1. Introduction
Chemically modified electrodes are some of the most intensively developed areas in modern electroanalysis. This trend is mainly caused by the appearance of a wide range of nanomaterials (different types of nanoscale carbon, metal, and metal oxide nanoparticles, nanostructured polymers, other nanosized compounds, and composites) that are used as effective electrode surface modifiers. One of the approaches for electrode surface modification is coverage with electropolymerized films. The non-conductive polymers based on phenolic compounds are of interest insofar as they give a highly sensitive and selective response to low-molecular-weight substances, including antioxidants [
1,
2,
3]. Further development in this field using a combination of such electropolymerized coatings with carbon nanomaterials provides conductivity of the electrode as well as high loading and more uniform coverage of the electrode surface [
1].
Among the wide range of analytes, natural phenolic antioxidants, being a part of the daily human diet and medicinal therapy, are of great interest and widely investigated in life sciences. Given that their antioxidant effects are caused by electron transfer reactions, electrochemical methods are often used for their determination [
4,
5]. Flavanones—flavonoids of
Citrus fruits [
6]—are less investigated and almost out of consideration in electroanalysis in comparison to other natural phenolics. The major natural flavanones are naringin and hesperidin (
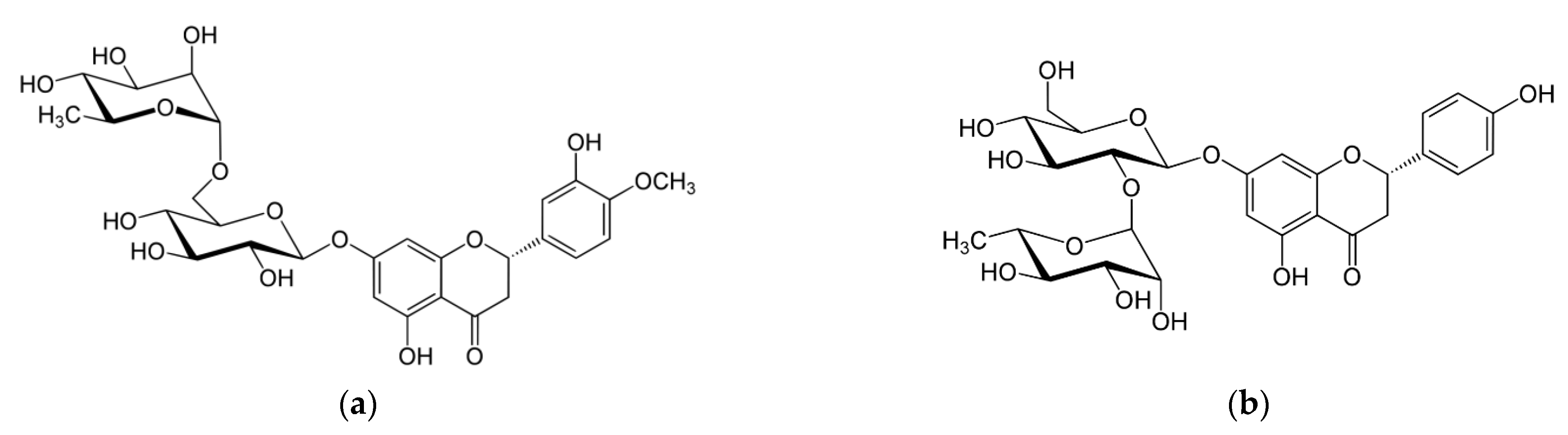
Figure 1), possessing a wide spectrum of biological activity, including antioxidant activity [
6]. Nevertheless, like other natural phenolic antioxidants [
7], they exhibit prooxidant properties when presented in high concentrations. Therefore, rigorous control of their contents in real samples is required.
