Bush Encroachment and Large Carnivore Predation Success in African Landscapes: A Review
Abstract
1. Introduction
2. Methodology
2.1. Search Methodology for Publication Trends
2.2. Search Methodology for Comprehensive Review
3. Current Knowledge
3.1. Search, Encounter, Capture and Kill Stages
3.1.1. Habitat Structure
Hunting Efficiency
Prey Accessibility
3.1.2. Prey Abundance and Distribution
3.2. Consumption Stage
4. Management Implications
5. Conclusions and Future Research
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Auken, O.W. Shrub invasions of North American semiarid grasslands. Annu. Rev. Ecol. Syst. 2000, 31, 197–215. [Google Scholar] [CrossRef]
- De Klerk, J.N. Bush encroachment in Namibia. Report on Phase 1 of the Bush Encroachment, Monitoring and Management Project; Ministry of Environment and Tourism: Windhoek, Namibia, 2004.
- Jeltsch, F.; Milton, S.J.; Dean, W.R.J.; Van Rooyen, N. Analysing shrub encroachment in the southern Kalahari: A grid-based modelling approach. J. Appl. Ecol. 1997, 34, 1497–1508. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Mooney, H.A. Community changes following shrub invasion of grassland. Oecologia 1986, 70, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Joshi, M.C. Ecology of the semi-arid regions of India with emphasis on land-use. Dev. Agric. Manag. For. Ecol. 1979, 7, 243–275. [Google Scholar]
- Maestre, F.T.; Bowker, M.A.; Puche, M.D.; Belén Hinojosa, M.; Martínez, I.; García-Palacios, P.; Castillo, A.P.; Soliveres, S.; Luzuriaga, A.L.; Sánchez, A.M.; et al. Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecol. Lett. 2009, 12, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Angassa, A. Effects of grazing intensity and bush encroachment on herbaceous species and rangeland condition in southern Ethiopia. Land Degrad. Dev. 2014, 25, 438–451. [Google Scholar] [CrossRef]
- Smit, I.P.; Prins, H.H. Predicting the effects of woody encroachment on mammal communities, grazing biomass and fire frequency in African savannas. PLoS ONE 2015, 10, e0137857. [Google Scholar] [CrossRef]
- Bakker, E.S.; Gill, J.L.; Johnson, C.N.; Vera, F.W.; Sandom, C.J.; Asner, G.P.; Svenning, J.C. Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl. Acad. Sci. USA 2016, 113, 847–855. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, G.F. A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob. Chang. Biol. 2000, 6, 865–869. [Google Scholar] [CrossRef]
- Augustine, D.J.; Mcnaughton, S.J. Regulation of shrub dynamics by native browsing ungulates on East African rangeland. J. Appl. Ecol. 2004, 41, 45–58. [Google Scholar] [CrossRef]
- Coetzee, B.W.; Tincani, L.; Wodu, Z.; Mwasi, S.M. Overgrazing and bush encroachment by Tarchonanthus camphoratus in a semi-arid savanna. Afr. J. Ecol. 2008, 46, 449–451. [Google Scholar] [CrossRef]
- Scheiter, S.; Higgins, S.I. Impacts of climate change on the vegetation of Africa: An adaptive dynamic vegetation modelling approach. Glob. Chang. Biol. 2009, 15, 2224–2246. [Google Scholar] [CrossRef]
- Groengroeft, A.; de Blécourt, M.; Classen, N.; Landschreiber, L.; Eschenbach, A. Acacia trees modify soil water dynamics and the potential groundwater recharge in savanna ecosystems. In Climate Change and Adaptive Land Management in Southern Africa-Assessments, Changes, Challenges, and Solutions; Revermann, R., Krewenka, K.M., Schmiedel, U., Olwoch, J.M., Helmschrot, J., Jürgens, N., Eds.; Klaus Hess Publishers: Göttingen, Germany; Windhoek, Namibia, 2018; pp. 177–186. [Google Scholar]
- Nghikembua, M.; Harris, J.; Tregenza, T.; Marker, L. Spatial and temporal habitat use by GPS collared male cheetahs in modified bushland habitat. Open J. For. 2016, 6, 269–280. [Google Scholar] [CrossRef][Green Version]
- Nghikembua, M.T.; Marker, L.L.; Brewer, B.; Mehtätalo, L.; Appiah, M.; Pappinen, A. Response of wildlife to bush thinning on the north central freehold farmlands of Namibia. For. Ecol. Manag. 2020, 473, 118330. [Google Scholar] [CrossRef]
- Durant, S.M. Competition refuges and coexistence: An example from Serengeti carnivores. J. Anim. Ecol. 1998, 67, 370–386. [Google Scholar] [CrossRef]
- Muntifering, J.R.; Dickman, A.J.; Perlow, L.M.; Hruska, T.; Ryan, P.G.; Marker, L.L.; Jeo, R.M. Managing the matrix for large carnivores: A novel approach and perspective from cheetah (Acinonyx jubatus) habitat suitability modelling. Anim. Conserv. 2006, 9, 103–112. [Google Scholar] [CrossRef]
