Author Contributions
All authors have read and agree to the published version of the manuscript. Conceptualization, E.K., A.Z. and M.M.; methodology, E.K. and M.M.; validation, E.K. and M.M.; formal analysis, E.K.; investigation, E.K., C.K. and S.P.; resources, A.Z. and M.M.; data curation, E.K. and S.P.; writing—original draft preparation, E.K.; writing—review and editing, A.Z., M.M. and S.P.; visualization, E.K.; supervision, A.Z. and M.M.; project administration, A.Z.; funding acquisition, A.Z. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Catalytic ozonation continuous flow pilot unit.
Figure 1.
Catalytic ozonation continuous flow pilot unit.
Figure 2.
Influence of pH in catalytic ozonation (C0, ozone: 2 mg/L, micropollutant (MP): p-CBA, C0, MP: 2 μΜ, contact time: 7 min, catalyst: PET).
Figure 2.
Influence of pH in catalytic ozonation (C0, ozone: 2 mg/L, micropollutant (MP): p-CBA, C0, MP: 2 μΜ, contact time: 7 min, catalyst: PET).
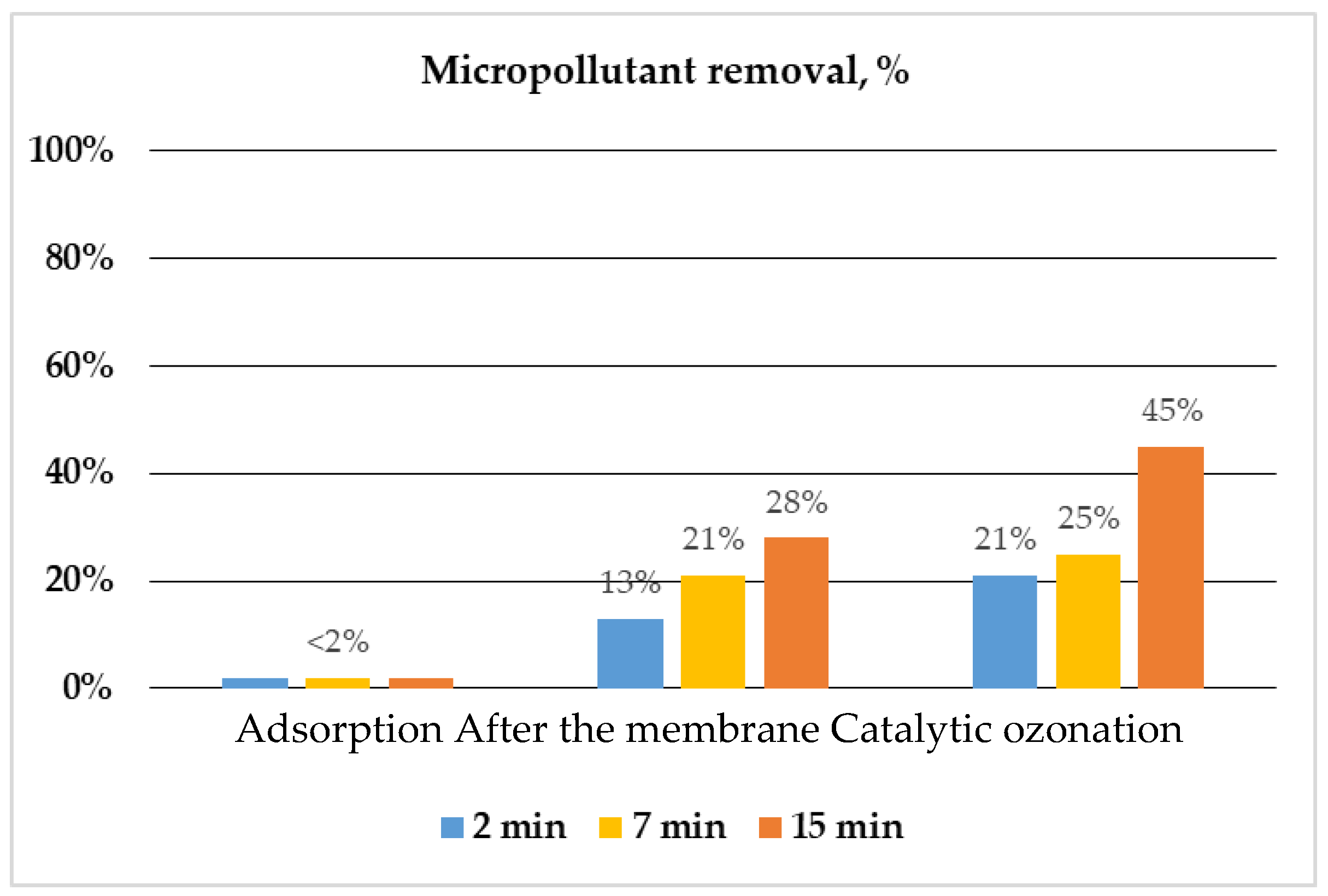
Figure 3.
Influence of contact time in catalytic ozonation (C0, ozone: 2 mg/L, MP: p-chlorobenzoic acid (p-CBA), C0, MP: 2 μΜ, pH 7, catalyst: goethite).
Figure 3.
Influence of contact time in catalytic ozonation (C0, ozone: 2 mg/L, MP: p-chlorobenzoic acid (p-CBA), C0, MP: 2 μΜ, pH 7, catalyst: goethite).
Table 1.
Mobile phase proportion for micropollutants detection by HPLC.
Table 1.