A limited number of chemically modified electrodes has been developed for the voltammetric quantification of hesperidin and naringin. Carbon nanomaterials [
8,
9], metal-based nanomaterials [
10,
11], tin dioxide [
12] and silica [
13] nanoparticles, amberlite-IRA 400 [
14,
15], and DNA [
16,
17], as well as poly-
o-aminophenol [
18]- and poly-
o-aminothiophenol [
19]-based molecularly imprinted polymers are used as a sensitive layers of modified electrodes. The analytical characteristics are typical, and the linear dynamic ranges mainly cover 10
−7–10
−5 M concentrations. In many cases, the linear dynamic ranges are narrow enough to limit the applicability of the electrodes. Another disadvantage of the electrodes is the selectivity, which is insufficient or fully out of consideration.
Therefore, further improvement of the flavanones’ analytical characteristics, as well as their simultaneous determination, is of practical interest. The current work is focused on the creation of novel modified electrodes based on a layer-by-layer combination of carbon nanotubes and electropolymerized coatings for the direct quantification of naringin and hesperidin. Ellagic acid or aluminon-containing phenolic fragments in their structure have been used as monomers. Conditions of their potentiodynamic electropolymerization (the monomer concentration, supporting electrolyte pH, potential scan rate and range, the number of cycles) have been found. The electrodes created have been studied by scanning electron microscopy and electrochemical methods. The individual quantification of naringin and the simultaneous determination of hesperidin and naringin with high sensitivity and selectivity have been achieved.
2. Materials and Methods
Ellagic acid (95% purity) from Sigma-Aldrich (Darmstadt, Germany) and aluminon from Sigma-Aldrich (Germany) were used. Their standard solutions (0.86 mM for ellagic acid and 10 mM for aluminon) were prepared in methanol (c.p. grade). Analytes (hesperidin of 94% purity and naringin (95%)) were purchased from Sigma-Aldrich (Germany). Stock solutions of 10 or 0.40 mM for naringin and 0.40 mM for hesperidin were prepared in methanol (c.p. grade) in 5.0 mL flasks. Less concentrated solutions were obtained by the exact dilution.
Multi-walled carbon nanotubes (MWCNTs) (outer diameter 40–60 nm, inner diameter 5–10 nm, and 0.5–500 μm length) from Aldrich and polyaminobenzene sulfonic acid functionalized single-walled carbon nanotubes (f-SWCNTs) (d × l is 1.1 nm × 0.5–1.0 μm) from Sigma-Aldrich (Steinheim, Germany) were used as a platform for the electrodeposition of polymeric coverages. Homogeneous suspensions of carbon nanomaterials (0.5 mg mL−1 of MWCNTs in 1% sodium dodecylsulfate (Panreac, Barcelona, Spain) and 1.0 mg mL−1 of f-SWCNTs in dimethylformamide) were obtained by 30 min sonication using an ultrasonic bath (WiseClean WUC-A03H (DAIHAN Scientific Co., Ltd., Wonju-si, Korea).
Chlorogenic (95%) and ferulic (99%) acids from Sigma-Aldrich (Germany); ascorbic (99%), gallic (99%), caffeic (98%), and p-coumaric (98%) acids and quercetin dihydrate (95%) and catechin hydrate (98%) from Sigma-Aldrich (Germany); rutin trihydrate (97%) from Alfa Aesar (United Kingdom); sinapic acid (97%) and tannin (Ph. Eur.) from Fluka (Germany) were used in the interference study. Their 10 mmol L−1 stock solutions in methanol were prepared in 5.0 mL flasks.
All reagents were c.p. grade. Distilled water was used for the measurements. The laboratory temperature was (25 ± 2 °C).
Electrochemical measurements were conducted on a μAutolab Type III (Eco Chemie B.V., Utrecht, The Netherlands) potentiostat/galvanostat supplied with GPES 4.9.005 software and Autolab PGSTAT 302N with the FRA 32M module (Eco Chemie B.V., Utrecht, The Netherlands) and NOVA 1.10.1.9 software. The glassy electrochemical cell of 10 mL volume was used. The tree-electrode system consisted of the working GCE of 3 mm diameter (CH Instruments, Inc., Bee Cave, TX, USA), or a modified electrode, an Ag/AgCl reference electrode, and a platinum wire as the auxiliary electrode.
