Chemical Elements in Hair and Their Association with Autism Spectrum Disorder: A Comprehensive Systematic Review
Abstract
:1. Introduction
1.1. Biomarkers of Exposure in Research and ASD
1.1.1. Blood
1.1.2. Teeth
1.1.3. Hair
1.2. Examples of Using HMA
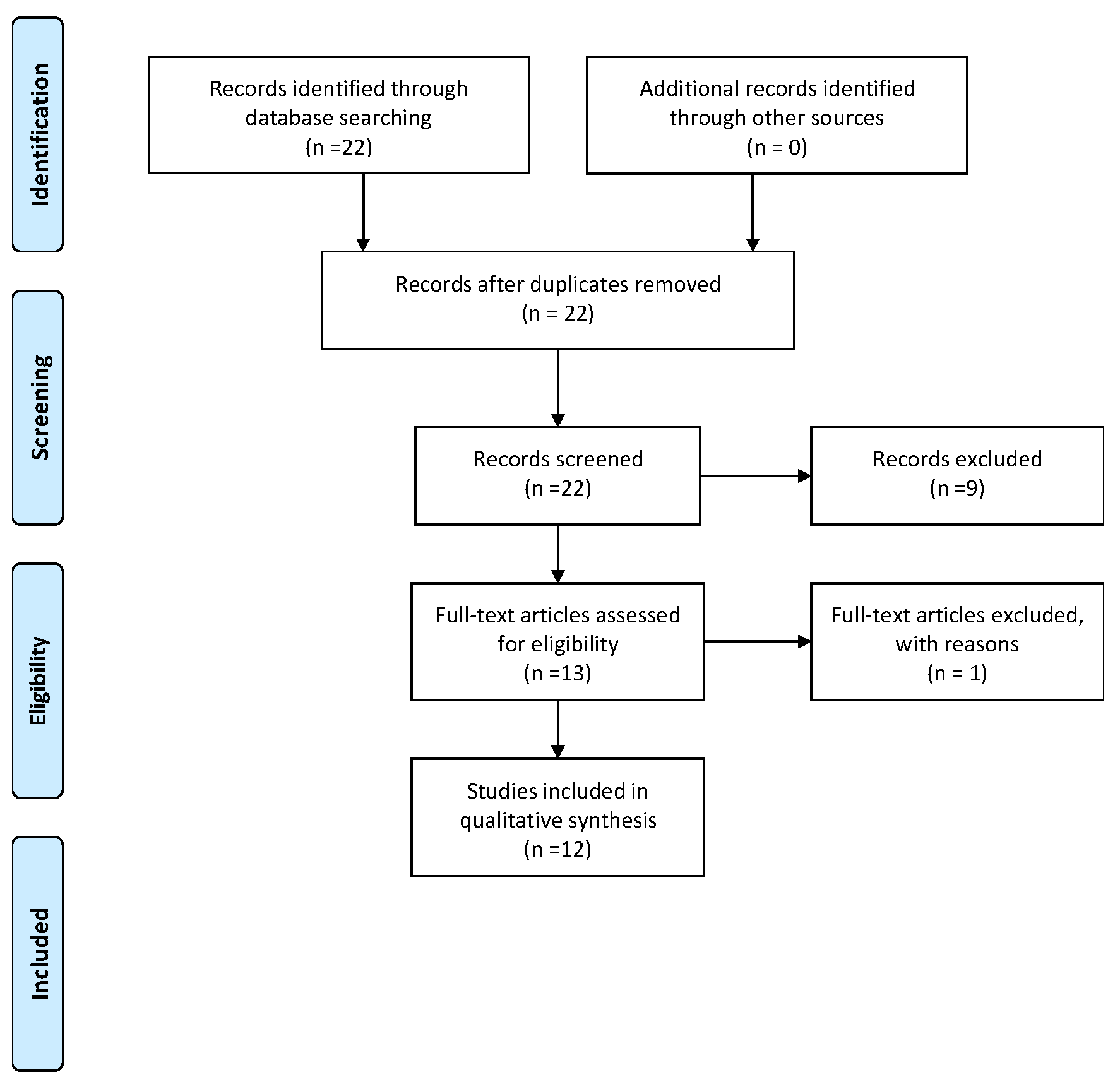
2. Materials and Methods
3. Results
| Ref. | Main Objective | No of Patients/Sex/Age | Analytical Technique/Unit | Main Conclusions |
|---|---|---|---|---|
| [33] | Evaluation of hair trace element and mineral levels in boys with ADHD, ASD, as well as ADHD with ASD. | M 52 (ASD) CTR 52 Age * 5.18 ± 1.00 | ICP-MS | The results obtained suggest that elements, such as Mg, Mn, and Zn may have an impact on the development of ADHD and ASD. |
| [34] | Associations between metal-based hair levels and essential elements and some specific characteristics of ASD. | ASD 48 M 34 F 14 Age: 6.5 ± 3.8 | ICP-MS | The results obtained show the relationship of metallomic analysis with the essential characteristics of ASD in order to identify potential environmental risk factors at the individual level. |
| Investigation of the trace element and gut microbiota profiles of Chinese autistic children. | ASD 78 M 56 F 22 58 CTR Age: 4.96 ± 1.01 | ICP-MS | The results obtained in Chinese children with ASD indicate significant changes in the profile of micronutrients and intestinal microbiota. | |
| [36] | Investigation of the association between catatonia in ASD and the levels of trace elements in hair and serum. | ASD 30 ASD + CAT 30 M 30 CTR 30 | ICP-MS | The results showed (regression) that Hg levels in hair were negatively associated with catatonia in ASD in raw and corrected models. |
| [37] | Evaluation of the levels of essential trace elements in hair and serum in children with autism spectrum disorder. | ASD 70 M40 F30 CTR 70 Age: 6.4 ± 2.9 Two groups: (2–5) (6–10) | ICP-DRC-MS | To develop a personal diet for patients with ASD, it is recommended to evaluate several bioindication matrices to critically assess the status of trace elements in patients. |
| [38] | Analysis of hair trace elements content in children with communication disorder and autism spectrum disorder. | ASD 33 ** M 33 CTR 33 Age: 5.0 ± 1.8 Two groups: (3–4) (5–8) | ICP-MS | On the basis of the obtained results, it can be hypothesized that children suffering from ASD are characterized by a deeper change in metal handling and excretion. |
| [39] | Investigation of hair trace elements content in children with ASD. | ASD 74 CTR 74 AGE: two groups: (2–4) (5–9) | ICP-MS | There was no significant difference between the groups in the content of mercury (Hg), zinc (Zn), and copper (Cu) in the hair. Children with ASD are characterized by lower values of not only essential, but also toxic trace elements in their hair. |
| [40] | Determination of the concentrations of trace elements. | ASD 1967 M 1553 F 414 Age: 0–15 | ICP-MS | Zinc and magnesium deficiency may play an important role in the pathogenesis of autism. |
| [41] | Examination of possible environmental risk factors and sources of exposure to mercury in children with autism. | ASD 25 M 22 F 3 CTR 25 Age: 3–9 | ICP-MS | The baseline levels of arsenic, cadmium, and cerium in the urine did not reflect statistically significant differences of these elements in the the mean levels in hair. |
