Abstract
Pinus brutia Ten. is distributed throughout the Eastern Mediterranean, mainly in Turkey and bordering countries. Pine essential oils (EOs), mainly comprised of terpenoid volatiles, have high commercial value and a wide range of biological applications as repellents, insecticides, antivirals, antimicrobials and antioxidants. The present work reviewed the chemical variability of EOs reported for P. brutia trees and related bioproducts. The major EO components (≥20%) reported were α-pinene, β-pinene and δ-3-carene, which generally comprised 50–90% of the EO composition. δ-3-Carene showed the highest variability, suggesting the occurrence of chemotypes. Assessing the variability of EOs extracted from different tree parts or tree bioproducts can provide useful information for guided P. brutia EO extraction according to its intended purpose.
Keywords:
chemical diversity; chemotypes; essential oil; Pinus brutia; volatiles; α-pinene; β-pinene; δ-3-carene 1. Introduction
In the Mediterranean, conifers play important ecological and cultural roles. Pines (Pinaceae family), are the most numerous and widespread conifers in forest ecosystems and occupy over 25% of the total forest area [1]. Five main species dominate the landscape: Pinus halepensis Mill., P. brutia Ten., P. pinea L., P. pinaster Aiton and P. sylvestris L. P. brutia is commonly known as Brutian pine, Calabrian pine or Turkish pine and is largely distributed throughout the Eastern Mediterranean, mainly in Turkey and the nearby countries of Azerbaijan, Bulgaria, Cyprus, Georgia, Greece, Iran, Iraq, Israel, Jordan, Lebanon and Syria. P. brutia often hybridizes with P. halepensis, despite the latter being distributed mainly in the Western Mediterranean [2]. Brutian pine is a rapidly growing tree that can reach 35 m in height, and presents a usually open crown of irregular branches. In its native range, P. brutia has a high economic value for the raw products it provides, namely wood, bark, cones and also the extracted resin [3]. In conifers, the release of oleoresin commonly occurs in response to abiotic factors, but occurs more pronouncedly in response to biotic attacks, such as the activity of herbivores, stem-boring insects, and pathogenic microorganisms. The production of oleoresin is stimulated by the wounds induced by these pathogens, and results in the release of strong chemical volatile deterrents and in the sealing of the open wounds [4]. Industrially, the oleoresin can be obtained by tapping the bark (also known as bark chipping) and collecting exudates from the pine trees. The crude oleoresin is then converted to turpentine (volatile fraction) and rosin (non-volatile fraction) by steam distillation, and can be further processed into valuable chemical industrial products, namely, adhesives, cleaners, coatings, disinfectants, flavorings, food gums, fragrances, pharmaceuticals and printing inks [1]. Pine essential oils (EOs), complex mixtures of volatile compounds, are another important bioproduct with high commercial value that can be obtained by hydro-, steam- or dry distillation from several parts of the pine tree. In addition to their cultural and ethnobotanical importance, pine EOs have strong biological activities as antimicrobials, antioxidants, antiparasitics, antivirals and insecticides [5].
2. Essential Oils
EOs are defined by the International Organization for Standardization (ISO 9235) as a product obtained from natural raw material of plant origin by hydro-, steam- or dry distillation, or by mechanical processes from the epicarp of citrus fruits. The result is a concentrated hydrophobic liquid at room temperature, containing volatile compounds and being slightly soluble in water and highly soluble in organic solvents [6]. The chemical composition of most EOs is dominated by mono- and sesquiterpenes and phenolic compounds (e.g., phenylpropanoids), although other groups of compounds can also occur in relevant amounts. Terpenes and terpenoids occurring in pine EOs have a high commercial value and a wide range of practical applications, such as industrial and household cleaning products, disinfectants, solvents, fragrances, medicine and aromatherapy [7]. Although pine EOs are usually comprised of one to three major components at relatively high concentrations (20–70%), great chemical variability can occur between and within species (e.g., the occurrence of chemotypes) [8].
The present work aimed at screening the EO variability in various parts of P. brutia trees and derived bioproducts reported in the literature. Mapping the chemical variability of EOs from different parts of the tree and related bioproducts can provide useful information for guided P. brutia EO extraction according to its intended purpose.