The pH measurements were carried out using an “Expert-001” pH meter (Econix-Expert Ltd., Moscow, Russian Federation) with a glassy electrode.
A MerlinTM (Carl Zeiss, Oberkochen, Germany) high-resolution field emission scanning electron microscope was applied for the electrode surface morphology characterization and operated at 5 kV accelerating voltage and a 300 pA emission current.
3. Results and Discussion
3.1. Polymer-Modified Electrodes Preparation and Characterization
Electropolymerization of ellagic acid and aluminon was carried out on the surface of GCE modified preliminarily with MWCNTs or f-SWCNTs by drop-casting technology (4.0 µL of MWCNTs or 2.0 µL f-SWCNTs suspensions were applied). This approach provided sufficient conductivity of the electrode as well as a high surface area. Polymeric coatings were electrodeposited in potentiodynamic mode. Both monomers were irreversibly oxidized at the electrode surface at 0.287 and 0.497 V for the ellagic acid at MWCNTs/GCE in phosphate buffer pH 7.0 and 0.50 V for aluminon at f-SWCNTs/GCE in 0.1 M NaOH. A decrease of the oxidation steps was observed for the following cycles, which means the formation of non-conducting polymer and typical for the phenolic compounds [
1]. The oxidation peaks of monomers almost disappeared after the seventh cycle for ellagic acid and the tenth cycle for aluminon. The conditions of electropolymerization (the monomer concentration, supporting electrolyte pH, potential scan rate and range, and the number of cycles) were optimized based on the response of target analytes (naringin on poly(ellagic acid)/MWCNTs/GCE and the hesperidin and naringin mixture on polyaluminon/f-SWCNTs/GCE). Electropolymerization conditions providing the best voltammetric characteristics of the analytes are presented in
