1. Introduction
Pesticides are agricultural drugs used to combat weeds, plants, and insects that are harmful to the product and affect the efficiency during the cultivation of plants. They are often used in modern agriculture to meet the nutrition needs of the growing population and to obtain more products. However, pesticides are highly toxic, carcinogenic, and mutagenic, even at low concentrations and pesticides persist in nature for a long period.
Pesticide manufacturing industry wastewater causes pollution problems due to its toxic components, high chemical oxygen demand (COD), biochemical oxygen demand (BOD), high total dissolved solids (TDS) and intensive color, disgusting odor, and generally low pH values [
1]. The chemical oxidation demand of pesticide wastewater is higher than the biological oxidation demand of it. Therefore, microorganisms could not easily degrade wastewater originating from the pesticide industry. Despite the low economic cost aspect of biological treatment, conventional biological methods have not been effective methods for treating or degrading refractory and toxic organics in pesticide wastewaters. Additionally, the pH value of pesticide wastewater is very low and this pH value cannot be suitable for the biological treatment process. Thus, pesticide wastewater could not be treated by biological processes directly. It is not feasible to use a single conventional biological method or conventional chemical-precipitation methods for treating pesticide wastewater.
New technologies need to develop for the effective treatment of pesticide-containing wastewaters. Depending on highly concentrated and refractory compounds in pesticide wastewater, an advanced oxidation process should be preferred as a pretreatment process before biological treatment methods to treat or degrade non-biodegradable pesticides in wastewater. Recently, advanced oxidation processes (AOPs) have been used as potentially powerful methods capable of transforming non-biodegradable pollutants into harmless substances. Almost all AOPs rely on the generation of reactive free radicals, such as the hydroxyl, OH
● with a redox potential of 2.8 V. Therefore, advanced oxidation is a promising alternative for the mineralization and reducing recalcitrant organic compounds in wastewaters. Photo-Fenton process as an advanced oxidation process is a very effective method to degrade pesticides in wastewater and to change the form of compounds, to convert easily degraded forms for microorganisms in biological treatment methods [
2]. Additionally, the photo-Fenton process has many advantages that it requires a short reaction time and a low number of oxidant and catalyst doses compared to other advanced oxidation methods.
The major objective of this study was to investigate the advanced oxidation of pesticide wastewaters using the photo-Fenton process as a pretreatment process. The effects of hydrogen peroxide (H2O2) and ferrous ion (Fe+2) concentrations and reaction times on the oxidation of pesticide wastewater were investigated with chemical oxidation demand (COD) removal by using a Box-Behnken statistical experimental design and surface response methodology.
2. Materials and Methods
The pesticide wastewater used in these experiments, was obtained from a pesticide factory in İzmir, Turkey. This factory produced many kinds of pesticides, such as azoxytrubin, imidacloprid, epoxinazole, and methoxy fenozide. The wastewater from the pesticide production industry, which contains many precursor chemicals and pesticides, was complicated. The COD concentration of the wastewater is around 150,000 mg/L. The pH value of the wastewater was around 5. Additionally, suspended solids are very high. Photo-Fenton experiments were carried out at room temperature (23 ± 2 °C) using different hydrogen peroxide and ferrous ions. Hydrogen peroxide solution (35% w/w) and ferrous sulfate obtained from Merck were used as an oxidant and catalyst, respectively.
2.1. Design of the Experiments
The effects of variables on the response were complicated and were observed by using the most known approach, which is altering one factor at a time for multivariable systems. However, this technique is not usable for the estimation of responses. Thus, some experimental stable design should be used for the optimization of the reaction conditions. To this aim, several significant parameters are determined by response surface methodology (RSM). Among all RSM designs, the Box-Behnken design needs fewer experimental runs and allows and shows efficiency at intermediate levels not experimentally studied [
3,
4].
2.2. Experimental Procedure
All batch photo-oxidation experiments were performed in a completely mixed, batch, cylindrical photochemical reactor containing a 16 W low-pressure mercury vapor lamp with a total volume of 2.2 L. A diagram of the laboratory-scale photochemical reactor used in an oxidation experiment was given in previous studies [
5]. In the experiments, the effects of hydrogen peroxide (H
2O
2) and ferrous ion (Fe
+2) concentrations and reaction times as independent variables on the oxidation of raw pesticide wastewater were evaluated in terms of chemical oxidation demand (COD) removal as dependent variables in Box-Behnken design.
3. Results and Discussion
Box-Behnken design approach was mentioned as an effective and useful method for optimization of the three variable response functions, predicting the response of the fitted model by the ANOVA tests. Reaction conditions for an independent variable; H
2O
2 concentration was 1000–5000 mg/L (X
1), Fe
+2 concentration was 100–500 mg/L (X
2), and reaction time was determined as 5–60 minutes (X
3). The experimental conditions of the Box-Behnken experiment design and results of the Photo-Fenton processes are presented in
Table 1.
This static design was preferred because of fewer combinations of the independent variables to estimate the second-order polynomial regression model. Coefficients in the regression model were determined by means of 15 runs. Nine coefficients were calculated such as one block term, three linear terms, three quadratic terms, and three interaction terms. The objective function for COD removal efficiency with the determined coefficient is presented in Equation (1).