3. Bibliographic Data
Research was performed with the Web of Science search engine in all available databases on published works reporting EO chemical composition, using the topics “Pinus”, “brutia” and “essential oil”.
A total of 17 works was retrieved reporting full EO compositions from various P. brutia tree parts and bioproducts. These reports were published in journals specializing in the scientific fields of plant sciences (24%), applied chemistry (18%), food science technology (18%) and materials science (paper, wood) (18%). Publications dated from 1995 to 2019. The listed publications were cited 394 times by a total of 283 works, with an average of 23 citations per work. These citations were included in journals specialized in the scientific fields of plant sciences (17%), biochemistry and molecular biology (17%) and food science technology (16%). Since 2011, the cumulative number of citations has greatly increased, revealing a growing research interest in P. brutia EOs (Figure 1).
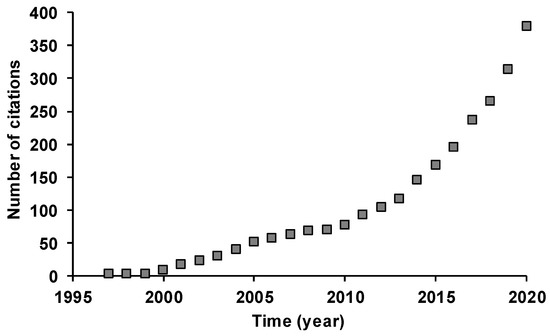
Figure 1.
Cumulative number of citations of works reporting on the chemical composition of essential oils of Pinus brutia tree parts and derived bioproducts, obtained from Web of Science (https://www.webofknowledge.com, accessed 29 March 2021), using the keywords “Pinus”, “brutia” and “essential oil”.
4. Pinus brutia EO Chemical Variability
The production of secondary metabolites in plants can be influenced by various factors, such as those related to plant genetics and physiology (e.g., plant parts and their developmental stage, the growth season and the type of secretory structures present), and those related to environmental conditions (e.g., geographic location, climatic and edaphic conditions, associated pests and diseases and environmental pollution) [9]. Among the genus Pinus, variability in secondary metabolites and in terpenoids in particular is heavily dependent on the species and on geographic locations (i.e., implying different environmental conditions). A high capacity for phenotypic plasticity can be observed through the common occurrence of different chemical phenotypes in some species, also known as chemotypes.
The occurrence of chemotypes has been strongly linked with geographical variation, reflecting the diverse environmental conditions to which pines are exposed [8,10,11,12]. Chemical diversity can lead to increased variability in the products obtained from these species and to an instability in production, for example in the production of extracted EOs [7]. In P. brutia, this degree of variability was reported for EOs obtained from different plant parts, but also among EOs obtained from the same plant part.
4.1. Between Plant Parts and Related Bioproducts
The compiled bibliography reported EOs from P. brutia needles, twigs, bark, flowers, seeds, extracted resin and sawdust from collected wood. The EOs’ main compositions (≥1%) were comprised of the compounds α-pinene, α-terpineol, α-terpinyl acetate, β-caryophyllene, β-myrcene, β-pinene, camphene, caryophyllene oxide, δ-2-carene, δ-3-carene, γ-terpinene, germacrene D, limonene, longifolene, neryl acetate, sylvestrene and terpinolene (Table 1).

Table 1.
Essential oil composition (≥1%) reported for Pinus brutia needles, twigs, resin, cones, flowers, bark, sawdust and seeds.
The monoterpenes α-pinene and β-pinene were present in all samples (Table 1). For α-pinene, the highest amounts were detected in resin, cones and sawdust samples (20–77%), while for β-pinene, needles, cones and seeds showed the highest amounts (38–48%). Other compounds present in relatively high amounts (≥15%) included the monoterpene δ-3-carene (25% in pine twigs), the sesquiterpene germacrene D (18% in pine needles), the monoterpene β-myrcene (15% in pine resin) and the sesquiterpene β-caryophyllene (15% in pine seeds) (Table 1).
