Pulsed-Supplied Water Electrolysis via Two-Switch Converter for PV Capacity Firming
Abstract
:1. Introduction
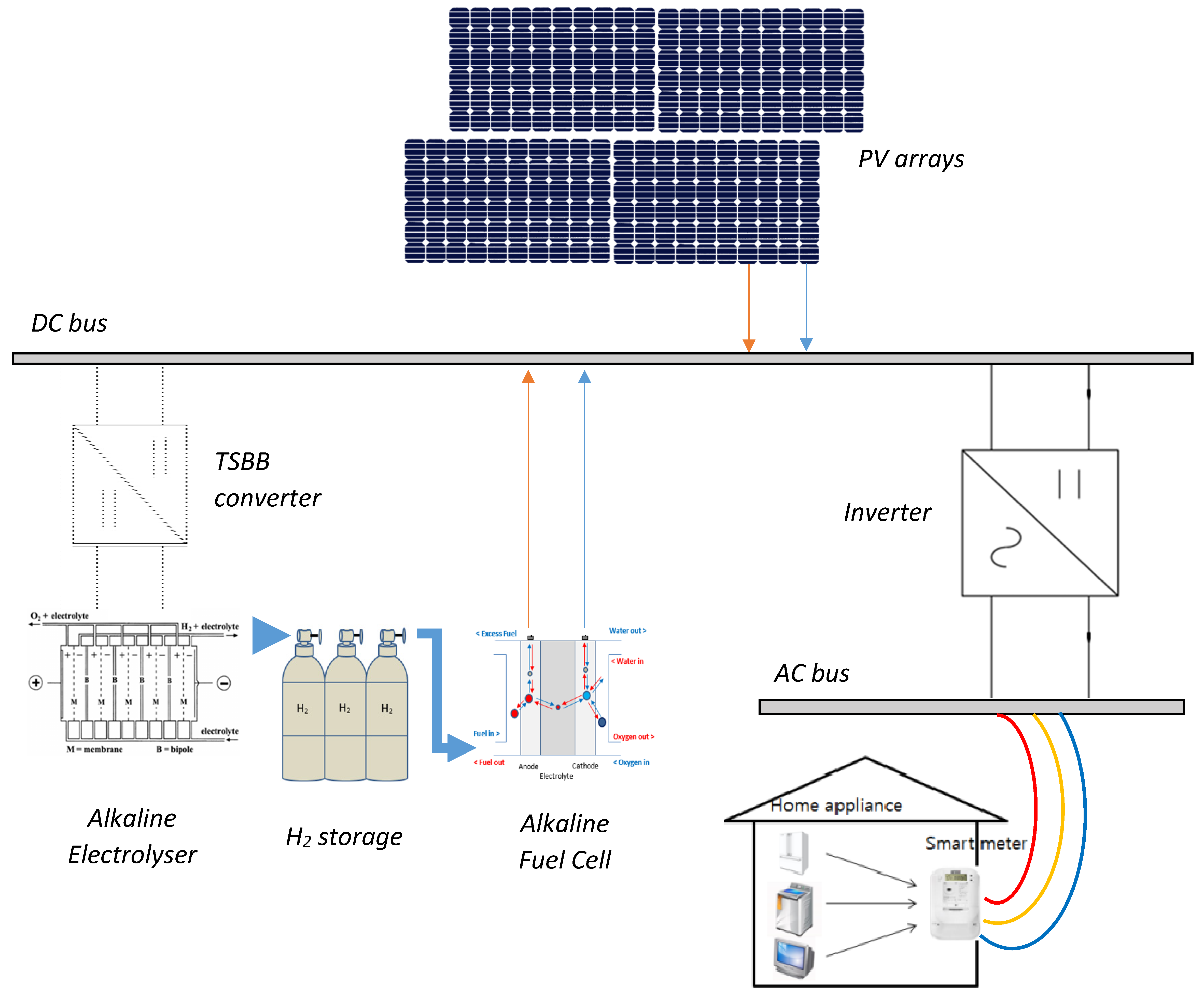
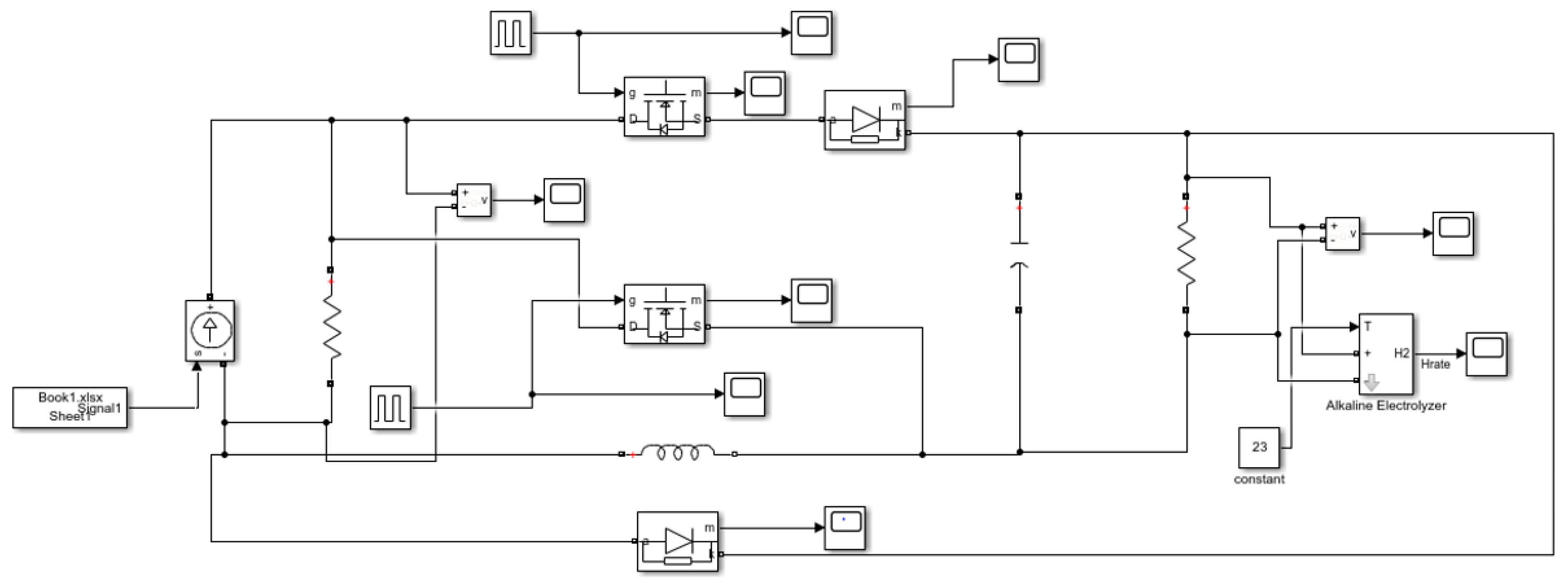
2. System Component Models
3. Objective Formulation
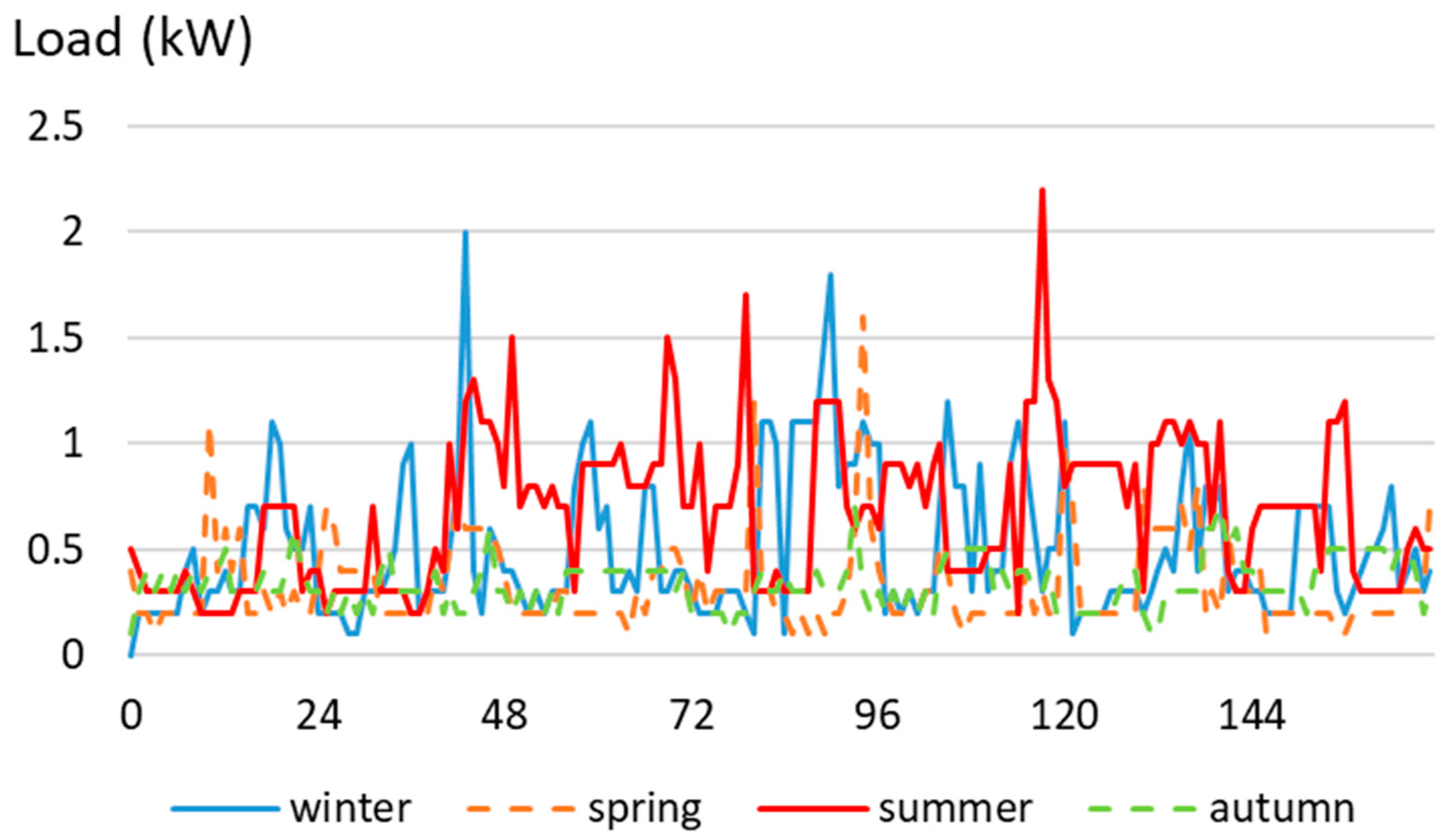
3.1. Domestic Load Profile
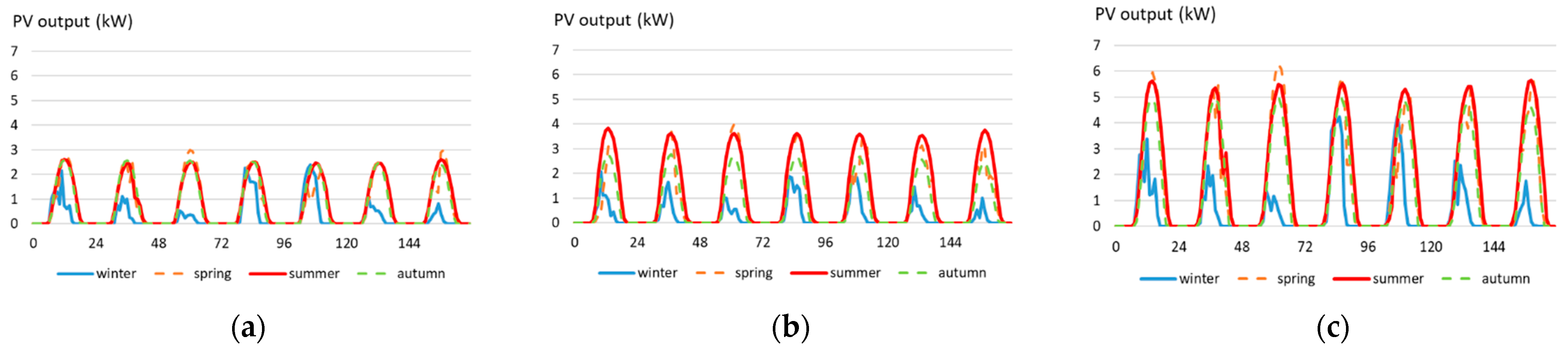
3.2. PV Generating System
3.3. Power-to-Hydrogen Module
3.4. Fuel Cell System
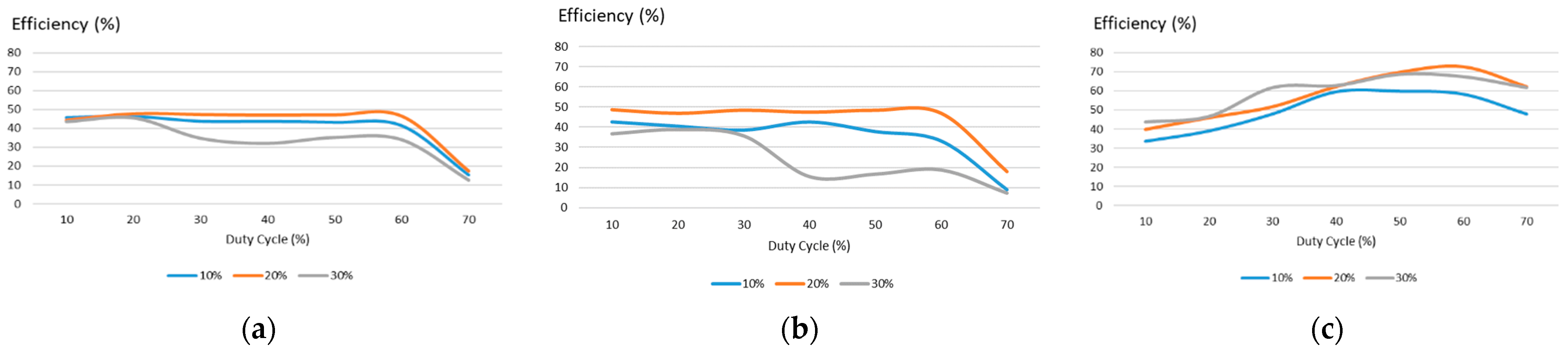
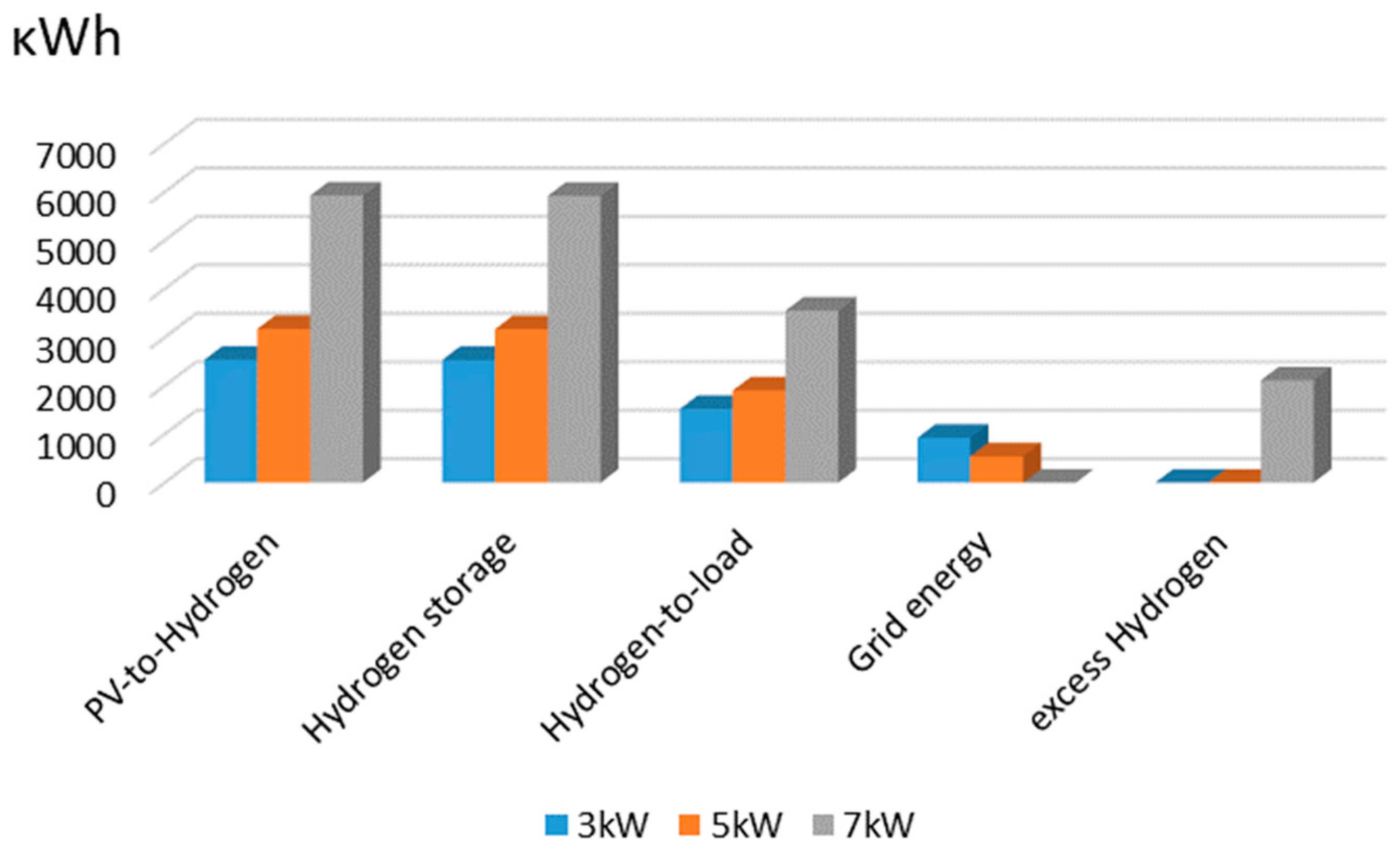
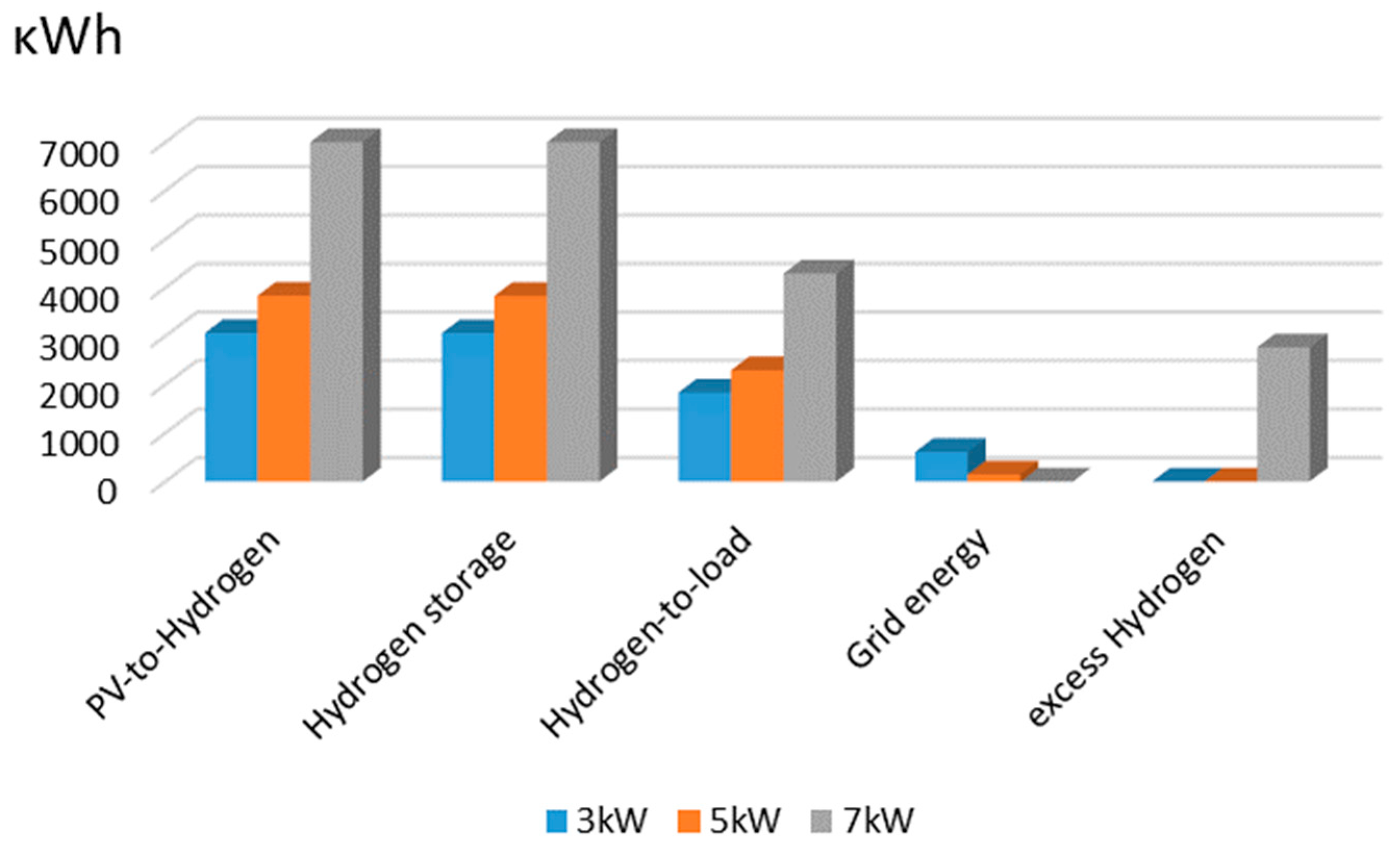
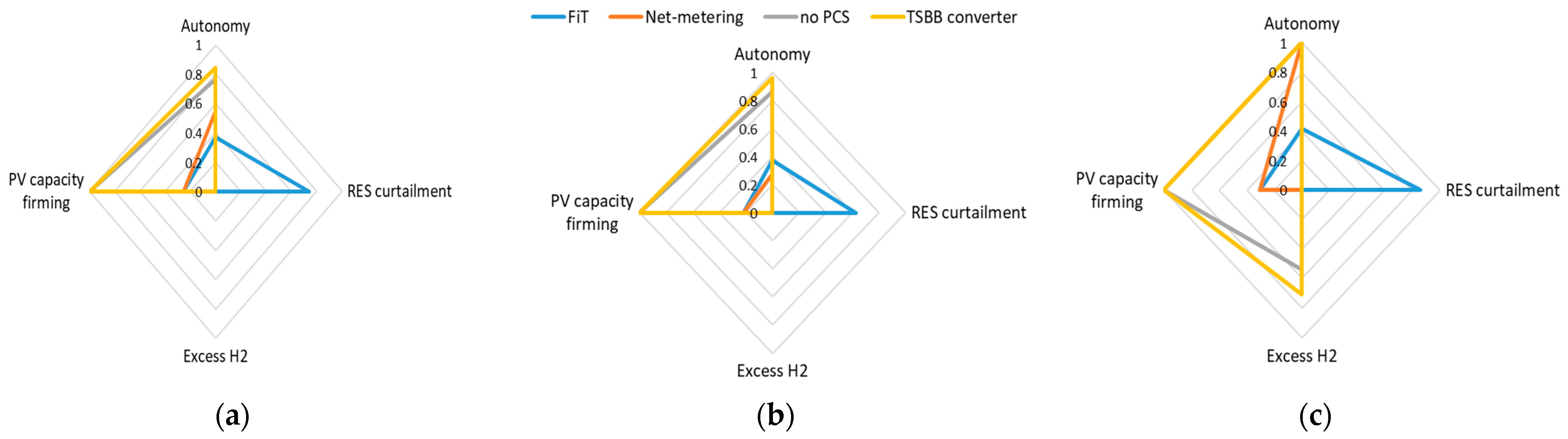
4. Experimental Evaluation
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nikolaidis, P.; Poullikkas, A. Sustainable Services to Enhance Flexibility in the Upcoming Smart Grids. In Sustaining Resources for Tomorrow; Stagner, J.A., Ting, D.S.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 245–274. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Chatzis, S.; Poullikkas, A. Life cycle cost analysis of electricity storage facilities in flexible power systems. Int. J. Sustain. Energy 2019, 38, 752–772. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Oldenbroek, V.; Wijtzes, S.; Blok, K.; van Wijk, A.J. Fuel cell electric vehicles and hydrogen balancing 100 percent renewable and integrated national transportation and energy systems. Energy Convers. Manag. X 2021, 9, 100077. [Google Scholar] [CrossRef]
- Bareiß, K.; de la Rúa, C.; Möckl, M.; Hamacher, T. Life cycle assessment of hydrogen from proton exchange membrane water electrolysis in future energy systems. Appl. Energy 2019, 237, 862–872. [Google Scholar] [CrossRef]
- Ajanovic, A.; Haas, R. Prospects and impediments for hydrogen and fuel cell vehicles in the transport sector. Int. J. Hydrogen Energy 2021, 46, 10049–10058. [Google Scholar] [CrossRef]
- Farahani, S.S.; Van Der Veen, R.; Oldenbroek, V.; Alavi, F.; Lee, E.H.P.; Van De Wouw, N.; Van Wijk, A.; De Schutter, B.; Lukszo, Z. A Hydrogen-Based Integrated Energy and Transport System: The Design and Analysis of the Car as Power Plant Concept. IEEE Syst. Man Cybern. Mag. 2019, 5, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Yamada, E.; Mashiba, T. Development of Technical Regulations for Fuel Cell Motorcycles in Japan—Hydrogen Safety. World Electr. Veh. J. 2019, 10, 48. [Google Scholar] [CrossRef] [Green Version]