Response functions with determined coefficients were used to estimate variations in response functions with the independent variables under different conditions.
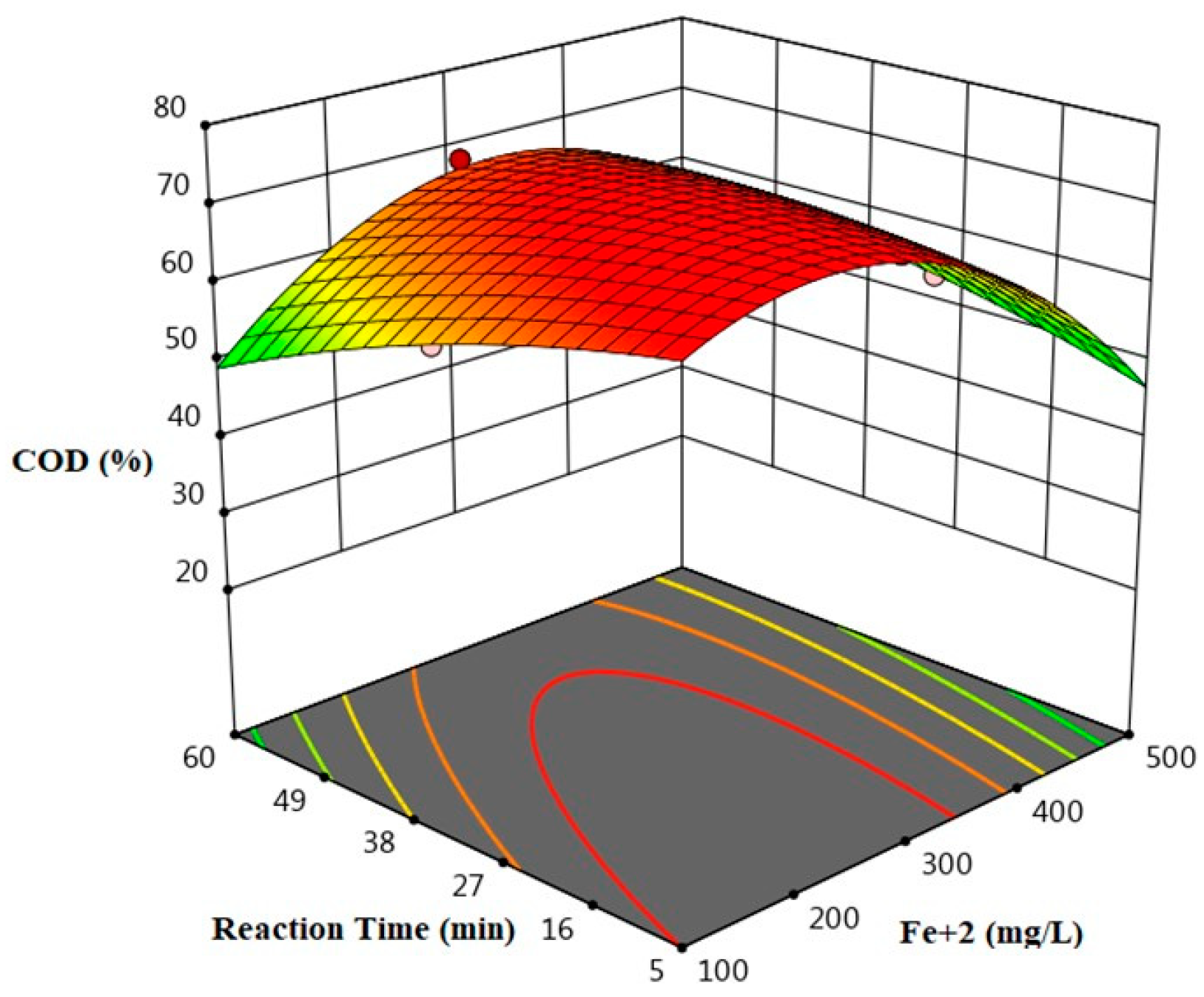
Figure 1 shows the effect of different Fe
+2 concentrations and different reaction times ions on percent COD removal at when the initial concentration of H
2O
2 1000 mg/L. As shown in
Figure 1, percent COD removal were 58, 65, and 48% when Fe
+2 concentrations of 100, 300, and 500 mg/L, respectively, at an H
2O
2 concentration of 1000 mg/L and reaction time of 5 min.
4. Conclusions
Real pesticide wastewater treatment with the photo-Fenton process as a pretreatment process was investigated. The effects of hydrogen peroxide (H2O2) and ferrous ion (Fe+2) concentrations and reaction times as independent variables on the oxidation of raw pesticide wastewater were evaluated in terms of chemical oxidation demand (COD) removal as dependent variables in the Box-Behnken design. The optimal H2O2/Fe+2/reaction time ratio resulting in the maximum COD (70%) removal was found to be 1000/325/35. Optimal values of the operating parameters maximizing COD removal were determined. Additionally, at these operating parameters, TOC and suspended solid removals were also observed as 20% and 32.5%, respectively. After the photo-Fenton process as a pretreatment process, treated pesticide wastewater characterization was converted to treatment with a biological treatment method. Therefore, the aim of the research succeeded.