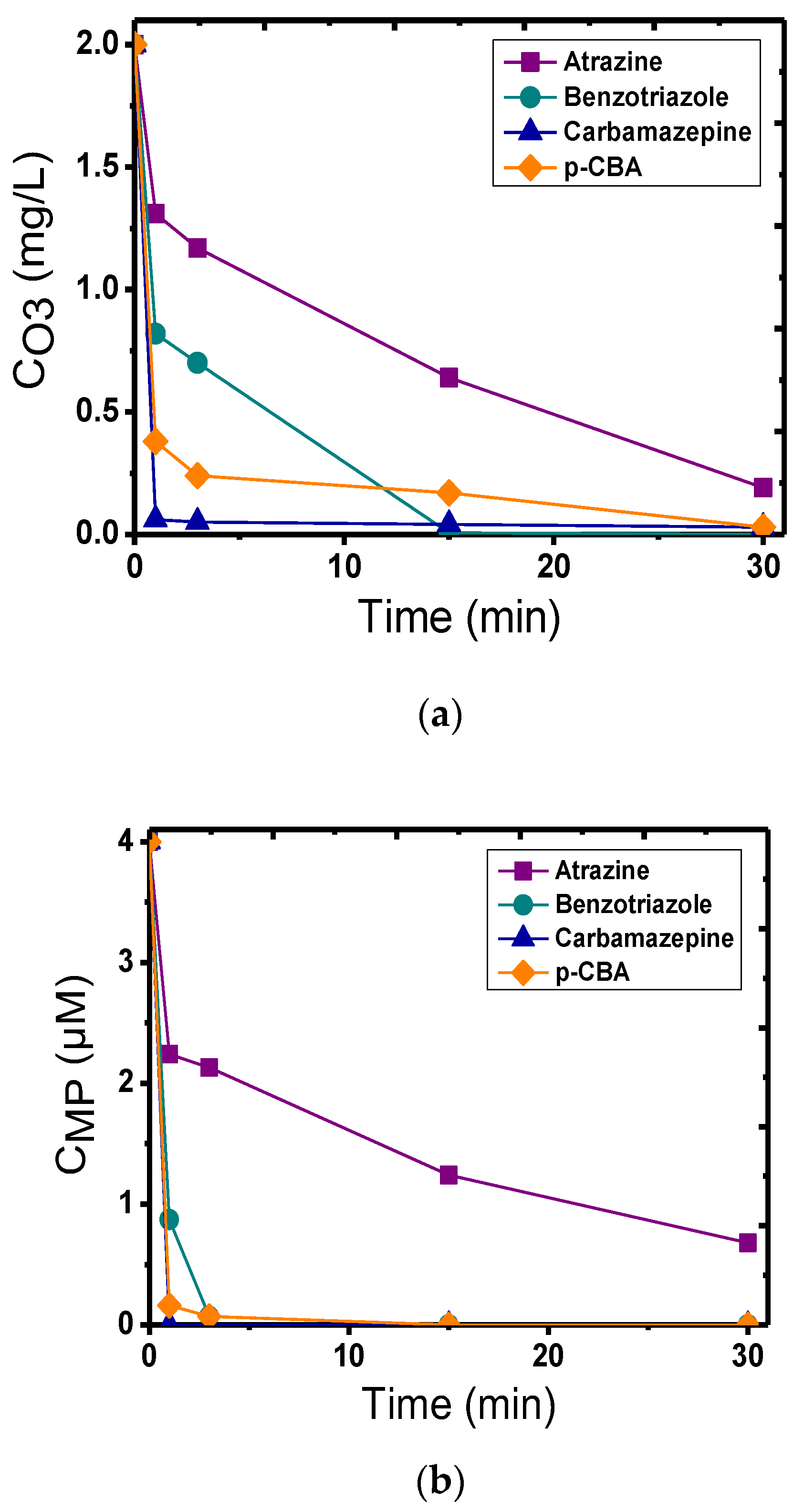
3.2. Calcite as a Catalyst in Heterogeneous Catalytic Ozonation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kapelewska, J.; Kotowska, U.; Karpi, J.; Kowalczuk, D.; Arciszewska, A.; Anna, Ś. Occurrence, removal, mass loading and environmental risk assessment of emerging organic contaminants in leachates, groundwaters and wastewaters. Microchem. J. 2018, 137, 292–301. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Z.; Huo, Y.; Yang, M.; Zheng, X.; Zhang, Y. Monitoring of 943 organic micropollutants in wastewater from municipal wastewater treatment plants with secondary and advanced treatment processes. J. Environ. Sci. 2018, 67, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Zoh, K.D. Occurrence and removals of micropollutants in water environment. Environ. Eng. Res. 2016, 21, 319–332. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment : Current knowledge, under studied areas and recommendations for future monitoring. Water Res. 2014, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water : A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Nawrocki, J. Catalytic ozonation in water: Controversies and questions. Discussion paper. Appl. Catal. B Environ. 2013, 142, 465–471. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Wang, B.; Deng, S.; Huang, J.; Yu, G.; Wang, Y. Prediction of micropollutant abatement during homogeneous catalytic ozonation by a chemical kinetic model. Water Res. 2018, 142, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, J.; Dong, W.; Ma, J.; Cao, J.; Li, T.; Li, J.; Gu, J.; Liu, P. Study on enhanced degradation of atrazine by ozonation in the presence of hydroxylamine. J. Hazard. Mater. 2016, 316, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Roshani, B.; McMaster, I.; Rezaei, E.; Soltan, J. Catalytic ozonation of benzotriazole over alumina supported transition metal oxide catalysts in water. Sep. Purif. Technol. 2014, 135, 158–164. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Gonzalo, M.S.; García-Calvo, E. Catalytic ozonation of naproxen and carbamazepine on titanium dioxide. Appl. Catal. B Environ. 2008, 84, 48–57. [Google Scholar] [CrossRef]