- Loggins, A.A.; Shrader, A.M.; Monadjem, A.; McCleery, R.A. Shrub cover homogenizes small mammals’ activity and perceived predation risk. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Otieno, T.O.; Goheen, J.R.; Webala, P.W.; Mwangi, A.; Osuga, I.M.; Ford, A.T. Human- and risk-mediated browsing pressure by sympatric antelope in an African savanna. Biol. Conserv. 2019, 232, 59–65. [Google Scholar] [CrossRef]
- Brashares, J.S.; Garland, T.; Arcese, P. Phylogenetic analysis of coadaptation in behavior, diet, and body size in the African antelope. Behav. Ecol. 2000, 11, 452–463. [Google Scholar] [CrossRef]
- Tambling, C.J.; Druce, D.J.; Hayward, M.W.; Castley, J.G.; Adendorff, J.; Kerley, G.I. Spatial and temporal changes in group dynamics and range use enable anti-predator responses in African buffalo. Ecology 2012, 93, 1297–1304. [Google Scholar] [CrossRef]
- Melian, C.J.; Bascompte, J. Food web structure and habitat loss. Ecol. Lett. 2002, 5, 37–46. [Google Scholar] [CrossRef]
- Sih, A.; Englund, G.; Wooster, D. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 1998, 13, 350–355. [Google Scholar] [CrossRef]
- Björklund, H.; Santangeli, A.; Blanchet, F.G.; Huitu, O.; Lehtoranta, H.; Lindén, H.; Valkama, J.; Laaksonen, T. Intraguild predation and competition impacts on a subordinate predator. Oecologia 2016, 181, 257–269. [Google Scholar] [CrossRef]
- Ward, D. Do we understand the causes of bush encroachment in African savannas? Afr. J. Range Forage Sci. 2005, 22, 101–105. [Google Scholar] [CrossRef]
- O’Connor, T.G.; Puttick, J.R.; Hoffman, M.T. Bush encroachment in southern Africa: Changes and causes. Afr. J. Range Forage Sci. 2014, 31, 67–88. [Google Scholar] [CrossRef]
- Belayneh, A.; Tessema, Z.K. Mechanisms of bush encroachment and its inter-connection with rangeland degradation in semi-arid African ecosystems: A review. J. Arid Land 2017, 9, 299–312. [Google Scholar] [CrossRef]
- Joubert, D.F.; Zimmermann, I. The impacts of wood harvesting of bush thickening species on biodiversity and ecological processes. In Proceedings of the First National Forestry Research Workshop, Windhoek, Namibia, 12–13 March 2002; Forestry Publication, Ministry of Environment and Tourism: Windhoek, Namibia, 2002; Volume 9, pp. 67–78. [Google Scholar]
- Andreka, G.; Linn, I.J.; Perrin, M.R.; Maddock, A.H. Range use by the wild dog in Hluhluwe Umfolozi Park, South Africa. S. Afr. J. Wildl. Res. 1999, 29, 1–9. [Google Scholar]
- Marker, L.L.; Dickman, A.J.; Mills, M.G.; Jeo, R.M.; Macdonald, D.W. Spatial ecology of cheetahs on north-central Namibian farmlands. J. Zool. 2008, 274, 226–238. [Google Scholar] [CrossRef]
- Hayward, M.W.; Kerley, G.I. Prey preferences of the lion (Panthera leo). J. Zool. 2005, 267, 309–322. [Google Scholar] [CrossRef]
- Hayward, M.W.; Hofmeyr, M.; O’Brien, J.; Kerley, G.I. Prey preferences of the cheetah (Acinonyx jubatus) (Felidae: Carnivora): Morphological limitations or the need to capture rapidly consumable prey before kleptoparasites arrive? J. Zool. 2006, 270, 615–627. [Google Scholar] [CrossRef]
- Hayward, M.W.; Henschel, P.; O’Brien, J.; Hofmeyr, M.; Balme, G.; Kerley, G.I. Prey preferences of the leopard (Panthera pardus). J. Zool. 2006, 270, 298–313. [Google Scholar] [CrossRef]
- Harzing, A.-W.; Alakangas, S. Google Scholar, Scopus and the Web of Science: A longitudinal and cross-disciplinary comparison. Scientometrics 2016, 106, 787–804. [Google Scholar] [CrossRef]
- Martín-Martín, A.; Orduna-Malea, E.; Thelwall, M.; López-Cózar, E.D. Google Scholar, Web of Science, and Scopus: A systematic comparison of citations in 252 subject categories. J. Informetr. 2018, 12, 1160–1177. [Google Scholar] [CrossRef]
- Hirt, M.R.; Tucker, M.; Müller, T.; Rosenbaum, B.; Brose, U. Rethinking trophic niches: Speed and body mass colimit prey space of mammalian predators. Ecol. Evol. 2020, 10, 7094–7105. [Google Scholar] [CrossRef]
- Chitwood, M.C.; Baruzzi, C.; Lashley, M.A. “Ecology of fear” in ungulates: Opportunities for improving conservation. Ecol. Evol. 2022, 12, e8657. [Google Scholar] [CrossRef]
- Cristescu, B.; Elbroch, L.M.; Dellinger, J.A.; Binder, W.; Wilmers, C.C.; Wittmer, H.U. Kill rates and associated ecological factors for an apex predator. Mamm. Biol. 2022, 102, 291–305. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. 2021. Available online: https://www.rstudio.com/products/rstudio/older-versions/ (accessed on 10 July 2022).