| [42] | Investigation of the levels of both toxic and nontoxic essential metals in the hair of autistic children. | ASD 44 M 37 F 7 CTR 61 Age: 9.00 ± 4.05 | ICP-MS | The results of the current study (as well as the previous one) assessing the concentration of elements in the hair-when it comes to Hg-are not meaningful. |
| [43] | Assessment of the levels of trace elements in hair and nail samples of autistic children. | ASD 45 LFA/MFA/HFA *** 15 each CTR 50 M:F = 4:1 Age: 4–12 | AAS | Zn showed a significantly different level in hair and nails, Cu, Pb, and Hg-increased concentration, and Mg and Se decreased concentration (hair, nails) in people with autism vs. control. |
| [44] | Concentration of toxic metals in the hair of children with autism. | ASD 40 M 40 40 CTR Age: 4–7 | ICP-MS | Lead, mercury and uranium-higher content in the hair of children with autism. |
| Ref. | Statistical Value/Unit | Cr | Co | Fe | ||
|---|---|---|---|---|---|---|
| [33] | Median/(μg/g) | 0.125 (0.092–0.184) | 0.008 (0.005–0.01) | 13.11 (9.81–15.68) | ||
| [34] | Median/(μg/g) | 1.151 (0.714–1.714) | 0.025 (0.025–0.025) | - | ||
| [35] | Median/(mg/kg) | - | - | 19.00 | ||
| [36] | Median/(μg/g) | 0.110 (0.085–0.150) | 0.008 (0.006–0.011) | 12.37 (9.744–14.85) | ||
| [37] | Median/(μg/g) | - | 0.0066 (0.0048–0.0104) * | 11.7 (9.1–18.5) * | ||
| [38] | Median/(μg/g) | - | 0.0111 (0.0086–0.0157) | 0.0068 (0.0048–0.0104) | 16.0 (11.5–22.9) | 10.5 (8.8–15.7) |
| [39] | Median/(μg/g) | 0.110 (0.082–0.156) * | 0.008 (0.006–0.011) * | 11.7 (9.6–18.0) * | ||
| [40] | ** | 12% ** | 2.0% ** | 17% ** | ||
| [41] | Mean + SD/(μg/g) | 0.09 ± 0.06 | 0.04 ± 0.05 | 10.89 ± 4.28 | ||
| [42] | Median/(μg/g) | 0.20 | 0.01 | 7.60 | ||
| [43] | Mean + SD/(μg/g) | LFA MFA HFA | - | - | ||
| [44] | Median/(μg/g) | - | - | - | ||
| Ref. | Statistical Value/Unit | Mn | Zn | Cu | Se | ||||
|---|---|---|---|---|---|---|---|---|---|
| [33] | Median/(μg/g) | 0.224 (0.161–0.404) | 122.3 (86.6–152.9) | 10.11 (8.46–11.99) | 0.422 (0.35–0.484) | ||||
| [34] | Median/(μg/g) | 0.144 (0.058–0.322) | 136 (107–168) | 9.226 (7.939–12.07) | 0.511 (0.465–0.610) | ||||
| [35] | Median/(mg/kg) | - | 78.00 | 8.30 | - | ||||
| [36] | Median/(μg/g) | 0.254 (0.194–0.325) | 121.59 (77.17–156.1) | 9.604 (8.454–11.02) | 0.341 (0.261–0.388) | ||||
| [37] | Median/(μg/g) | 0.2233 (0.1384–0.3220) * | 122 (77–169) * | 10.9 (9.7–13.3) * | 0.4061 (0.3427–0.4598) * | ||||
| [38] | Median/(μg/g) | 0.326 (0.195–0.469) | 0.241 (0.174–0.340) | 77.0 (63.8–141.8) | 153.2 (117.3–202.0) | 10.6 (8.8–12.5) | 8.7 (8.2–11.1) | 0.364 (0.317–0.407) | 0.406 (0.253–0.437) |
| [39] | Median/(μg/g) | 0.230 (0.170–0.327) * | - | 10.2 (8.7–12.5) * | 0.365 (0.315–0.424) * | ||||
| [40] | ** | 4% ** | 29.7% ** | 4% ** | - | ||||
| [41] | Mean + SD/(μg/g) | 0.38 ± 0.24 | 101.042 ± 52.0 | 21.94 ± 21.7 | 0.80 ± 0.25 | ||||
| [42] | Median/(μg/g) | 0.20 | 149.00 | 10.20 | 0.90 | ||||
| [43] | Mean + SD/(μg/g) | - | 130.46 ± 15.65 172.81 ± 20.73 171.92 ± 20.63 | 36.62 ± 4.39 23.16 ± 2.77 12.35 ± 1.48 | 0.57 ± 0.06 1.98 ± 0.23 2.55 ± 0.30 | ||||
| [44] | Median/(μg/g) | - | - | - | - | ||||
| Ref. | Li | Be | Al | Ni | As | ||||