Table 1.
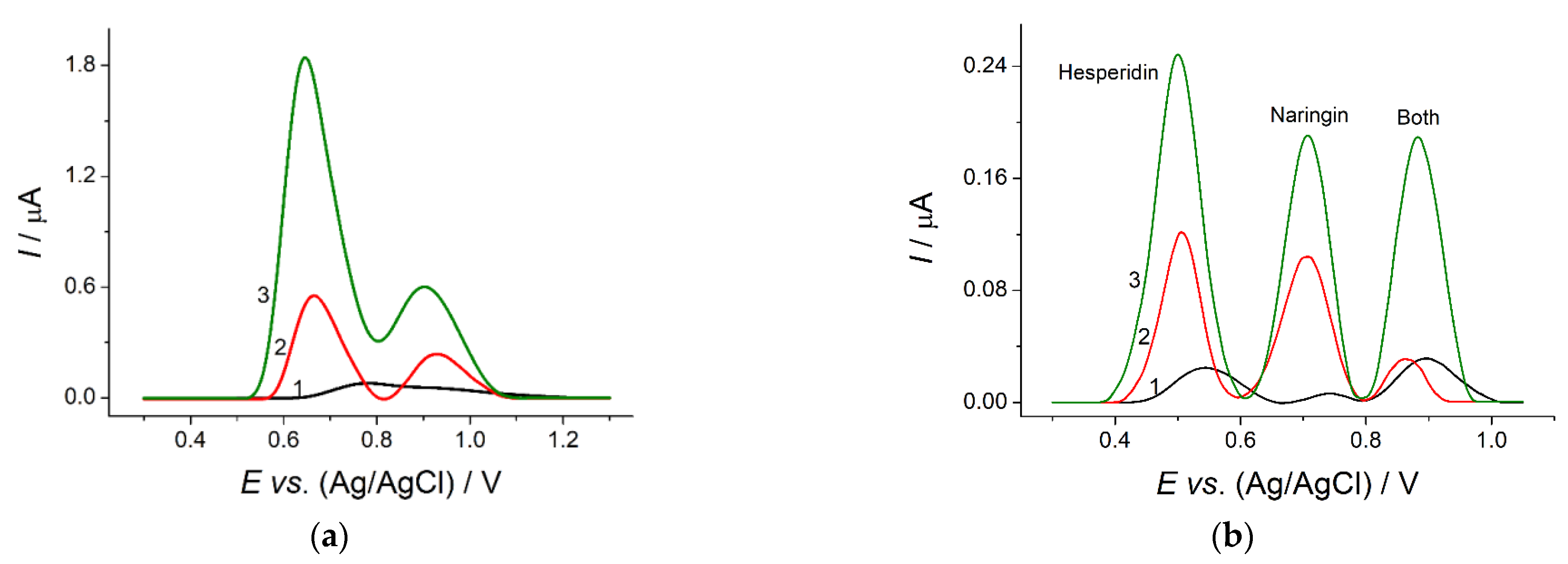
The suggested modification of the electrode surface provided significant improvement of the voltammetric response of the flavanones under consideration. The shifts of oxidation potentials to less positive values (
Figure 2) confirmed the increase of the electron transfer rate at the modified electrodes, which was proved by charge transfer resistance data obtained by electrochemical impedance spectroscopy in the presence of a 1.0 mM equimolar mixture of hexacyanoferrate(II)/(III) ions (
Table 2). A statistically significant increase of the flavanones’ oxidation currents (
Figure 2) was caused by the increase of the electroactive surface area of polymer-modified electrodes, as confirmed by electrochemical data based on the electrooxidation of hexacyanoferrate(II) ions (
Table 2).
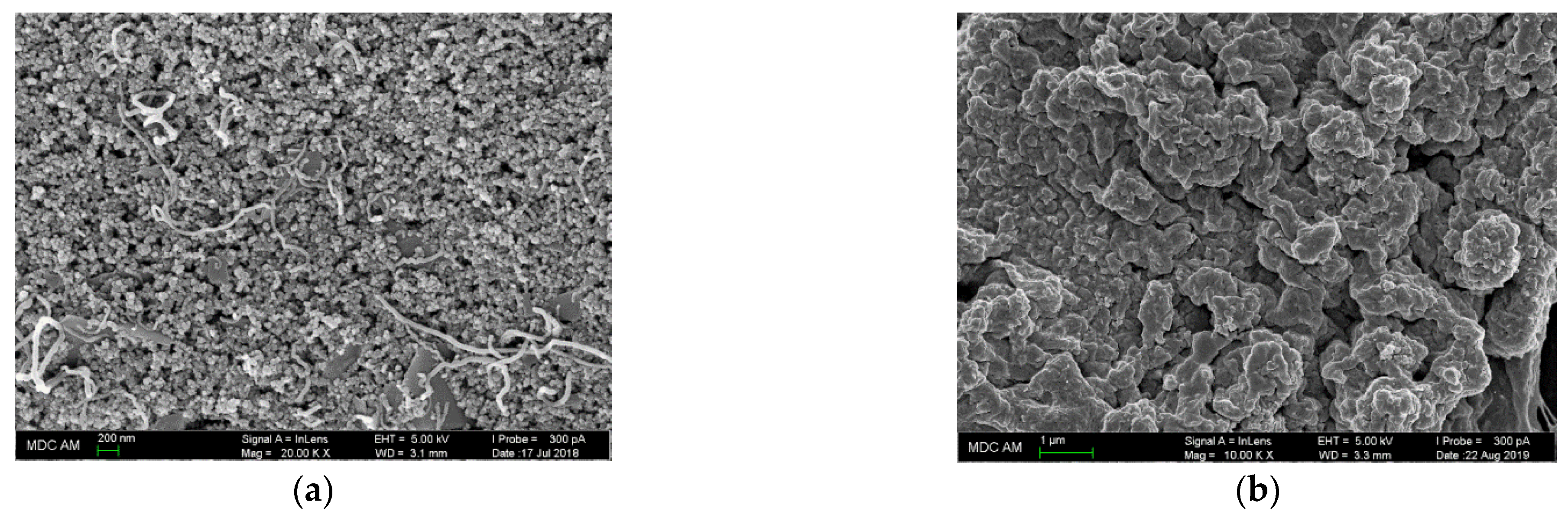
The electrode surface morphology was studied by scanning electron microscopy (
Figure 3). The polymeric coatings exhibited a porous structure with the shape of spherical particles of 30–50 nm in diameter for poly(ellagic acid) (
Figure 3a) and a folded structure with channels and cavities for polyaluminon (
Figure 3b), confirming successful electropolymerization as well as a high roughness of the electrode surface. These results agree well with those reported for other phenol-based polymeric coatings [
1,
20,
21].