- Luna, Á.; Romero-Vidal, P.; Arrondo, E. Predation and scavenging in the city: A review of spatio-temporal trends in research. Diversity 2021, 13, 46. [Google Scholar] [CrossRef]
- Wirsing, A.J.; Newsome, T.M. Scavenging effects of large Canids. Integr. Comp. Biol. 2021, 61, 117–131. [Google Scholar] [CrossRef]
- Karandikar, H.; Serota, M.W.; Sherman, W.C.; Green, J.R.; Verta, G.; Kremen, C.; Middleton, A.D. Dietary patterns of a versatile large carnivore, the puma (Puma concolor). Ecol. Evol. 2022, 12, e9002. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. QGIS Association. Open Source Geospatial Foundation Project. 2020. Available online: http://qgis.osgeo.org (accessed on 3 July 2022).
- ICPAC GeoPortal. Africa Shapefiles. openAFRICA. Available online: https://open.africa/dataset/africa-shapefiles (accessed on 3 July 2022).
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Saleem, M. An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 2017, 67, 534–545. [Google Scholar] [CrossRef]
- Endler, J.A. Defense against predators. In Predator-Prey Relationships: Perspective and Approaches from the Study of Lower Vertebrates; Federand, M.E., Lauder, G.V., Eds.; University of Chicago Press: Chicago, IL, USA, 1986; pp. 109–134. [Google Scholar]
- Gorini, L.; Linnell, J.D.; May, R.; Panzacchi, M.; Boitani, L.; Odden, M.; Nilsen, E.B. Habitat heterogeneity and mammalian predator–prey interactions. Mammal Rev. 2012, 42, 55–77. [Google Scholar] [CrossRef]
- Blaum, N.; Rossmanith, E.; Popp, A.; Jeltsch, F. Shrub encroachment affects mammalian carnivore abundance and species richness in semiarid rangelands. Acta Oecologica 2007, 31, 86–92. [Google Scholar] [CrossRef]
- Marker-Kraus, L.; Kraus, D.; Barnett, D.; Hurlbut, S. Cheetah Survival on Namibian Farmlands, 3rd ed.; Solitaire Press: Windhoek, Namibia, 2003. [Google Scholar]
- Arbieu, U.; Grünewald, C.; Schleuning, M.; Böhning-Gaese, K. The importance of vegetation density for tourists’ wildlife viewing experience and satisfaction in African savannah ecosystems. PLoS ONE 2017, 12, e0185793. [Google Scholar] [CrossRef]
- Hopcraft, J.G.C.; Sinclair, A.R.E.; Packer, C. Planning for success: Serengeti lions seek prey accessibility rather than abundance. J. Anim. Ecol. 2005, 74, 559–566. [Google Scholar] [CrossRef]
- Davies, A.B.; Tambling, C.J.; Kerley, G.I.; Asner, G.P. Effects of vegetation structure on the location of lion kill sites in African thicket. PLoS ONE 2016, 11, e0149098. [Google Scholar] [CrossRef]
- Martin, J.; Owen-Smith, N. Habitat selectivity influences the reactive responses of African ungulates to encounters with lions. Anim. Behav. 2016, 116, 163–170. [Google Scholar] [CrossRef]
- Hunter, L.T.B. The Behavioural Ecology of Reintroduced Lions and Cheetah in the Phinda Resource Reserve, Kwazulu-Natal, South Africa. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 1998. [Google Scholar]
- Purchase, G.K.; Du Toit, J.T. The use of space and prey by cheetahs in Matusadona National Park, Zimbabwe. S. Afr. J. Wildl. Res. 2000, 30, 139–144. [Google Scholar]
- Broomhall, L.S.; Mills, M.G.L.; Du Toit, J.T. Home range and habitat use by cheetahs (Acinonyx jubatus) in the Kruger National Park. J. Zool. 2003, 261, 119–128. [Google Scholar] [CrossRef]
- Mills, M.G.L.; Broomhall, L.S.; du Toit, J.T. Cheetah Acinonyx jubatus feeding ecology in the Kruger National Park and a comparison across African savanna habitats: Is the cheetah only a successful hunter on open grassland plains? Wildl. Biol. 2004, 10, 177–186. [Google Scholar] [CrossRef]
- Bissett, C.; Bernard, R.T.F. Habitat selection and feeding ecology of the cheetah (Acinonyx jubatus) in thicket vegetation: Is the cheetah a savanna specialist? J. Zool. 2007, 271, 310–317. [Google Scholar] [CrossRef]