- Bendary, A.F.; Ismail, M.M. Battery Charge Management for Hybrid PV/Wind/Fuel Cell with Storage Battery. Energy Procedia 2019, 162, 107–116. [Google Scholar] [CrossRef]
- Stern, A.G. A new sustainable hydrogen clean energy paradigm. Int. J. Hydrogen Energy 2018, 43, 4244–4255. [Google Scholar] [CrossRef]
- Maleki, A.; Rosen, M.A.; Pourfayaz, F. Optimal Operation of a Grid-Connected Hybrid Renewable Energy System for Residential Applications. Sustainability 2017, 9, 1314. [Google Scholar] [CrossRef] [Green Version]
- Anifantis, A.S.; Colantoni, A.; Pascuzzi, S.; Santoro, F. Photovoltaic and Hydrogen Plant Integrated with a Gas Heat Pump for Greenhouse Heating: A Mathematical Study. Sustainability 2018, 10, 378. [Google Scholar] [CrossRef] [Green Version]
- Lewandowska-Bernat, A.; Desideri, U. Opportunities of Power-to-Gas technology. Energy Procedia 2017, 105, 4569–4574. [Google Scholar] [CrossRef]
- Liu, W.; Wang, D.; Yu, X.; Jia, H.; Wang, W.; Yang, X.; Zhi, Y. Optimal Scheduling of Multi-Source Microgrid Considering Power to Gas Technology and Wind Power Uncertainty. Energy Procedia 2017, 143, 668–673. [Google Scholar] [CrossRef]