4.2. Among Samples of the Same Plant Part
Variability in the amounts of volatile compounds present in P. brutia EO was determined as the range of value percentage of the maximum value in the plant parts where more than one sample was reported (namely needles, twigs, resin or cones). Apart from longifolene, all compounds showed at least 20% variability between samples (Figure 2). The highest variabilities (≥80%) were detected for δ-3-carene (94%) and limonene (89%) in twig EOs, and β-myrcene in EOs from resin (86%) and twigs (81%).
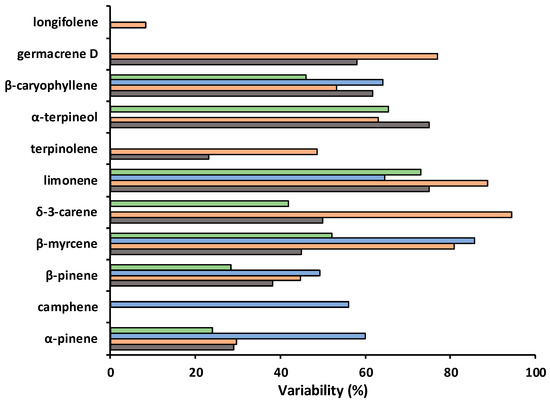
Figure 2.
Variability (range percentage of the maximum value {100 × [(Max − Min)/Max]}) of essential oil compounds in Pinus brutia needles (grey bars), twigs (orange bars), resin (blue bars) and cones (green bars).
It is noteworthy that the compositions of EOs extracted from P. brutia resin samples share a resemblance with other resin-producing pines, such as P. pinea, being rich in α-pinene (21% to 25%), β-pinene (10%) and caryophyllene (5% to 9%) [4]. Additionally, both types of EOs were seen to possess similar antimicrobial, insecticidal, phytotoxic and antioxidant activities.
5. Conclusions
Analysis of the chemical composition of EOs extracted from various tree parts and bioproducts obtained from P. brutia allows the selection of the most suitable raw material according to the desired volatile components. A higher number of studies are necessary for a detailed description of the anatomic volatile composition of Pinus brutia.
Author Contributions
Conceptualization, J.M.S.F.; methodology, J.M.S.F.; software, J.M.S.F.; investigation, J.M.S.F.; writing—original draft preparation, J.M.S.F. and A.M.R.; writing—review and editing, J.M.S.F., A.M.R. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the findings of this study are available from the corresponding author (Jorge M. S. Faria) upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bagci, E.; Hayta, S.; Dogan, G. Chemical composition of essential oils from bark and leaves of Pinus brutia Ten. from Turkey. Asian J. Chem. 2011, 23, 2782–2784. [Google Scholar]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Menichini, F.; Bonesi, M.; Statti, G.A.; Menichini, F. Chemical composition and antimicrobial activity of essential oils from Pinus brutia (calabrian pine) growing in Lebanon. Chem. Nat. Compd. 2008, 44, 784–786. [Google Scholar] [CrossRef]
- Öz, M.; Deniz, İ.; Okan, O.T.; Fidan, M.S. Chemical Composition of Oleoresin and Larvae Gallery Resin of Pinus Brutia Attacked by Dioryctria Sylvestrella Ratz. Drv. Ind. 2015, 66, 179–188. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Karabörklü, S.; Bozok, F.; Ates, B.; Erdogan, S.; Cenet, M.; Karaaslan, M.G. Chemical composition, antimicrobial, insecticidal, phytotoxic and antioxidant activities of Mediterranean Pinus brutia and Pinus pinea resin essential oils. Chin. J. Nat. Med. 2014, 12, 901–910. [Google Scholar] [CrossRef]
- Karaogul, E.; Hakki Alma, M. Solvent-free microwave and hydro-distillation extraction of essential oils from the Sawdust of Pines: Correlation with heat-map. BioResources 2019, 14, 8229–8240. [Google Scholar] [CrossRef]
- Faria, J.M.S. Essential Oils as Anti-Nematode Agents and Their Influence on In Vitro Nematode/Plant Co-Cultures. Ph.D. Thesis, Faculdade de Ciências da Universidade de Lisboa, Lisboa, Portugal, 2015. [Google Scholar]