- SAIEA: Southern African Institute for Environmental Assessment. Strategic Environmental Assessment of Large-Scale Bush Thinning and Value-Addition Activities in Namibia; John Meinert Printing: Windhoek, Namibia, 2016. [Google Scholar]
- Laurenson, M.K. High juvenile mortality in cheetahs (Acinonyx jubatus) and its consequences for maternal care. J. Zool. 1994, 234, 387–408. [Google Scholar] [CrossRef]
- Hubel, T.Y.; Myatt, J.P.; Jordan, N.R.; Dewhirst, O.P.; McNutt, J.W.; Wilson, A.M. Additive opportunistic capture explains group hunting benefits in African wild dogs. Nat. Commun. 2016, 7, 11033. [Google Scholar] [CrossRef]
- Soto-Shoender, J.R.; McCleery, R.A.; Monadjem, A.; Gwinn, D.C. The importance of grass cover for mammalian diversity and habitat associations in a bush encroached savanna. Biol. Conserv. 2018, 221, 127–136. [Google Scholar] [CrossRef]
- Cusack, J.J.; Dickman, A.J.; Rowcliffe, J.M.; Carbone, C.; Macdonald, D.W.; Coulson, T. Random versus game trail-based camera trap placement strategy for monitoring terrestrial mammal communities. PLoS ONE 2015, 10, e0126373. [Google Scholar] [CrossRef]
- Walker, E.H.; Nghikembua, M.; Bibles, B.; Marker, L. Scent-post preference of free-ranging Namibian cheetahs. Glob. Ecol. Conserv. 2016, 8, 55–57. [Google Scholar] [CrossRef]
- Fabiano, E.; Boast, L.K.; Fuller, A.K.; Sutherland, C. The use of remote camera trapping to study cheetahs: Past reflections and future directions. In Cheetahs: Biology and Conservation: Biodiversity of the World: Conservation from Genes to Landscapes; Marker, L., Boast, L.K., Schmidt-Küntzel, A., Eds.; John Fedor: London, UK, 2018; pp. 415–425. [Google Scholar]
- Rafiq, K.; Jordan, N.R.; Meloro, C.; Wilson, A.M.; Hayward, M.W.; Wich, S.A.; McNutt, J.W. Scent-marking strategies of a solitary carnivore: Boundary and road scent marking in the leopard. Anim. Behav. 2020, 161, 115–126. [Google Scholar] [CrossRef]
- Caro, T. Cheetahs of the Serengeti Plains: Group Living of an Asocial Species; University of Chicago Press: Chicago, IL, USA, 1994. [Google Scholar]
- Whittington-Jones, B.M.; Parker, D.M.; Bernard, R.T.; Davies-Mostert, H.T. Habitat selection by transient African wild dogs (Lycaon pictus) in northern KwaZulu-Natal, South Africa: Implications for range expansion. S. Afr. J. Wildl. Res. 2014, 44, 135–147. [Google Scholar] [CrossRef]
- Mills, M.G.L.; Gorman, M.L. Factors affecting the density and distribution of wild dogs in the Kruger National Park. Conserv. Biol. 1997, 11, 1397–1406. [Google Scholar] [CrossRef]
- Krüger, S.C.; Lawes, M.J.; Maddock, A.H. Diet choice and capture success of wild dog Lycaon pictus in Hluhluwe-Umfolozi Park, South Africa. J. Zool. 1999, 248, 543–555. [Google Scholar]
- Malcolm, J.R.; van Lawick, H. Notes on wild dogs (Lycaon pictus) hunting zebras. J. Mammal. 1975, 39, 231–240. [Google Scholar] [CrossRef]
- Fuller, T.K.; Kat, P.W. Movements, activity, and prey relationships of Africa wild dogs (Lycaon pictus) near Aitong, southwestern Kenya. Afr. J. Ecol. 1990, 28, 330–350. [Google Scholar] [CrossRef]
- Fanshawe, J.H.; Fitzgibbon, C.D. Factors influencing hunting success of an African wild dog pack. Anim. Behav. 1993, 45, 479–490. [Google Scholar] [CrossRef]
- Creel, S.R.; Creel, N.M. Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim. Behav. 1995, 50, 1325–1339. [Google Scholar] [CrossRef]
- Sunquist, M.; Sunquist, F. Wild Cats of the World; The University of Chicago Press: Chicago, IL, USA, 2002. [Google Scholar]
- Yoganand, K.; Owen-Smith, N. Restricted habitat use by an African savanna herbivore through the seasonal cycle: Key resources concept expanded. Ecography 2014, 37, 969–982. [Google Scholar] [CrossRef]