Mobile phase proportion for micropollutants detection by HPLC.
| Micropollutant (MP) | 10 mM H3PO4, % v/v | ACN, % v/v |
|---|
| Atrazine | 5 | 50 |
| Benzotriazole | 75 | 25 |
| Carbamazepine | 60 | 40 |
| p-CBA | 60 | 40 |
Table 2.
Main physicochemical characteristics of materials tested as catalysts
Table 2.
Main physicochemical characteristics of materials tested as catalysts
| Catalyst | PZC | IEP | SBET, (m2/g) | Pore volume (mL/g) |
|---|
| Alumina | 8.5 | 7.5 | 150 | 0.512 |
| Calcite | 9.7 | 8.2 | 6.3 | 0.038 |
| Dolomite | 10.1 | 9.3 | 5.1 | 0.030 |
| Goethite | 7.8 | 7.4 | 135 | 0.265 |
| Pearlite | / | / | 1.92 | 0.035 |
| PET | 6.2 | / | / | / |
| Zeolite | 6.8 | 2.2 | 21 | 0.164 |
Table 3.
Micropollutant removal and ozone decomposition in catalytic ozonation with alumina (C0, eozon: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
Table 3.
Micropollutant removal and ozone decomposition in catalytic ozonation with alumina (C0, eozon: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
| ALUMINA |
|---|
| Micropollutant Removal, % |
|---|
| Micropollutant | Adsorption | Catalytic ozonation |
| p-CBA | <2% | 29% |
| ATZ | 5% | 43% |
| BTA | 5% | 95% |
| CBZ | <2% | >99% |
| Ozone decomposition in catalytic ozonation, %. |
| p-CBA | ATZ | BTA | CBZ |
| >99% | >99% | >99% | >99% |
Table 4.
Micropollutant removal and ozone decomposition in catalytic ozonation with calcite (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
Table 4.
Micropollutant removal and ozone decomposition in catalytic ozonation with calcite (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
| CALCITE |
|---|
| Micropollutant Removal, % |
|---|
| Micropollutant | Adsorption | Catalytic ozonation |
| p-CBA | 5% | 30% |
| ATZ | <2% | 42% |
| BTA | 5% | 92% |
| CBZ | <2% | >99% |
| Ozone decomposition in catalytic ozonation, % |
| p-CBA | ATZ | BTA | CBZ |
| 90% | 79% | 83% | >99% |
Table 5.
Micropollutant removal and ozone decomposition in catalytic ozonation with dolomite (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
Table 5.
Micropollutant removal and ozone decomposition in catalytic ozonation with dolomite (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
| DOLOMITE |
|---|
| Micropollutant Removal, % |
|---|
| Micropollutant | Adsorption | Catalytic ozonation |
| p-CBA | 5% | 31% |
| ATZ | <2% | 43% |
| BTA | 5% | 90% |
| CBZ | <2% | >99% |
| Ozone decomposition in catalytic ozonation, % |
| p-CBA | ATZ | BTA | CBZ |
| 89% | 77% | 85% | >99% |
Table 6.
Micropollutant removal and ozone decomposition in catalytic ozonation with goethite (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
Table 6.
Micropollutant removal and ozone decomposition in catalytic ozonation with goethite (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
| GOETHITE |
|---|
| Micropollutant Removal, % |
|---|
| Micropollutant | Adsorption | Catalytic ozonation |
| p-CBA | <2% | 25% |
| ATZ | <2% | 31% |
| BTA | <2% | >99% |
| CBZ | <2% | >99% |
| Ozone decomposition in catalytic ozonation, % |
| p-CBA | ATZ | BTA | CBZ |
| >99% | >99% | >99% | >99% |
Table 7.
Micropollutant removal and ozone decomposition in catalytic ozonation with pearlite (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
Table 7.
Micropollutant removal and ozone decomposition in catalytic ozonation with pearlite (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
| PEARLITE |
|---|
| Micropollutant Removal, % |
|---|
| Micropollutant | Adsorption | Catalytic ozonation |
| p-CBA | 3% | 31% |
| ATZ | 5% | 28% |
| BTA | 5% | 98% |
| CBZ | <2% | >99% |
| Ozone decomposition in catalytic ozonation, % |
| p-CBA | ATZ | BTA | CBZ |
| 70% | | 76% | >99% |
Table 8.
Micropollutant removal and ozone decomposition in catalytic ozonation with PET (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
Table 8.
Micropollutant removal and ozone decomposition in catalytic ozonation with PET (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
| PET |
|---|
| Micropollutant Removal, % |
|---|
| Micropollutant | Adsorption | Catalytic ozonation |
| p-CBA | 5% | 35% |
| ATZ | <2% | 53% |
| BTA | <2% | >99% |
| CBZ | <2% | >99% |
| Ozone decomposition in catalytic ozonation, % |
| p-CBA | ATZ | BTA | CBZ |
| 95% | 95% | 95% | >99% |
Table 9.
Micropollutant removal and ozone decomposition in catalytic ozonation with zeolite (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
Table 9.
Micropollutant removal and ozone decomposition in catalytic ozonation with zeolite (C0, ozone: 2 mg/L, C0, MP: 2 μΜ, pH: 7, contact time: 7 min).
| ZEOLITE |
|---|
| Micropollutant Removal, % |
|---|
| Micropollutant | Adsorption | Catalytic ozonation |
| p-CBA | 4% | 47% |
| ATZ | 4% | 67% |
| BTA | 5% | >99% |
| CBZ | <2% | >99% |
| Ozone decomposition in catalytic ozonation, % |
| p-CBA | ATZ | BTA | CBZ |
| 92% | 84% | >99% | 92% |