- Li, B.; Roche, R.; Paire, D.; Miraoui, A. Sizing of a stand-alone microgrid considering electric power, cooling/heating, hydrogen loads and hydrogen storage degradation. Appl. Energy 2017, 205, 1244–1259. [Google Scholar] [CrossRef] [Green Version]
- Suresh, V.; Muralidhar, M.; Kiranmayi, R. Modelling and optimization of an off-grid hybrid renewable energy system for electrification in a rural areas. Energy Rep. 2020, 6, 594–604. [Google Scholar] [CrossRef]
- Kebede, M.H.; Beyene, G.B. Feasibility Study of PV-Wind-Fuel Cell Hybrid Power System for Electrification of a Rural Village in Ethiopia. J. Electr. Comput. Eng. 2018, 2018, 4015354. [Google Scholar] [CrossRef]
- Acakpovi, A.; Adjei, P.; Nwulu, N.; Asabere, N.Y. Optimal Hybrid Renewable Energy System: A Comparative Study of Wind/Hydrogen/Fuel-Cell and Wind/Battery Storage. J. Electr. Comput. Eng. 2020, 2020, 1756503. [Google Scholar] [CrossRef]
- Rezk, H.; Sayed, E.; Al-Dhaifallah, M.; Obaid, M.; El-Sayed, A.H.M.; Abdelkareem, M.A.; Olabi, A. Fuel cell as an effective energy storage in reverse osmosis desalination plant powered by photovoltaic system. Energy 2019, 175, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Ge, P.; Hu, Q.; Wu, Q.; Dou, X.; Wu, Z.; Ding, Y. Increasing operational flexibility of integrated energy systems by introducing power to hydrogen. IET Renew. Power Gener. 2020, 14, 372–380. [Google Scholar] [CrossRef]