3.2. Analytical Application of the Electrodes
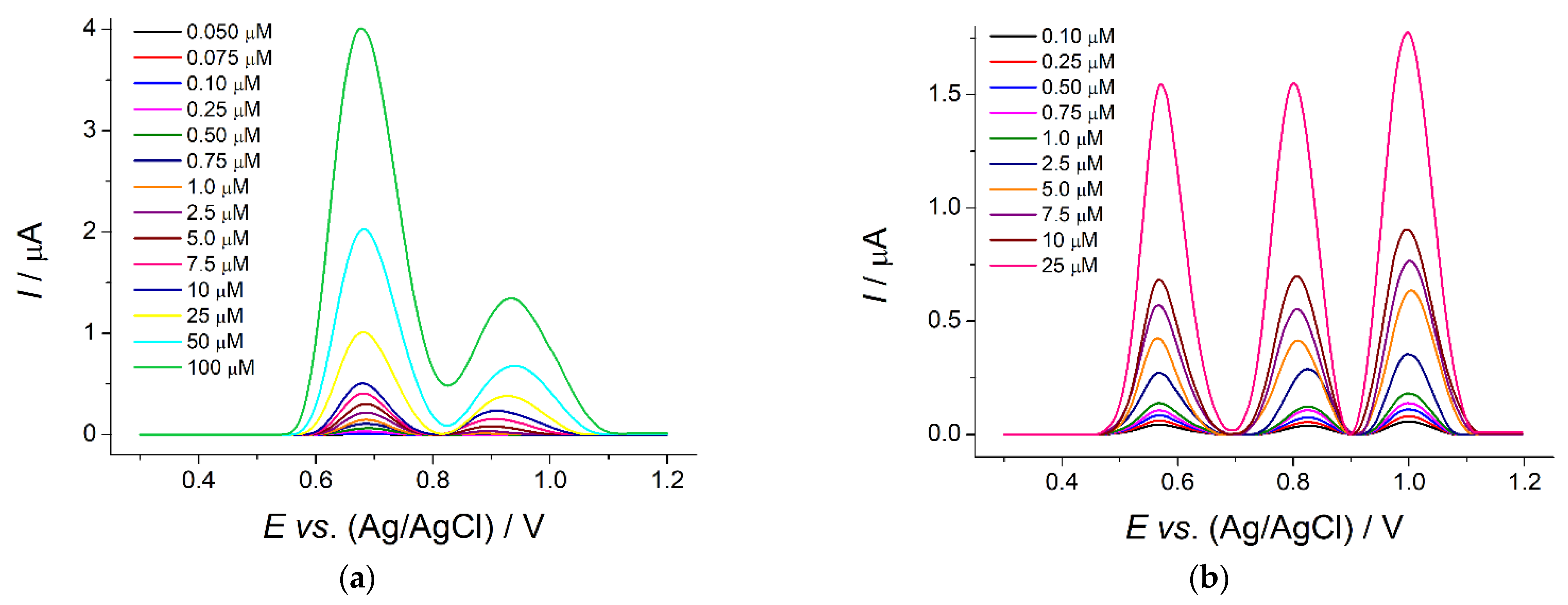
The electrodes created were used for analytical purposes in the differential pulse mode. The best responses of flavanones were observed in phosphate buffer of pH 6.5 for naringin at the poly(ellagic acid)/MWCNTs/GCE and of pH 5.0 for hesperidin and naringin at the polyaluminon/f-SWNTs/GCE. There were well-pronounced oxidation peaks on the voltammograms (
Figure 4).