- Underwood, R. Vigilance behaviour in grazing African antelopes. Behaviour 1982, 79, 81–107. [Google Scholar] [CrossRef]
- Ford, A.T.; Goheen, J.R.; Otieno, T.O.; Bidner, L.; Isbell, L.A.; Palmer, T.M.; Ward, D.; Woodroffe, R.; Pringle, R.M. Large carnivores make savanna tree communities less thorny. Science 2014, 346, 346–349. [Google Scholar] [CrossRef]
- Riginos, C. Climate and the landscape of fear in an African savanna. J. Anim. Ecol. 2015, 84, 124–133. [Google Scholar] [CrossRef]
- Le Roux, E.; Kerley, G.I.; Cromsigt, J.P. Megaherbivores modify trophic cascades triggered by fear of predation in an African savanna ecosystem. Curr. Biol. 2018, 28, 2493–2499. [Google Scholar] [CrossRef]
- Graf, J.A.; Somers, M.J.; Gunther, M.S.; Slotow, R. Heterogeneity in the density of spotted hyaenas in Hluhluwe-iMfolozi Park, South Africa. Acta Theriol. 2009, 54, 333–343. [Google Scholar] [CrossRef]
- Grange, S.; Owen-Smith, N.; Gaillard, J.M.; Druce, D.J.; Moleón, M.; Mgobozi, M. Changes of population trends and mortality patterns in response to the reintroduction of large predators: The case study of African ungulates. Acta Oecologica 2012, 42, 16–29. [Google Scholar] [CrossRef]
- Riginos, C.; Grace, J.B. Savanna tree density, herbivores, and the herbaceous community: Bottom-up vs. top-down effects. Ecology 2008, 89, 2228–2238. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.T.; Stahler, D.R.; Metz, M.C.; Forester, J.D.; Kauffman, M.J.; Varley, N.; White, P.J.; Smith, D.W.; MacNulty, D.R. Diel predator activity drives a dynamic landscape of fear. Ecol. Monogr. 2018, 88, 638–652. [Google Scholar] [CrossRef]
- Bergstrom, B.J.; Sensenig, R.L.; Augustine, D.J.; Young, T.P. Searching for cover: Soil enrichment and herbivore exclusion, not fire, enhance African savanna small-mammal abundance. Ecosphere 2018, 9, e02519. [Google Scholar] [CrossRef]
- Ott, T.; Kerley, G.I.; Boshoff, A.F. Preliminary observations on the diet of leopards (Panthera pardus) from a conservation area and adjacent rangelands in the Baviaanskloof region, South Africa. Afr. Zool. 2007, 42, 31–37. [Google Scholar] [CrossRef]
- Mbizah, M.M.; Marino, J.; Groom, R.J. Diet of four sympatric carnivores in Savé Valley Conservancy, Zimbabwe: Implications for conservation of the African wild dog (Lycaon pictus). S. Afr. J. Wildl. Res. 2012, 42, 94–103. [Google Scholar] [CrossRef]
- Fuller, T.; Sievert, P. Carnivore demography and the consequences of changes in prey availability. In Carnivore Conservation; Gittleman, J.L., Funk, S.M., Macdonald, D., Wayne, R.K., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 163–179. [Google Scholar]
- Palomares, F.; Caro, T.M. Interspecific killing among mammalian carnivores. Am. Nat. 1999, 153, 492–508. [Google Scholar] [CrossRef]
- Lima, S.L. Putting predators back into behavioral predator–prey interactions. Trends Ecol. Evol. 2002, 17, 70–75. [Google Scholar] [CrossRef]
- Kavwele, C.M.; Kimanzi, J.K.; Kinyanjui, M.J. Impacts of bush encroachment on wildlife species diversity, composition, and habitat Preference in Ol Pejeta Conservancy, Laikipia, Kenya. Int. J. Ecol 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Kiffner, C.; Rheault, H.; Miller, E.; Scheetz, T.; Enriquez, V.; Swafford, R.; Kioko, J.; Prins, H.H. Long-term population dynamics in a multi-species assemblage of large herbivores in East Africa. Ecosphere 2017, 8, e02027. [Google Scholar] [CrossRef]
- Schwarz, K.; Finckh, M.; Stolter, C. Influence of differently managed bush-encroached sites on the large herbivore distribution in the Namibian Savannah. Afr. J. Ecol. 2018, 56, 290–300. [Google Scholar] [CrossRef]
