Application of Ferrocene in the Treatment of Winery Wastewater in a Heterogeneous Photo-Fenton Process †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Winery Wastewater Sampling
2.2. Analytical Determinations
2.3. Heterogeneous Photo-Fenton Experimental Set-Up
2.4. Statistical Analysis
3. Results
3.1. Characterization of Ferrocene
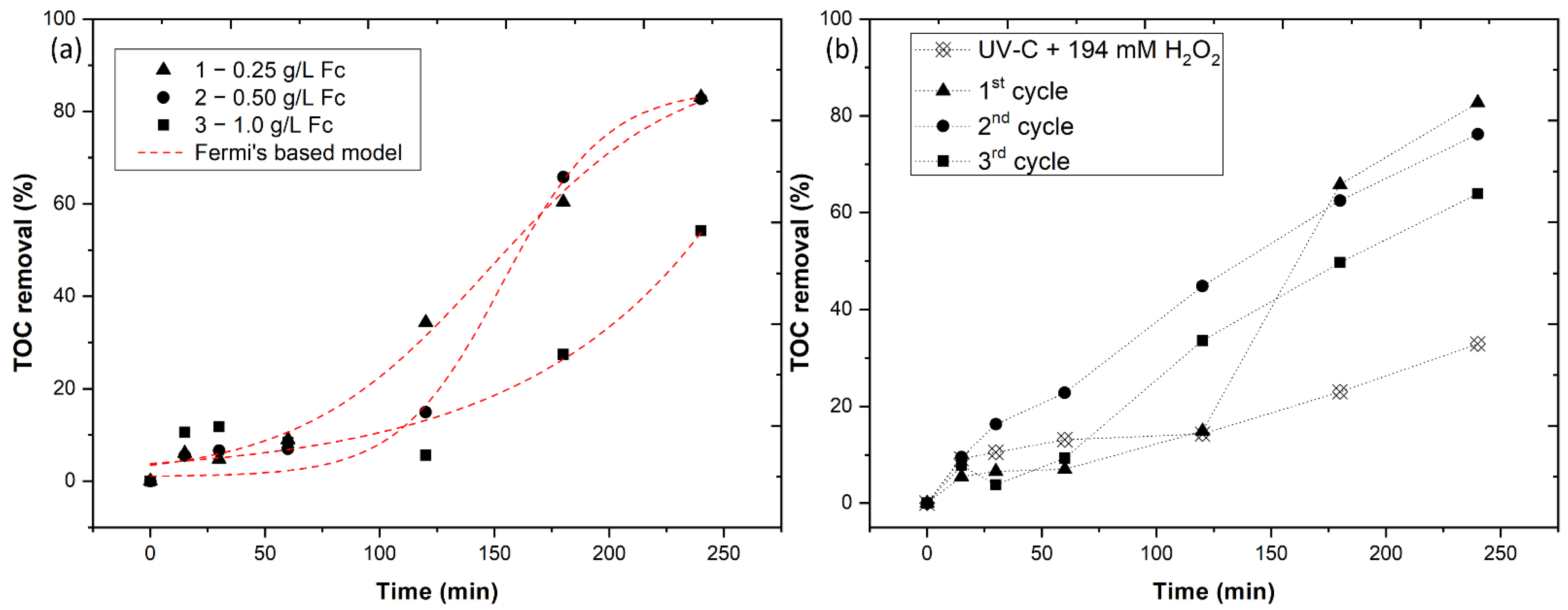
3.2. Heterogeneous Photo-Fenton Optimization
3.3. Kinetic Analysis
4. Conclusions
- (1)
- Ferrocene can be used as a source of iron in heterogeneous catalysis in winery wastewater treatment;
- (2)
- Under the optimal conditions, the heterogeneous photo-Fenton achieves an 82.7% TOC removal;
- (3)
- Fermi’s kinetic model shows the best operational condition at = 4.77 × 10−2 min−1;
- (4)
- Ferrocene can be reused for three consecutive cycles with a TOC removal of 82.7, 76.2 and 63.9% for the first, second and third cycles, respectively.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ioannou, L.A.; Puma, G.L.; Fatta-Kassinos, D. Treatment of Winery Wastewater by Physicochemical, Biological and Advanced Processes: A Review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Petruccioli, M.; Duarte, J.C.; Federici, F. High-Rate Aerobic Treatment of Winery Wastewater Using Bioreactors with Free and Immobilized Activated Sludge. J. Biosci. Bioeng. 