- Zappa, W.; Junginger, M.; Broek, M.V.D. Is a 100% renewable European power system feasible by 2050? Appl. Energy 2019, 233–234, 1027–1050. [Google Scholar] [CrossRef]
- Mathiesen, B.V.; Lund, H.; Karlsson, K.B. 100% Renewable energy systems, climate mitigation and economic growth. Appl. Energy 2011, 88, 488–501. [Google Scholar] [CrossRef]
- Connolly, D.; Lund, H.; Mathiesen, B.; Leahy, M. The first step towards a 100% renewable energy-system for Ireland. Appl. Energy 2011, 88, 502–507. [Google Scholar] [CrossRef]
- Tezer, T.; Yaman, R.; Yaman, G. Evaluation of approaches used for optimization of stand-alone hybrid renewable energy systems. Renew. Sustain. Energy Rev. 2017, 73, 840–853. [Google Scholar] [CrossRef]
- Diesendorf, M.; Elliston, B. The feasibility of 100% renewable electricity systems: A response to critics. Renew. Sustain. Energy Rev. 2018, 93, 318–330. [Google Scholar] [CrossRef]
- Singh, A.; Baredar, P.; Gupta, B. Techno-economic feasibility analysis of hydrogen fuel cell and solar photovoltaic hybrid renewable energy system for academic research building. Energy Convers. Manag. 2017, 145, 398–414. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Dawood, F.; Shafiullah, G.; Anda, M. Stand-Alone Microgrid with 100% Renewable Energy: A Case Study with Hybrid Solar PV-Battery-Hydrogen. Sustainability 2020, 12, 2047. [Google Scholar] [CrossRef] [Green Version]
- Nikolaidis, P.; Poullikkas, A. A comparative review of electrical energy storage systems for better sustainability. J. Power Technol. 2011, 97, 220–245. [Google Scholar]
- Chakik, F.E.; Kaddami, M.; Mikou, M. Effect of operating parameters on hydrogen production by electrolysis of water. Int. J. Hydrogen Energy 2017, 42, 25550–25557. [Google Scholar] [CrossRef]
- Samy, M.; Barakat, S.; Ramadan, H. A flower pollination optimization algorithm for an off-grid PV-Fuel cell hybrid renewable system. Int. J. Hydrogen Energy 2019, 44, 2141–2152. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, S.H.; Moon, B.; Lee, S.-H. A New Circuit Design of Two-Switch Buck-Boost Converter. IEEE Access 2018, 6, 47415–47423. [Google Scholar] [CrossRef]