|---|---|---|---|---|---|---|---|---|---|
| [33] | 0.023 (0.017–0.034) | - | - | - | - | ||||
| [34] | 0.025 (0.025–0.025) | 0.025 (0.025–0.025) | 7.738 (5.797–12.79) | 0.158 (0.067–0.365) | 0.082 (0.030–0.165) | ||||
| [35] | - | - | - | - | 0.21 | ||||
| [36] | - | - | - | - | 0.033 (0.023–0.039) | ||||
| [37] | - | - | - | - | - | ||||
| [38] | 0.0165 (0.0119–0.0470) | 0.0202 (0.0115–0.0290) | 0.0005 (0.0003–0.0007) | 0.0006 (0.0003–0.0008) | 8.8 (7.4–19.1) | 9.2 (5.3–11.8) | - | 0.0391 (0.0284–0.0591) | 0.0301 (0.0250–0.0408) |
| [39] | 0.020 (0.012–0.033) | 0.0004 (0.0001–0.0010) | 8.0 (5.3–11.7) | - | 0.034 (0.021–0.044) | ||||
| [40] | - | - | - | - | - | ||||
| [41] | 0.002 ± 0.004 | 0.0001 ± 0.0003 | 8.89 ±6.16 | 0.55 ± 0.83 | 0.20 ± 0.26 | ||||
| [42] | 0.008 | 11.65 | 0.20 | 0.03 | |||||
| [43] | LFA MFA HFA | - | - | - | - | ||||
| [44] | - | 0.05 (0.01, 0.10) | 61.0 (59.0, 70.0) | - | 0.13 (0.12, 0.18) | ||||
| Ref. | Mo | Cd | Hg | U | Pb | |||
|---|---|---|---|---|---|---|---|---|
| [33] | - | - | - | - | - | |||
| [34] | 0.117 (0.051–0.179) | 0.021 (0.010–0.035) | 0.338 (0.121–1.255) | 0.047 (0.026–0.086) | 0.542 (0.316–1.690) | |||
| [35] | - | 0.04 | 0.41 | - | 2.0 | |||
| [36] | - | 0.022 (0.015–0.030) | 0.229 (0.072–0.393) | - | - | |||
| [37] | - | - | - | - | - | |||
| [38] | - | 0.0277 (0.0224–0.0617) | 0.0222 (0.0149–0.0297) | 0.116 (0.066–0.203) | 0.097 (0.049–0.168) | - | 0.717 (0.547–1.273) | 0.463 (0.210–0.612) |
| [39] | - | 0.023 (0.014–0.035) | 0.127 (0.049–0.250) | - | 0.506 (0.330–0.673) | |||
| [40] | - | - | - | - | - | |||
| [41] | 0.08 ± 0.1 | 0.23 ± 0.27 | 0.47 ± 0.42 | 0.02 ± 0.01 | 0.01 ± 0.02 | |||
| [42] | 0.04 | 0.01 | 0.50 | 0.03 | 1.30 | |||
| [43] | - | - | 3.09 ± 0.37 1.10 ± 0.13 0.65 ± 0.07 | 17.97 ± 2.15 3.24 ± 0.38 2.04 ± 0.24 | ||||
| [44] | - | 0.14 (0.12, 0.16) | 4.50 (4.10, 4.90) | 0.42 (0.40, 0.50) | 6.75 (5.70, 7.00) | |||
4. Discussion
Microelements and ASD
5. Conclusions
- -
- Future research can center on the development and validation of standardized methods to ensure consistency and comparability across studies,
- -
- Since alterations in both essential and non-essential/toxic elements have been noted in children with ASD, a comparative analysis studying the impact and significance of these alterations could be of immense value,
- -
- Genetic sequencing coupled with environmental exposure data could be employed to unravel these interactions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | Attention Deficit Hyperactivity Disorder |
| ASD | Autism Spectrum Disorder |
| HMA | Hair Mineral Analysis |
References
- Virolainen, S.; Hussien, W.; Dalibalta, S. Autism spectrum disorder in the United Arab Emirates: Potential environmental links. Rev. Environ. Health 2020, 35, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Ijomone, O.M.; Olung, N.F.; Akingbade, G.T.; Okoh, C.O.; Aschner, M. Environmental influence on neurodevelopmental disorders: Potential association of heavy metal exposure and autism. J. Trace Elements Med. Biol. 2020, 62, 126638. [Google Scholar] [CrossRef] [PubMed]