2000, 90, 381–386. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Guimarães, V.; Lucas, M.S.; Peres, J.A. Treatment of Winery Wastewater with a Combination of Adsorption and Thermocatalytic Processes. Processes 2022, 10, 75. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Matos, C.C.; Lucas, M.S.; Peres, J.A. Combination of Coagulation–Flocculation–Decantation and Ozonation Processes for Winery Wastewater Treatment. Int. J. Environ. Res. Public Health 2021, 18, 8882. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.S.; Mouta, M.; Pirra, A.; Peres, J.A. Winery Wastewater Treatment by a Combined Process: Long Term Aerated Storage and Fenton’s Reagent. Water Sci. Technol. 2009, 60, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, V.; Teixeira, A.R.; Lucas, M.S.; Silva, A.M.T.; Peres, J.A. Pillared Interlayered Natural Clays as Heterogeneous Photocatalysts for H2O2-Assisted Treatment of a Winery Wastewater. Sep. Purif. Technol. 2019, 228, 115768. [Google Scholar] [CrossRef]
- Da̧browski, M.; Misterkiewicz, B.; Sporzyński, A. Solubilities of Substituted Ferrocenes in Organic Solvents. J. Chem. Eng. Data 2001, 46, 1627–1631. [Google Scholar] [CrossRef]