The oxidation currents of flavanones were linearly dependent on their concentration. The analytical characteristics obtained (
Table 3) were significantly improved or comparable with those reported with other modified electrodes. Simultaneous determination of hesperidin and naringin was performed for the first time.
The electrodes developed were characterized by high accuracy of flavanone determination (recovery of 99.3–100.3%), as shown with the model systems. The relative standard deviation of 0.55–3.1% confirmed the absence of random errors of determination, as well as the high reproducibility of the analytical signal of flavanones, since the surface of the electrodes was renewed before each measurement.
The interference study showed excellent selectivity of polymer-based electrodes towards flavanones. Typical interferences (1000-fold excesses of K+, Mg2+, Ca2+, NO3−, Cl−, and SO42−, and 100-fold excesses of glucose, rhamnose, sucrose, and ascorbic acid) did not show interference effects. Structurally related natural phenolics were the major potential interferences and were widely distributed in Citrus fruits. Poly(ellagic acid)/MWCNTs/GCE showed a selective response towards naringin in the presence of 10-fold excesses of phenolic acids (gallic, ferulic, caffeic, and chlorogenic acids) and hesperidin. In the case of polyaluminon/f-SWNTs/GCE, 10-fold excesses of gallic, caffeic, and chlorogenic acids, as well as tannin, 1.0 µM of ferulic, sinapic and p-coumaric acids, catechin, quercetin, and rutin did not interfere with hesperidin and naringin response. Sample dilution could be used for the masking of the interference signals, while the target flavanones’ response was still sufficient.
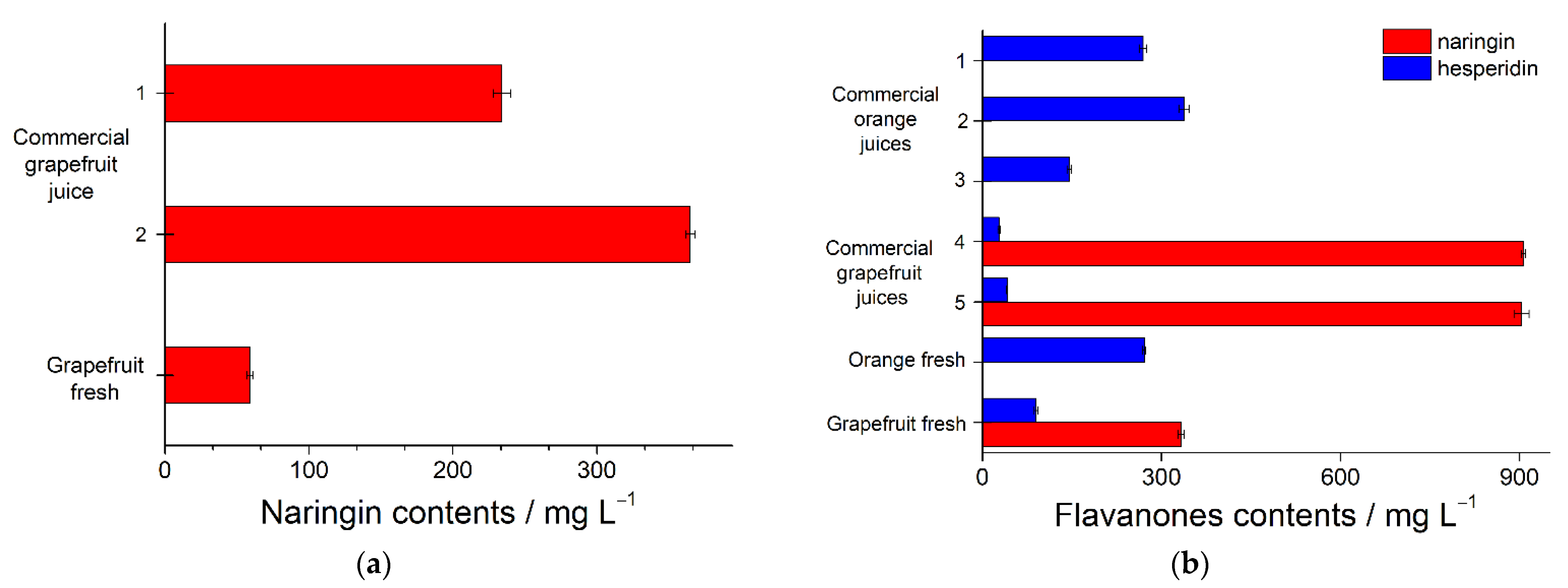
The electrodes developed were successfully applied to
Citrus (fresh and commercial) juice analysis. The standard addition method was used for the confirmation of the signal-forming compounds. The absence of matrix effects in the determination of flavanones was confirmed by recoveries of 98–101%. Grapefruit and orange juice analysis results are presented in
Figure 5. The data obtained agreed well with the results of the independent methods (
F-test confirms similar accuracy of the methods).
4. Conclusions
Novel modified electrodes based on electropolymerized ellagic acid and aluminon are highly sensitive and selective to flavanones, allowing their direct quantification. The simplicity of electrode fabrication, reliability, and cost-efficiency are important advantages of the electrodes developed. Real samples analysis data confirm the applicability of the electrodes in routine practice as an alternative to chromatographic methods.