- Currenti, S.A. Understanding and Determining the Etiology of Autism. Cell. Mol. Neurobiol. 2010, 30, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Geier, D.A.; Sykes, L.K.; Haley, B.E.; Geier, M.R. The relationship between mercury and autism: A comprehensive review and discussion. J. Trace Elements Med. Biol. 2016, 37, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Semenova, Y.; Zhunussov, Y.; Pivina, L.; Abisheva, A.; Tinkov, A.; Belikhina, T.; Skalny, A.; Zhanaspayev, M.; Bulegenov, T.; Glushkova, N.; et al. Trace element biomonitoring in hair and blood of occupationally unexposed population residing in polluted areas of East Kazakhstan and Pavlodar regions. J. Trace Elements Med. Biol. 2019, 56, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Savinov, S.S.; Sharypova, R.M.; Drobyshev, A.I. Determination of the Trace Element Composition of Human Nails. J. Anal. Chem. 2020, 75, 409–415. [Google Scholar] [CrossRef]
- Adams, J.B.; Holloway, C.E.; George, F.; Quig, D. Analyses of Toxic Metals and Essential Minerals in the Hair of Arizona Children with Autism and Associated Conditions, and Their Mothers. Biol. Trace Element Res. 2006, 110, 193–210. [Google Scholar] [CrossRef]
- Watts, C.; Sun, J.; Jones, P.D.; Peng, H.; Giesy, J.P. Monthly variations of unregulated brominated disinfection by-products in chlorinated Water are correlated with total Bromine. Eco-Environ. Health 2022, 1, 147–155. [Google Scholar] [CrossRef]
- Esplugas, R.; Mari, M.; Marquès, M.; Schuhmacher, M.; Domingo, J.L.; Nadal, M. Biomonitoring of trace elements in hair of schoolchildren living near a hazardous waste incinerator—A 20 years follow-up. Toxics 2019, 7, 52. [Google Scholar] [CrossRef]
- de Oliveira, L.M.; Das, S.; da Silva, E.B.; Gao, P.; Gress, J.; Liu, Y.; Ma, L.Q. Metal concentrations in traditional and herbal teas and their potential risks to human health. Sci. Total. Environ. 2018, 633, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Teitelbaum, S.L.; Dolios, G.; Dang, L.-H.T.; Tu, P.; Wolff, M.S.; Petrick, L.M. Molecular Gatekeeper Discovery: Workflow for Linking Multiple Exposure Biomarkers to Metabolomics. Environ. Sci. Technol. 2021, 56, 6162–6171. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.A. Individual and collective identification in contemporary forensics. Biosocieties 2020, 15, 350–375. [Google Scholar] [CrossRef]
- Alekseenko, S.I.; Skalny, A.V.; Karpischenko, S.A.; Tinkov, A.A. Serum, Whole Blood, Hair, and Mucosal Essential Trace Element and Mineral Levels in Children with Verified Chronic Rhinosinusitis Undergoing Functional Endoscopic Sinus Surgery. Biol. Trace Element Res. 2020, 199, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Çelik, B.; Nalçacıoğlu, H.; Karakükçü, Ç.; Aslaner, H.; Şahiner, Ü.M. Assessment of Hair Zinc in the School Children in Kayseri, Turkey. Biol. Trace Element Res. 2020, 196, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Sazakli, E.; Leotsinidis, M. Hair biomonitoring and health status of a general population exposed to Nickel. J. Trace Elements Med. Biol. 2017, 43, 161–168. [Google Scholar] [CrossRef]
- Shin, W.-J.; Jung, M.; Ryu, J.-S.; Hwang, J.; Lee, K.-S. Revisited digestion methods for trace element analysis in human hair. J. Anal. Sci. Technol. 2020, 11, 1. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, Y.; Ma, S.; Chen, F.; Zhang, D.; Hu, Z.; Zhang, Z.; Jin, H.; Guo, L. Highly accurate determination of Zn and Cu in human hair by ultrasound-assisted alkali dissolution combined with laser-induced breakdown spectroscopy. Microchem. J. 2020, 157, 105018. [Google Scholar] [CrossRef]