- Khorozyan, I.; Ghoddousi, A.; Soofi, M.; Waltert, M. Big cats kill more livestock when wild prey reaches a minimum threshold. Biol. Conserv. 2015, 192, 268–275. [Google Scholar] [CrossRef]
- Gordon, I.J.; Prins, H.H.T. Grazers and browsers in a changing world: Conclusions. In The Ecology of Browsing and Grazing. Ecological Studies; Gordeon, I.J., Prins, H.H.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 195, pp. 309–321. [Google Scholar]
- Prins, H.H.; van der Jeugd, H.P. Herbivore population crashes and woodland structure in East Africa. J. Ecol. 1993, 81, 305–314. [Google Scholar] [CrossRef]
- Ogutu, J.O.; Piepho, H.P.; Said, M.Y.; Kifugo, S.C. Herbivore dynamics and range contraction in Kajiado County Kenya: Climate and land use changes, population pressures, governance, policy and human-wildlife conflicts. Open Ecol. J. 2014, 7, 9–31. [Google Scholar] [CrossRef]
- Hayward, M.W.; Kerley, G.I. Prey preferences and dietary overlap amongst Africa’s large predators. S. Afr. J. Wildl. Res. 2008, 38, 93–108. [Google Scholar] [CrossRef]
- Brockmann, H.J.; Barnard, C.J. Kleptoparasitism in birds. Anim. Behav. 1979, 27, 487–514. [Google Scholar] [CrossRef]
- Hunter, J.S.; Durant, S.M.; Caro, T.M. To flee or not to flee: Predator avoidance by cheetahs at kills. Behav. Ecol. Sociobiol. 2007, 61, 1033–1042. [Google Scholar] [CrossRef]
- Mills, M.G.L.; Mills, M.E.J. Cheetah cub survival revisited: A re-evaluation of the role of predation, especially by lions, and implications for conservation. J. Zool. 2014, 292, 136–141. [Google Scholar] [CrossRef]
- Dröge, E.; Creel, S.; Becker, M.S.; M’soka, J. Spatial and temporal avoidance of risk within a large carnivore guild. Ecol. Evol. 2017, 7, 189–199. [Google Scholar] [CrossRef]
- Loarie, S.R.; Tambling, C.J.; Asner, G.P. Lion hunting behaviour and vegetation structure in an African savanna. Anim. Behav. 2013, 85, 899–906. [Google Scholar] [CrossRef]
- Ramesh, T.; Kalle, R.; Rosenlund, H.; Downs, C.T. Low leopard populations in protected areas of Maputaland: A consequence of poaching, habitat condition, abundance of prey, and a top predator. Ecol. Evol. 2017, 7, 1964–1973. [Google Scholar] [CrossRef]
- Gigliotti, L.C.; Slotow, R.; Hunter, L.T.; Fattebert, J.; Sholto-Douglas, C.; Jachowski, D.S. Habitat complexity and lifetime predation risk influence mesopredator survival in a multi-predator system. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Creel, S.R.; Creel, N.M. Limitation of African wild dogs by competition with larger carnivores. Conserv. Biol. 1996, 10, 526–538. [Google Scholar] [CrossRef]
- Gorman, M.L.; Mills, M.G.L.; Raath, J.P.; Speakman, J.R. High hunting costs make African wild dogs vulnerable to kleptoparasitism by hyaenas. Nature 1998, 391, 479–481. [Google Scholar] [CrossRef]
- Woodroffe, R. Ranging behaviour of African wild dog packs in a human-dominated landscape. J. Zool. 2010, 11, 1–10. [Google Scholar] [CrossRef]
- Michel, M.J.; Adams, M.M. Differential effects of structural complexity on predator foraging behavior. Behav. Ecol. 2009, 20, 313–317. [Google Scholar] [CrossRef]
- Blake, L.W.; Gese, E.M. Resource selection by cougars: Influence of behavioral state and season. J. Wildl. Manag. 2016, 80, 1205–1217. [Google Scholar] [CrossRef][Green Version]
- Hay, C.T.; Cross, P.C.; Funston, P.J. Trade-offs of predation and foraging explain sexual segregation in African buffalo. J. Anim. Ecol. 2008, 77, 850–858. [Google Scholar] [CrossRef]
- Trinkel, M.; Kastberger, G. Competitive interactions between spotted hyenas and lions in the Etosha National Park, Namibia. Afr. J. Ecol. 2005, 43, 220–224. [Google Scholar] [CrossRef]