- Lan, B.; Huang, R.; Li, L.; Yan, H.; Liao, G.; Wang, X.; Zhang, Q. Catalytic ozonation of p-chlorobenzoic acid in aqueous solution using Fe-MCM-41 as catalyst. Chem. Eng. J. 2013, 219, 346–354. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Psaltou, S.; Stylianou, S.; Mitrakas, M.; Zouboulis, A.I. Heterogeneous Catalytic Ozonation of p-Chlorobenzoic Acid in Aqueous Solution by FeMnOOH and PET. Separations 2018, 5, 42. [Google Scholar] [CrossRef]
- Clesceri, S.L.; Greenberg, E.A.; Trussel, R.R. Standard Methods for Examination of Water and Wastewater, 17th ed.; Inorganic Nonmetals; American Public Health Association: Washington, DC, USA, 1989; pp. 162–165. ISBN 0-87553-161-X.
- Kosmulski, M. Surface Charging and Points Zero Charge; CRC: Boca Raton, FL, USA, 2009; p. 145. ISBN 978-1-4200-51889-9. [Google Scholar]
- Mandal, S. Reaction Rate Constants of Hydroxyl Radicals with Micropollutants and Their Significance in Advanced Oxidation Processes. J. Adv. Oxid. Technol. 2018, 21. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Han, K.N. (Eds.) Principles of Mineral Processing, Society for Mining, Metallurgy, and Exploration; Inc. (SME): Littleton, CO, USA, 2003; Volume 18, ISBN 0-87335-167-3. [Google Scholar]
- Pines, D.S.; Reckhow, D.A. Solid Phase Catalytic Ozonation Process for the Destruction of a Model Pollutant. Ozone Sci. Eng. 2016, 5, 25–39. [Google Scholar] [CrossRef]
- Andreozzi, R.; Marotta, R.; Pinto, G.; Pollio, A. Carbamazepine in water : Persistence in the environment, ozonation treatment and preliminary assessment on algal toxicity. Water Res. 2002, 36, 2869–2877. [Google Scholar] [CrossRef]
- Aguilar, C.M.; Vazquez-arenas, J.; Castillo-araiza, O.O.; Rodríguez, J.L.; Salinas, E.; Poznyak, T. Improving ozonation to remove carbamazepine through ozone-assisted catalysis using different NiO concentrations. Environ. Sci. Pollut. Res. 2020, 27, 22184–22194. [Google Scholar] [CrossRef]
- Fan, X.; Restivo, J.; Órfão, J.J.M.; Fernando, M.; Pereira, R.; Lapkin, A.A. The role of multiwalled carbon nanotubes (MWCNTs) in the catalytic ozonation of atrazine. Chem. Eng. J. 2014, 241, 66–76. [Google Scholar] [CrossRef]
- Kermani, M.; Kakavandi, B.; Farzadkia, M.; Esrafili, A.; Jokandan, S.F.; Shahsavani, A. Catalytic ozonation of high concentrations of catechol over TiO2@Fe3O4 magnetic core-shell nanocatalyst: Optimization, toxicity and degradation pathway studies. J. Clean. Prod. 2018, 192, 597–607. [Google Scholar] [CrossRef]