- Bocato, M.Z.; Ximenez, J.P.B.; Hoffmann, C.; Barbosa, F. An overview of the current progress, challenges, and prospects of human biomonitoring and exposome studies. J. Toxicol. Environ. Health B Crit. Rev. 2019, 22, 131–156. [Google Scholar] [CrossRef]
- Peña-Fernández, A.; del Carmen Lobo-Bedmar, M.; González-Muñoz, M.J. Effects of sex on the levels of metals and metalloids in the hair of a group of healthy Spanish adolescents (13 to 16 years old). Environ. Sci. Pollut. Res. 2017, 24, 23666–23678. [Google Scholar] [CrossRef]
- Bjørklund, G.; Tinkov, A.A.; Hosnedlová, B.; Kizek, R.; Ajsuvakova, O.P.; Chirumbolo, S.; Skalnaya, M.G.; Peana, M.; Dadar, M.; El-Ansary, A.; et al. The role of glutathione redox imbalance in autism spectrum disorder: A review. Free. Radic. Biol. Med. 2020, 160, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guo, S.; Zhao, J.; Gao, Y.; Li, Y.-F. Human Biological Monitoring of Mercury through Hair Samples in China. Bull. Environ. Contam. Toxicol. 2019, 102, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Astolfi, M.L.; Marconi, E.; Antonucci, A.; Canepari, S.; Piamonti, D.; Brunori, M.; Vitali, M. Occupational Exposure Assessment of Major and Trace Elements in Human Scalp Hair Among a Group of Eritrean Workers. Biol. Trace Element Res. 2019, 197, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Noreen, F.; Sajjad, A.; Mahmood, K.; Anwar, M.; Zahra, M.; Waseem, A. Human Biomonitoring of Trace Elements in Scalp Hair from Healthy Population of Pakistan. Biol. Trace Element Res. 2020, 196, 37–46. [Google Scholar] [CrossRef]
- Wei, B.; Yu, J.; Wang, J.; Li, H.; Yang, L.; Kong, C. Trace Metals in the Urine and Hair of a Population in an Endemic Arsenism Area. Biol. Trace Element Res. 2018, 182, 209–216. [Google Scholar] [CrossRef]
- Wongsasuluk, P.; Chotpantarat, S.; Siriwong, W.; Robson, M. Using hair and fingernails in binary logistic regression for bio-monitoring of heavy metals/metalloid in groundwater in intensively agricultural areas, Thailand. Environ. Res. 2018, 162, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Vinnikov, D.; Semizhon, S.; Rybina, T.; Zaitsev, V.; Pleshkova, A.; Rybina, A. Occupational exposure to metals and other elements in the tractor production. PLoS ONE 2018, 13, e0208932. [Google Scholar] [CrossRef]
- Mauriello, M.C.; Sbordone, C.; Montuori, P.; Alfano, R.; Triassi, M.; Iavicoli, I.; Manno, M. Biomonitoring of toxic metals in incinerator workers: A systematic review. Toxicol. Lett. 2017, 272, 8–28. [Google Scholar] [CrossRef]
- Ding, M.; Shi, S.; Qie, S.; Li, J.; Xi, X. Association between heavy metals exposure (cadmium, lead, arsenic, mercury) and child autistic disorder: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1169733. [Google Scholar] [CrossRef]
- Shiani, A.; Sharafi, K.; Omer, A.K.; Kiani, A.; Karamimatin, B.; Massahi, T.; Ebrahimzadeh, G. A systematic literature review on the association between exposures to toxic elements and an autism spectrum disorder. Sci. Total Environ. 2023, 857, 159246. [Google Scholar] [CrossRef]