- Stein, A.B.; Bourquin, S.L.; McNutt, J.W. Avoiding intraguild competition: Leopard feeding ecology and prey caching in northern Botswana. Afr. J. Wildl. Res. 2015, 45, 247–257. [Google Scholar] [CrossRef]
- Balme, G.A.; Miller, J.R.; Pitman, R.T.; Hunter, L.T. Caching reduces kleptoparasitism in a solitary, large felid. J. Anim. Ecol. 2017, 86, 634–644. [Google Scholar] [CrossRef]
- Yarnell, R.W.; Phipps, W.L.; Burgess, L.P.; Ellis, J.A.; Harrison, S.W.; Dell, S.; Scott, D.M. The influence of large predators on the feeding ecology of two African mesocarnivores: The black-backed jackal and the brown hyaena. Afr. J. Wildl. Res. 2013, 43, 155–166. [Google Scholar] [CrossRef]
- Mills, M.G. Kalahari Hyaenas; Unwin Hyman: London, UK, 1990. [Google Scholar]
- Mills, M.G.L.; Funston, P.J. Large carnivores and savannah heterogeneity. In The Kruger Experience: Ecology and Management of Savanna Heterogeneity; du Toit, J.T., Rogers, K.H., Bigg, H.C., Eds.; Island Press: Washington, DC, USA, 2003; pp. 370–388. [Google Scholar]
- Dube, K.; Nhamo, G. Evidence and impact of climate change on South African national parks. Potential implications for tourism in the Kruger National Park. Environ. Dev. 2020, 33, 100485. [Google Scholar] [CrossRef]
- Stander, P.E. Cooperative hunting in lions: The role of the individual. Behav. Ecol. Sociobiol. 1992, 29, 445–454. [Google Scholar] [CrossRef]
- Brashares, J.S.; Prugh, L.R.; Stoner, C.J.; Epps, C.W. Ecological and conservation implications of mesopredator release. In Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature; Terborgh, J., Estes, J.A., Eds.; Island Press: Washington, DC, USA, 2010; pp. 221–240. [Google Scholar]
- Salo, P.; Korpimäki, E.; Banks, P.B.; Nordström, M.; Dickman, C.R. Alien predators are more dangerous than native predators to prey populations. Proc. Royal Soc. B Biol. Sci. 2007, 274, 1237–1243. [Google Scholar] [CrossRef]
- Nghikembua, M.T.; Marker, L.L.; Brewer, B.; Leinonen, A.; Mehtätalo, L.; Appiah, M.; Pappinen, A. Restoration thinning reduces bush encroachment on freehold farmlands in north-central Namibia. For. Int. J. For. Res. 2021, 94, 551–564. [Google Scholar] [CrossRef]
- Cristescu, B.; Bernard, R.T.; Krause, J. Partitioning of space, habitat, and timing of activity by large felids in an enclosed South African system. J. Ethol. 2013, 31, 285–298. [Google Scholar] [CrossRef]
- Wessels, K.; Mathieu, R.; Knox, N.; Main, R.; Naidoo, L.; Steenkamp, K. Mapping and monitoring fractional woody vegetation cover in the Arid Savannas of Namibia Using LiDAR training data, machine learning, and ALOS PALSAR data. Remote Sens. 2019, 11, 2633. [Google Scholar] [CrossRef]
- Atkinson, H.; Cristescu, B.; Marker, L.; Rooney, N. Habitat thresholds for successful predation under landscape change. Landsc. Ecol. 2022. [Google Scholar] [CrossRef]
 cheetah,
cheetah,  leopard,
leopard,  brown hyena,
brown hyena,  spotted hyena,
spotted hyena,  lion,
lion,  African wild dog. Data used to create map available in Table S1. Map generated in QGIS 3.10 (QGIS Association) [44]; map of Africa layer from ICPAC GeoPortal [45]; Ecoregion layer produced by Dinerstein et al. [46].
African wild dog. Data used to create map available in Table S1. Map generated in QGIS 3.10 (QGIS Association) [44]; map of Africa layer from ICPAC GeoPortal [45]; Ecoregion layer produced by Dinerstein et al. [46].
 cheetah,
cheetah,  leopard,
leopard,  brown hyena,
brown hyena,  spotted hyena,
spotted hyena,  lion,
lion,  African wild dog. Data used to create map available in Table S1. Map generated in QGIS 3.10 (QGIS Association) [44]; map of Africa layer from ICPAC GeoPortal [45]; Ecoregion layer produced by Dinerstein et al. [46].