Author Contributions
Conceptualization, G.Z.; methodology, G.Z.; validation, G.Z. and E.Y.; investigation, E.Y. and A.Z.; writing—original draft preparation, G.Z.; writing—review and editing, G.Z.; visualization, G.Z., E.Y. and A.Z.; supervision, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank Aleksei Rogov (Laboratory of Scanning Electron Microscopy, Interdisciplinary Center for Analytical Microscopy, Kazan Federal University) for the scanning electron microscopy measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ziyatdinova, G.; Guss, E.; Yakupova, E. Electrochemical sensors based on the electropolymerized natural phenolic antioxidants and their analytical application. Sensors 2021, 21, 8385. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.K.; Zhupanova, A.S.; Budnikov, H.C. Electrochemical sensors for the simultaneous detection of phenolic antioxidants. J. Anal. Chem. 2022, 77, 155–172. [Google Scholar] [CrossRef]
- da Silva, L.V.; de Almeida, A.K.A.; Xavier, J.A.; Lopes, C.B.; dos Santos Silva, F.A.; Lima, P.R.; dos Santos, N.D.; Kubota, L.T.; Goulart, M.O.F. Phenol based redox mediators in electroanalysis. J. Electroanal. Chem. 2018, 827, 230–252. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.-M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Budnikov, H. Analytical capabilities of coulometric sensor systems in the antioxidants analysis. Chemosensors 2021, 9, 91. [Google Scholar] [CrossRef]
- Khan, M.K.; Zill-E-Huma; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Budnikov, H. Natural phenolic antioxidants in bioanalytical chemistry: State of the art and prospects of development. Russ. Chem. Rev. 2015, 84, 194–224. [Google Scholar] [CrossRef]
- Sims, M.J.; Li, Q.; Kachoosangi, R.T.; Wildgoose, G.G.; Compton, R.G. Using multiwalled carbon nanotube modified electrodes for the adsorptive striping voltammetric determination of hesperidin. Electrochim. Acta 2009, 54, 5030–5034. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Yakupova, E.; Ziganshina, E.; Budnikov, H. First order derivative voltammetry on the in situ surfactant modified electrode for naringin quantification. Electroanalysis 2019, 31, 2130–2137. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Wang, H.; Lu, W.; Guo, M. Highly sensitive detection of hesperidin using AuNPs/rGO modified glassy carbon electrode. Analyst 2018, 143, 297–303. [Google Scholar] [CrossRef]