- Blaurock-Busch, E.; Dessoki, H.H.; Rabah, T. Toxic Metals and Essential Elements in Hair and Severity of Symptoms among Children with Autism. Maedica 2012, 7, 38–48. [Google Scholar] [PubMed]
- Amadi, C.N.; Orish, C.N.; Frazzoli, C.; Orisakwe, O.E. Association of autism with toxic metals: A systematic review of case-control studies. Pharmacol. Biochem. Behav. 2022, 212, 173313. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Notova, S.V.; Chernova, L.N.; Skalny, A.A.; Burtseva, T.I.; Tinkov, A.A. Hair trace element concentrations in autism spectrum disorder (ASD) and attention deficit/hyperactivity disorder (ADHD). J. Trace Elements Med. Biol. 2020, 61, 126539. [Google Scholar] [CrossRef]
- Fiore, M.; Barone, R.; Copat, C.; Grasso, A.; Cristaldi, A.; Rizzo, R.; Ferrante, M. Metal and essential element levels in hair and association with autism severity. J. Trace Elements Med. Biol. 2020, 57, 126409. [Google Scholar] [CrossRef]
- Zhai, Q.; Cen, S.; Jiang, J.; Zhao, J.; Zhang, H.; Chen, W. Disturbance of trace element and gut microbiota profiles as indicators of autism spectrum disorder: A pilot study of Chinese children. Environ. Res. 2019, 171, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Skalnaya, M.G.; Simashkova, N.V.; Klyushnik, T.P.; Skalnaya, A.A.; Bjørklund, G.; Notova, S.V.; Kiyaeva, E.V.; Skalny, A.V. Association between catatonia and levels of hair and serum trace elements and minerals in autism spectrum disorder. Biomed. Pharmacother. 2019, 109, 174–180. [Google Scholar] [CrossRef]
- Skalny, A.V.; Simashkova, N.V.; Skalnaya, A.A.; Klyushnik, T.P.; Bjørklund, G.; Skalnaya, M.G.; Tinkov, A.A. Assessment of gender and age effects on serum and hair trace element levels in children with autism spectrum disorder. Metab. Brain Dis. 2017, 32, 1675–1684. [Google Scholar] [CrossRef]
- Skalny, A.V.; Simashkova, N.V.; Klyushnik, T.P.; Grabeklis, A.R.; Radysh, I.V.; Skalnaya, M.G.; Tinkov, A.A. Analysis of Hair Trace Elements in Children with Autism Spectrum Disorders and Communication Disorders. Biol. Trace Element Res. 2016, 177, 215–223. [Google Scholar] [CrossRef]
- Skalny, A.V.; Simashkova, N.V.; Klyushnik, T.P.; Grabeklis, A.R.; Bjørklund, G.; Skalnaya, M.G.; Nikonorov, A.A.; Tinkov, A.A. Hair toxic and essential trace elements in children with autism spectrum disorder. Metab. Brain Dis. 2017, 32, 195–202. [Google Scholar] [CrossRef]
- Yasuda, H.; Yoshida, K.; Yasuda, Y.; Tsutsui, T. Infantile zinc deficiency: Association with autism spectrum disorders. Sci. Rep. 2011, 1, 129. [Google Scholar] [CrossRef]
- Blaurock-Busch, E.; Amin, O.R.; Rabah, T. Heavy metals and trace elements in hair and urine of a sample of arab children with autistic spectrum disorder. Maedica 2011, 4, 247–257. [Google Scholar] [CrossRef]
- De Palma, G.; Catalani, S.; Franco, A.; Brighenti, M.; Apostoli, P. Lack of correlation between metallic elements analyzed in hair by ICP-MS and autism. J. Autism Dev. Disord. 2012, 42, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Priya, M.D.; Geetha, A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol. Trace Element Res. 2011, 142, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Fido, A.; Al-Saad, S. Toxic trace elements in the hair of children with autism. Autism 2005, 9, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Nabgha-e-Amen; Eqani, S.A.M.A.S.; Khuram, F.; Alamdar, A.; Tahir, A.; Shah, S.T.A.; Nasir, A.; Javed, S.; Bibi, N.; Hussain, A.; et al. Environmental exposure pathway analysis of trace elements and autism risk in Pakistani children population. Sci. Total. Environ. 2020, 712, 136471. [Google Scholar] [CrossRef] [PubMed]