- Shahama, Y.D.; Sadeghi, M.; Shahryari, A.; Okhovat, N.; Asl, F.B.; Baneshi, M.R. Heterogeneous catalytic ozonation of 2,4-dinitrophenol in aqueous solution by magnetic carbonaceous nanocomposite: catalytic activity and mechanism. Desalin. Water Treat. 2015, 57, 20447–20456. [Google Scholar]
| Micropollutant | 10 mM H3PO4 (% v/v) | ACN (% v/v) |
|---|---|---|
| Atrazine | 50 | 50 |
| Benzotriazole | 75 | 25 |
| Carbamazepine | 60 | 40 |
| p-CBA | 60 | 40 |
| Micropollutant | Structure | MW | Log D at pH 8 | pKa | kO3 (M−1s−1) | k•OH(M−1s−1) |
|---|---|---|---|---|---|---|
| Atrazine | 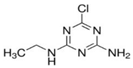 | 215.7 | 2.20 | 3.2 | 6[8] | 2.4×109 [16] |
| Benzotriazole | 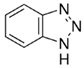 | 119.1 | 1.21 | 9.04 | 20[9] | 7.6×109 [16] |
| Carbamazepine | 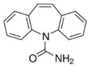 | 236.3 | 2.77 | 16 | 3×105 [10] | 8.8×109 [16] |
| p-CBA | 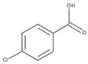 | 156.6 | −1.15 | 4.07 | 0.15[11] | 5×109 [16] |
| Parameter | Atrazine | Benzotriazole | Carbamazepine | p-CBA |
|---|---|---|---|---|
| q (μg MP/g calcite) | 289.0 | 59.6 | 104.0 | 55.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psaltou, S.; Kaprara, E.; Mitrakas, M.; Zouboulis, A. Calcite Mineral Catalyst Capable of Enhancing Micropollutant Degradation during the Ozonation Process at pH7. Environ. Sci. Proc. 2020, 2, 26. https://doi.org/10.3390/environsciproc2020002026
Psaltou S, Kaprara E, Mitrakas M, Zouboulis A. Calcite Mineral Catalyst Capable of Enhancing Micropollutant Degradation during the Ozonation Process at pH7. Environmental Sciences Proceedings. 2020; 2(1):26. https://doi.org/10.3390/environsciproc2020002026
Chicago/Turabian StylePsaltou, Savvina, Efthimia Kaprara, Manassis Mitrakas, and Anastasios Zouboulis. 2020. "Calcite Mineral Catalyst Capable of Enhancing Micropollutant Degradation during the Ozonation Process at pH7" Environmental Sciences Proceedings 2, no. 1: 26. https://doi.org/10.3390/environsciproc2020002026
APA StylePsaltou, S., Kaprara, E., Mitrakas, M., & Zouboulis, A. (2020). Calcite Mineral Catalyst Capable of Enhancing Micropollutant Degradation during the Ozonation Process at pH7. Environmental Sciences Proceedings, 2(1), 26. https://doi.org/10.3390/environsciproc2020002026