- Martinez, D.; Zamora, R. MATLAB simscape model of an alkaline electrolyser and its simulation with a directly coupled PV module. Int. J. Renew. Energy Res. 2018, 8, 552–560. [Google Scholar]
- Demir, N.; Kaya, M.F.; Albawabiji, M.S. Effect of pulse potential on alkaline water electrolysis performance. Int. J. Hydrogen Energy 2018, 43, 17013–17020. [Google Scholar] [CrossRef]
- Burton, N.; Padilla, R.; Rose, A.; Habibullah, H. Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew. Sustain. Energy Rev. 2021, 135, 110255. [Google Scholar] [CrossRef]


| Mode | M1 | M2 | D1 | D2 | Vo |
|---|---|---|---|---|---|
| Buck | On | Off | Off | On | |
| Off | Off | On | Off | ||
| Boost | On | On | Off | Off | |
| On | Off | Off | On | ||
| Buck-Boost | Off | On | Off | Off | |
| Off | Off | On | Off |
| Appliance | Power (W) | Consumption (kWh) | Energy Category | Operation Time |
|---|---|---|---|---|
| Washing machine | 1020 | 2.14 | A | 2.1 h |
| Dish washer | 1050 | 1.5 | A | 86 min |
| Dryer | 4300 | 4.3 | C | 1 h |
| Refrigerator | 90 | 1.35 | B | 24 h |
| Freezer | 110 | 1.65 | A | 24 h |
| Ceramic hob | 2000 | 2 | A | 1 h |
| Electric oven | 890 | 0.89 | A | 1 h |
| Toaster | 1500 | 0.75 | - | 30 min |
| Mixer | 1200 | 0.6 | - | 30 min |