- Scassellati, C.; Bonvicini, C.; Benussi, L.; Ghidoni, R.; Squitti, R. Neurodevelopmental disorders: Metallomics studies for the identification of potential biomarkers associated to diagnosis and treatment. J. Trace Elements Med. Biol. 2020, 60, 126499. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Skalny, A.V.; Rahman, M.; Dadar, M.; Yassa, H.A.; Aaseth, J.; Chirumbolo, S.; Skalnaya, M.G.; Tinkov, A.A. Toxic metal(loid)-based pollutants and their possible role in autism spectrum disorder. Environ. Res. 2018, 166, 234–250. [Google Scholar] [CrossRef]
- Petrova, M.V.; Ourgaud, M.; Boavida, J.R.; Dufour, A.; Onrubia, J.A.T.; Lozingot, A.; Heimbürger-Boavida, L.-E. Human mercury exposure levels and fish consumption at the French Riviera. Chemosphere 2020, 258, 127232. [Google Scholar] [CrossRef]
- Tseng, P.-T.; Cheng, Y.-S.; Chen, Y.-W.; Stubbs, B.; Whiteley, P.; Carvalho, A.F.; Li, D.-J.; Chen, T.-Y.; Tang, C.-H.; Chu, C.-S.; et al. Peripheral iron levels in children with autism spectrum disorders vs controls: A systematic review and meta-analysis. Nutr. Res. 2018, 50, 44–52. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Ahangari, N.; Hendi, K.; Saleh, F.; Rezaei, N. Status of essential elements in autism spectrum disorder: Systematic review and meta-analysis. Rev. Neurosci. 2017, 28, 783–809. [Google Scholar] [CrossRef]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Chao, J.C.-J.; Chernova, L.N.; Shakieva, R.A.; Kopylov, P.Y.; Skalny, A.A.; et al. Serum zinc, copper, zinc-to-copper ratio, and other essential elements and minerals in children with attention deficit/hyperactivity disorder (ADHD). J. Trace Elements Med. Biol. 2020, 58, 126445. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Ajsuvakova, O.P.; Skalny, A.V. A Case-Control Study of Essential and Toxic Trace Elements and Minerals in Hair of 0–4-Year-Old Children with Cerebral Palsy. Biol. Trace Element Res. 2020, 195, 399–408. [Google Scholar] [CrossRef] [PubMed]
| Metal | Chemical Form | Mechanism of ASD Contribution |
|---|---|---|
| Hg (Mercury) | - | Higher levels of antineuronal antibodies; neurological, motor, immune, and sensory dysfunctions. Exposure can occur through fish contaminated with methylmercury or through fungicides used as grain preservatives in bread. Children with ASD exhibit higher levels of Hg in primary teeth and blood. Hg induces metallothionein dysfunction, related to Zn deficit. |
| Mercury ions (Hg2+) | Nephrotoxic and causes damage to muscle tissue. | |
| Methylmercury (CH3Hg+) | Most toxic form, can cross the blood-brain barrier due to its lipophilic nature, binding to neurons and causing high neurotoxicity. Main sources for humans include fish, bacteria, and algae, leading to the biotransformation of elemental Hg to methylmercury. | |