- Beluomini, M.A.; Stradiotto, N.R.; Zanoni, M.V.B. Simultaneous detection of hesperidin and narirutin in residual water using nanoporous platinum electrosynthesized by alloying-dealloying mechanism. J. Electroanal. Chem. 2022, 904, 115866. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Yakupova, E.; Davletshin, R. Voltammetric determination of hesperidin on the electrode modified with SnO2 nanoparticles and surfactants. Electroanalysis 2021, 33, 2417–2427. [Google Scholar] [CrossRef]
- Sun, D.; Wang, F.; Wu, K.; Chen, J.; Zhou, Y. Electrochemical determination of hesperidin using mesoporous SiO2 modified electrode. Microchim. Acta 2009, 167, 35–39. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mishra, P.; Senapati, M.; Sahu, P.P. A novel electrochemical device for naringin quantification and removal from bitter variety of citrus fruits. J. Food Eng. 2021, 306, 110637. [Google Scholar] [CrossRef]
- Gupta, A.K.; Das, S.; Sahu, P.P.; Mishra, P. Design and development of IDE sensor for naringin quantification in pomelo juice: An indicator of citrus maturity. Food Chem. 2022, 377, 131947. [Google Scholar] [CrossRef]
- Tığ, G.A.; Bolat, E.Ö.; Zeybek, B.; Pekyardımcı, Ş. Hesperidin-dsDNA interaction based on electrochemically reduced graphene oxide and poly-(2,6-pyridinedicarboxylic acid) modified glassy carbon electrode. Hacet. J. Biol. Chem. 2016, 44, 487–497. [Google Scholar]
- Ensafi, A.A.; Karbalaei, S.; Heydari-Bafrooei, E.; Rezaei, B. Biosensing of naringin in marketed fruits and juices based on its interaction with DNA. J. Iran Chem. Soc. 2016, 13, 19–27. [Google Scholar] [CrossRef]
- Ma, X.-L.; Chen, R.-Y.; Zheng, X.; Chen, X.; Chen, Z. Preparation and application of naringin sensor based on molecularly imprinting technique. Chin. J. Anal. Chem. 2010, 38, 100–104. [Google Scholar] [CrossRef]
- Sun, B.; Hou, X.; Li, D.; Gou, Y.; Hu, F.; Li, W.; Shi, X. Electrochemical sensing and high selective detection of hesperidin with molecularly imprinted polymer based on ultrafine activated carbon. J. Electrochem. Soc. 2019, 166, B1644–B1652. [Google Scholar] [CrossRef]
- da Silva, L.V.; Lopes, C.B.; da Silva, W.C.; de Paiva, Y.G.; dos Santos Silva, F.A.; Lima, P.R.; Kubota, L.T.; Goulart, M.O.F. Electropolymerization of ferulic acid on multi-walled carbon nanotubes modified glassy carbon electrode as a versatile platform for NADH, dopamine and epinephrine separate detection. Microchem. J. 2017, 133, 460–467. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kozlova, E.; Morozova, E.; Budnikov, H. Chronocoulometric method for the evaluation of antioxidant capacity of medicinal plant tinctures. Anal. Methods 2018, 10, 4995–5003. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).