African wild dog. Data used to create map available in Table S1. Map generated in QGIS 3.10 (QGIS Association) [44]; map of Africa layer from ICPAC GeoPortal [45]; Ecoregion layer produced by Dinerstein et al. [46].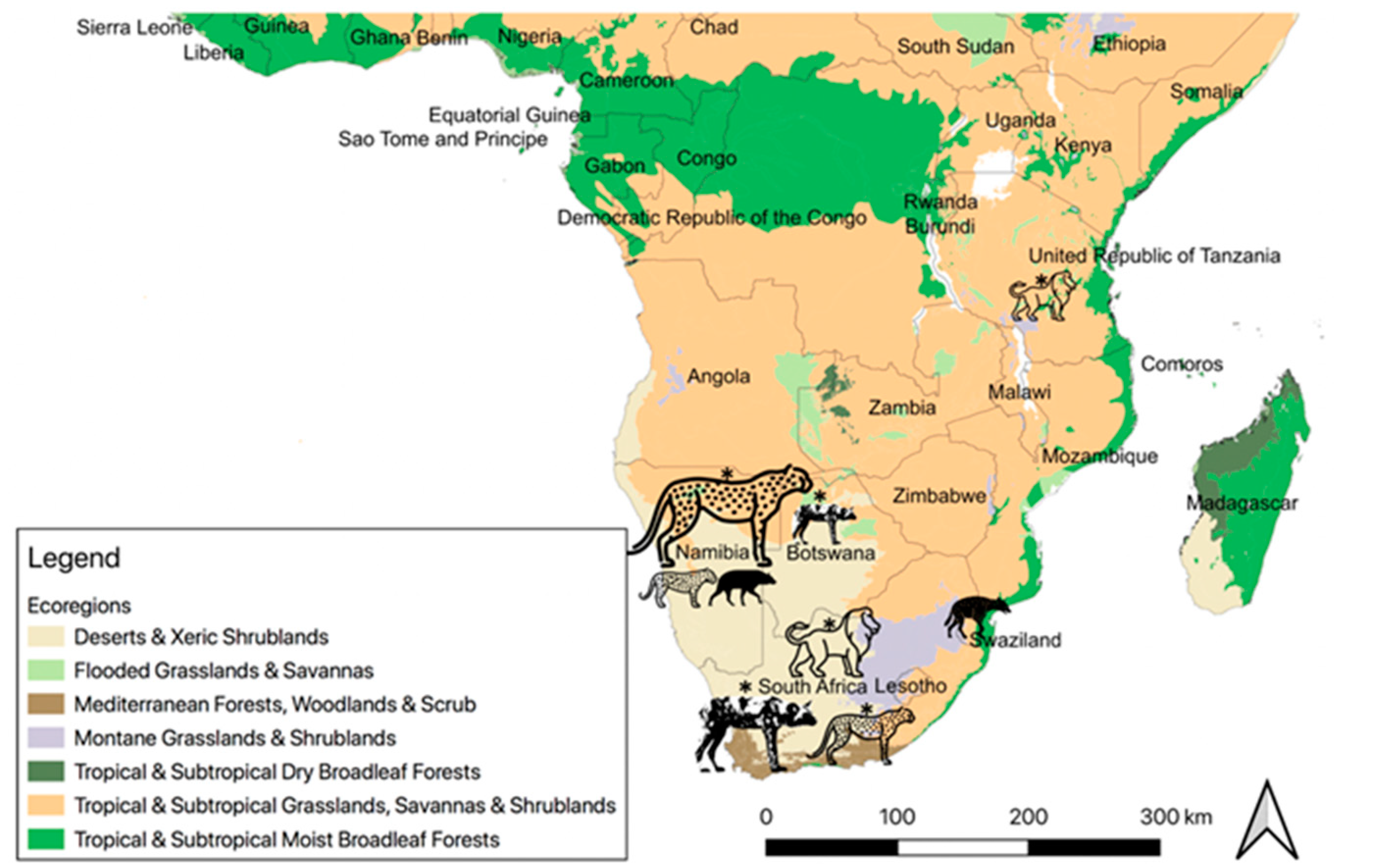
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atkinson, H.; Cristescu, B.; Marker, L.; Rooney, N. Bush Encroachment and Large Carnivore Predation Success in African Landscapes: A Review. Earth 2022, 3, 1010-1026. https://doi.org/10.3390/earth3030058
Atkinson H, Cristescu B, Marker L, Rooney N. Bush Encroachment and Large Carnivore Predation Success in African Landscapes: A Review. Earth. 2022; 3(3):1010-1026. https://doi.org/10.3390/earth3030058
Chicago/Turabian StyleAtkinson, Holly, Bogdan Cristescu, Laurie Marker, and Nicola Rooney. 2022. "Bush Encroachment and Large Carnivore Predation Success in African Landscapes: A Review" Earth 3, no. 3: 1010-1026. https://doi.org/10.3390/earth3030058
APA StyleAtkinson, H., Cristescu, B., Marker, L., & Rooney, N. (2022). Bush Encroachment and Large Carnivore Predation Success in African Landscapes: A Review. Earth, 3(3), 1010-1026. https://doi.org/10.3390/earth3030058