| As (Arsenic) | - | Alters brain morphology and causes Mcl-1 depression in the cerebral cortex. Induces gliosis degeneration and up-regulates Bax and Bak expression. Impairs neurite growth through suppression of AMPK kinase activation and inhibits the Wnt/β-catenin signaling pathway. |
| Pb (Lead) | - | Induces neuroinflammation and autoimmunity, stimulating the synthesis of anti-ribosomal P antibodies. Exposure to Pb from leaded gasoline, used in the past, is another theorized pathogenesis of autism. |
| Al (Aluminum) | - | Interacts with glycolytic enzymes and inhibits cellular energy synthesis. Intensifies neurotoxic effects through Al3+ ion by oxygen-based ligands and activates microglia producing IL-6, TNF-α, iNOS, NOS-2, neuroinflammatory PICs, and ROS. |
| U (Uranium) | - | Uranium from coal combustion and phosphate fertilizers can contribute to autism. Elevated levels of non-radioactive isotope of uranium have been found in the hair of autistic children compared to controls. |
| Metal | Mechanism of ASD Contribution |
|---|---|
| Zn | Zinc is crucial for the scaffolding of ProSAP/Shank proteins, linked to excitatory synapses. Imbalances, either excess or deficiency, are associated with epileptogenesis, depression, and ASD, respectively, confirming the role of abnormal zinc levels in brain dysfunctions. Elevated levels of copper, being antagonistic to zinc, lead to synaptic dysfunction [50]. Zn is integral to the active site of 300 enzymes. |
| Mg | Magnesium ions are pivotal for the synthesis of the key neurotransmitter gamma-aminobutyric acid (GABA), thus playing a crucial role in ASD as they regulate GABA activity [50]. |
| Fe | Iron is imperative for proper brain functioning, playing a key role in gene expression and myelination. Disruption in iron homeostasis is observed in various neurodegenerative diseases. Iron deficiency can lead to conditions such as depression and anxiety, influencing social and emotional behavior and contributing to the development of ASD [50]. |
| Zn/Cu | Research indicates that the Zn/Cu ratio and zinc content are significantly lower in children with ASD compared to healthy children, underlining the significant role of the Zn to Cu ratio in ASD [37]. |
| Other elements | No associations with ASD were found in the case of the content of Cr, I, Se, in hair [37]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojnacka, K.; Mikulewicz, M. Chemical Elements in Hair and Their Association with Autism Spectrum Disorder: A Comprehensive Systematic Review. Pollutants 2023, 3, 587-602. https://doi.org/10.3390/pollutants3040038
Chojnacka K, Mikulewicz M. Chemical Elements in Hair and Their Association with Autism Spectrum Disorder: A Comprehensive Systematic Review. Pollutants. 2023; 3(4):587-602. https://doi.org/10.3390/pollutants3040038
Chicago/Turabian StyleChojnacka, Katarzyna, and Marcin Mikulewicz. 2023. "Chemical Elements in Hair and Their Association with Autism Spectrum Disorder: A Comprehensive Systematic Review" Pollutants 3, no. 4: 587-602. https://doi.org/10.3390/pollutants3040038
APA StyleChojnacka, K., & Mikulewicz, M. (2023). Chemical Elements in Hair and Their Association with Autism Spectrum Disorder: A Comprehensive Systematic Review. Pollutants, 3(4), 587-602. https://doi.org/10.3390/pollutants3040038