| Electric iron | 2400 | 2.4 | - | 1 h |
| LCD TV set | 200 | 0.8 | - | 4 h |
| AC unit 12,000 BTU | 3519 | 10.557 | A | 3 h |
| AC unit 22,000 BTU | 6452 | 12.904 | A | 2 h |
| Blow dryer | 2000 | 1 | - | 30 min |
| Water heater | 4000 | 4 | - | 1 h |
| Vaccum cleaner | 2000 | 2 | - | 1 h |
| Computer | 300 | 0.6 | - | 2 h |
| Printer | 150 | 0.05 | - | 20 min |
| Stereo sytem | 60 | 0.06 | - | 1 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaidis, P. Pulsed-Supplied Water Electrolysis via Two-Switch Converter for PV Capacity Firming. Electricity 2022, 3, 131-144. https://doi.org/10.3390/electricity3010008
Nikolaidis P. Pulsed-Supplied Water Electrolysis via Two-Switch Converter for PV Capacity Firming. Electricity. 2022; 3(1):131-144. https://doi.org/10.3390/electricity3010008
Chicago/Turabian StyleNikolaidis, Pavlos. 2022. "Pulsed-Supplied Water Electrolysis via Two-Switch Converter for PV Capacity Firming" Electricity 3, no. 1: 131-144. https://doi.org/10.3390/electricity3010008
APA StyleNikolaidis, P. (2022). Pulsed-Supplied Water Electrolysis via Two-Switch Converter for PV Capacity Firming. Electricity, 3(1), 131-144. https://doi.org/10.3390/electricity3010008