- Faria, J.M.S.; Rodrigues, A.M. Metabolomic Variability in the Volatile Composition of Essential Oils from Pinus pinea and P. pinaster. Biol. Life Sci. Forum 2021, 2, 14. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Mendes, M.D.; Lima, A.S.; Barbosa, P.M.; Ascensão, L.; Barroso, J.G.; Pedro, L.G.; Mota, M.M.; Figueiredo, A.C. Pinus halepensis, Pinus pinaster, Pinus pinea and Pinus sylvestris essential oils chemotypes and monoterpene hydrocarbon enantiomers, before and after inoculation with the pinewood nematode Bursaphelenchus xylophilus. Chem. Biodivers. 2017, 14, e1600153. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Arrabal, C.; García-Vallejo, M.C.; Cadahia, E.; Cortijo, M.; de Simón, B.F. Characterization of two chemotypes of Pinus pinaster by their terpene and acid patterns in needles. Plant Syst. Evol. 2012, 298, 511–522. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G.; Trindade, H.; Sanches, J.; Oliveira, C.; Correia, M. Pinus pinaster Aiton e Pinus pinea L. Agrotec 2014, 12, 14–18. [Google Scholar]
- Rodrigues, A.M.; Miguel, C.; Chaves, I.; António, C. Mass spectrometry—Based forest tree metabolomics. Mass Spectrom. Rev. 2019, 40, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Roussis, V.; Petrakis, P.V.; Ortiz, A.; Mazomenos, B.E. Volatile constituents of needles of five Pinus species grown in Greece. Phytochemistry 1995, 39, 357–361. [Google Scholar] [CrossRef]
- Hmamouchi, M.; Hamamouchi, J.; Zouhdi, M.; Bessiere, J.M. Chemical and antimicrobial properties of essential oils of five Moroccan pinaceae. J. Essent. Oil Res. 2001, 13, 298–302. [Google Scholar] [CrossRef]
- Lahlou, M. Composition and molluscicidal properties of essential oils of five Moroccan pinaceae. Pharm. Biol. 2003, 41, 207–210. [Google Scholar] [CrossRef]
- Ustun, O.; Senol, F.S.; Kurkcuoglu, M.; Orhan, I.E.; Kartal, M.; Baser, K.H.C. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oils of Turkish Pinus species and pycnogenol. Ind. Crops Prod. 2012, 38, 115–123. [Google Scholar] [CrossRef]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Yener, H.O.; Saygideger, S.D.; Sarikurkcu, C.; Yumrutas, O. Evaluation of antioxidant activities of essential oils and methanol extracts of Pinus species. J. Essent. Oil-Bear. Plants 2014, 17, 295–302. [Google Scholar] [CrossRef]
- Ghosn, M.W.; Saliba, N.A.; Talhouk, S.Y. Chemical Composition of the Needle-Twig Oils of Pinus brutia Ten. J. Essent. Oil Res. 2006, 18, 445–447. [Google Scholar] [CrossRef]
- Koutsaviti, K.; Giatropoulos, A.; Pitarokili, D.; Papachristos, D.; Michaelakis, A.; Tzakou, O. Greek Pinus essential oils: Larvicidal activity and repellency against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2015, 114, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Roussis, V.; Papadogianni, K.; Vagias, C.; Harvala, C.; Petrakis, P.V.; Ortiz, A. Volatile Constituents of Three Pinus Species Grown in Greece. J. Essent. Oil Res. 2001, 13, 118–121. [Google Scholar] [CrossRef]
- Yasar, S.; Beram, A.; Guler, G. Effect of extraction technique on composition of volatile constituents of oleoresin from Pinus brutia Ten. Drv. Ind. 2018, 69, 239–245. [Google Scholar] [CrossRef]
- Tumen, I.; Hafizoglu, H.; Kilic, A.; Dönmez, I.E.; Sivrikaya, H.; Reunanen, M. Yields and constituents of Essential Oil from cones of Pinaceae spp. Natively grown in Turkey. Molecules 2010, 15, 5797–5806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).