- Nie, Y.; Hu, C.; Qu, J.; Hu, X. Efficient Photodegradation of Acid Red B by Immobilized Ferrocene in the Presence of UVA and H2O2. J. Hazard. Mater. 2008, 154, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Amor, C.; Fernandes, J.R.; Lucas, M.S.; Peres, J.A. Hydroxyl and Sulfate Radical Advanced Oxidation Processes: Application to an Agro-Industrial Wastewater. Environ. Technol. Innov. 2020, 21, 101183. [Google Scholar] [CrossRef]
- De Souza, A.C.; Pires, A.T.N.; Soldi, V. Thermal Stability of Ferrocene Derivatives and Ferrocene-Containing Polyamides. J. Therm. Anal. Calorim. 2002, 70, 405–414. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Zhao, X.; Zhai, Q.; Yin, D.; Sun, Y. The Marriage of Ferrocene and Silicotungstate: An Ingenious Heterogeneous Fenton-like Synergistic Photocatalyst. Appl. Catal. B Environ. 2016, 193, 47–57. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Cun, J.; Ning, P. Degradation of Methylene Blue Using a Heterogeneous Fenton Process Catalyzed by Ferrocene. Desalin. Water Treat. 2013, 28, 5821–5830. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Amor, C.; Silva, T.; Dionysiou, D.D.; Li, G.; Lucas, M.S.; Peres, J.A. Treatment of Winery Wastewater by Sulphate Radicals: HSO5-/Transition Metal/UV-A LEDs. Chem. Eng. J. 2017, 310, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Rache, M.L.; García, A.R.; Zea, H.R.; Silva, A.M.T.; Madeira, L.M.; Ramírez, J.H. Azo-Dye Orange II Degradation by the Heterogeneous Fenton-like Process Using a Zeolite Y-Fe Catalyst—Kinetics with a Model Based on the Fermi’s Equation. Appl. Catal. B Environ. 2014, 146, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Jorge, N.; Teixeira, A.R.; Lucas, M.S.; Peres, J.A. Combination of Adsorption in Natural Clays and Photo-Catalytic Processes for Winery Wastewater Treatment. In Advances in Geoethics and Groundwater Management: Theory and Practice for a Sustainable Development; Abrunhosa, M., Chambel, A., Peppoloni, S., Chaminé, H.I., Eds.; Springer, Cham: Switzerland, 2021; pp. 291–294. ISBN 978-3-030-59320-9. [Google Scholar]
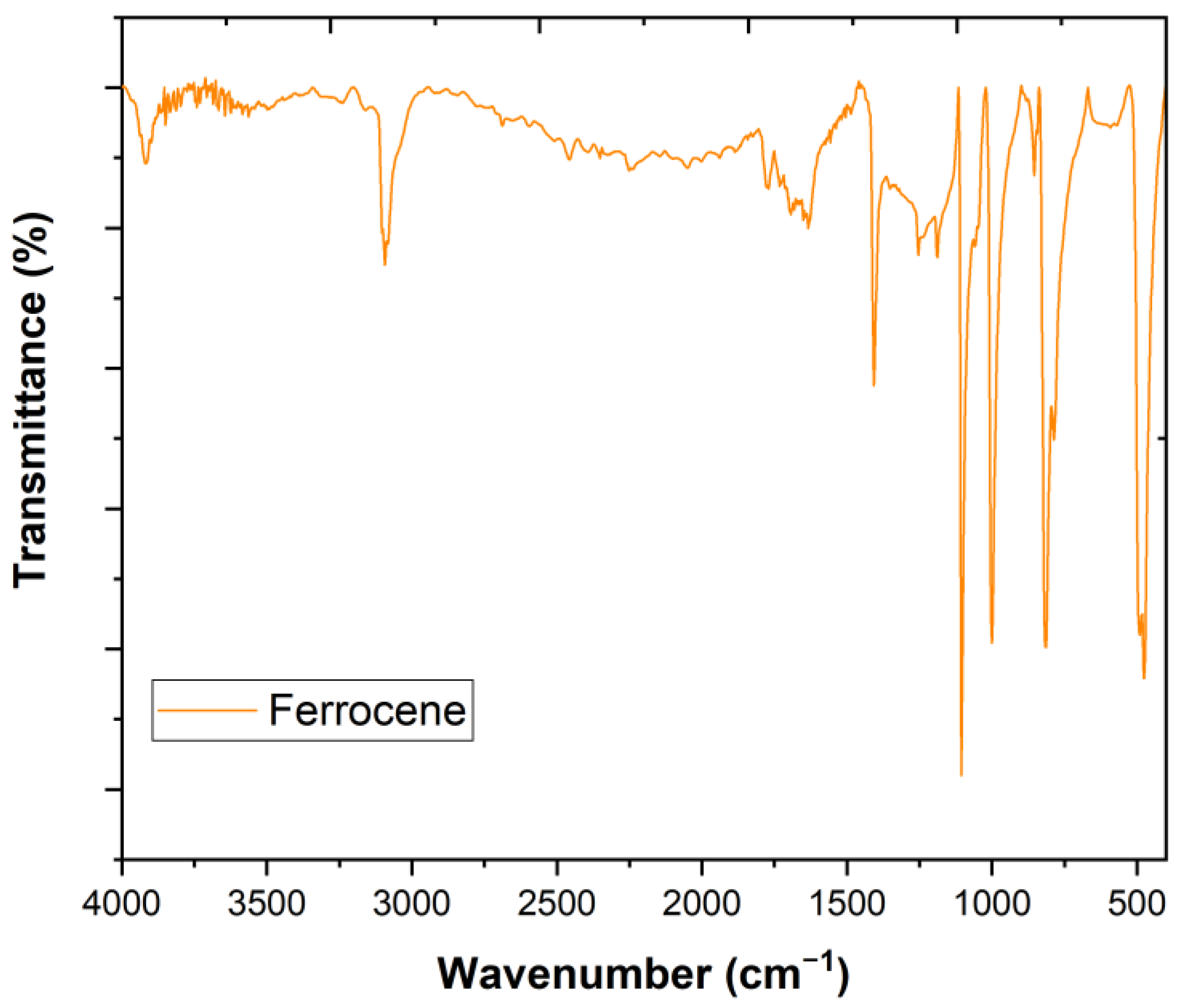
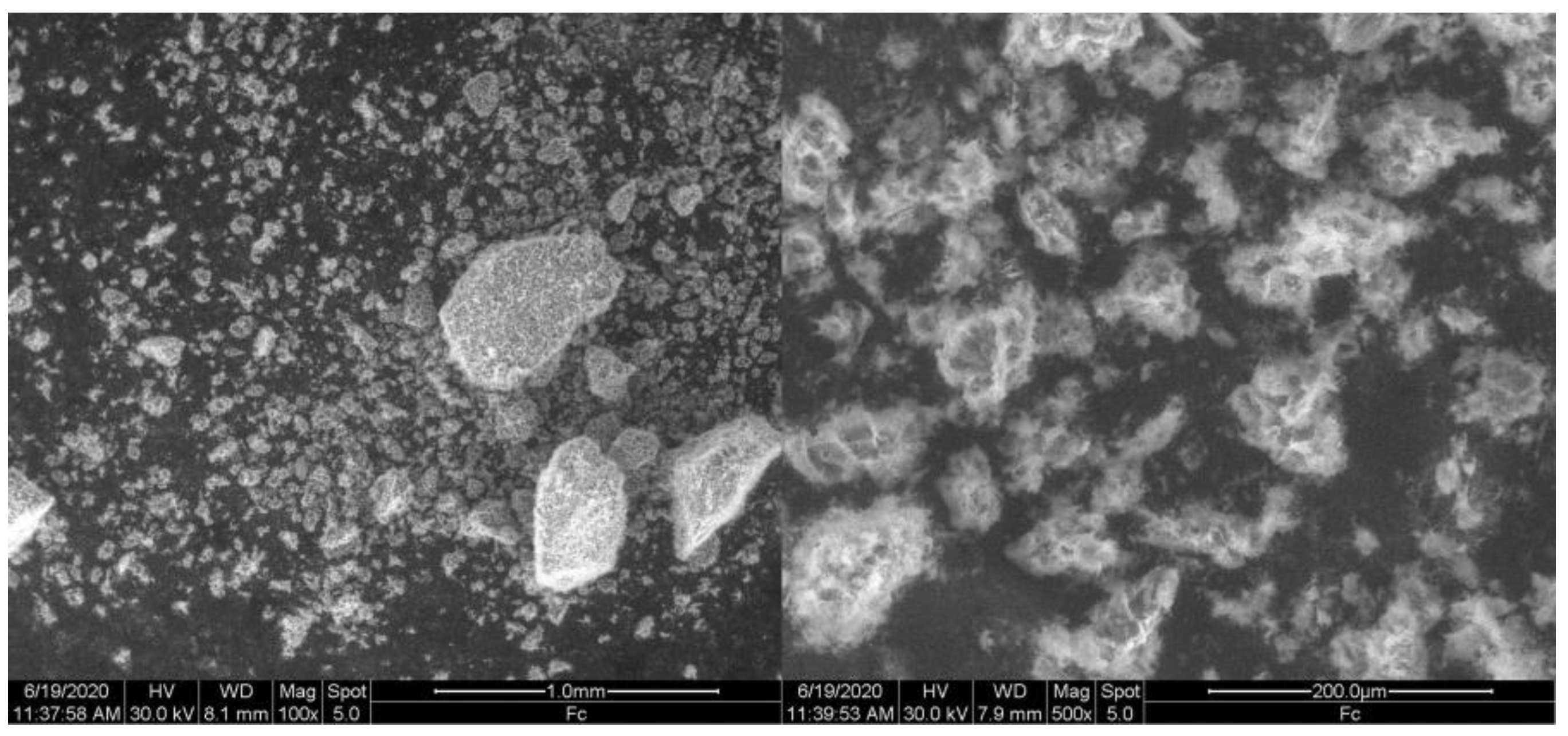
| Parameters | Winery Wastewater | Portuguese Decree Law No. 236/98 |
|---|---|---|
| pH | 4.0 | 6.0–9.0 |
| Conductivity (μS/cm) | 62.5 | |
| Turbidity (NTU) | 296 | |
| Total suspended solids (mg/L) | 750 | 60 |
| Chemical Oxygen Demand—COD (mg O2/L) | 2145 | 150 |
| Biochemical Oxygen Demand—BOD5 (mg O2/L) | 550 | 40 |
| Total Organic Carbon (mg C/L) | 400 | |
| Total polyphenols (mg gallic acid/L) | 22.6 | 0.5 |
| Ferrous iron (mg Fe/L) | 0.05 | 2.0 |
| Biodegradability—BOD5/COD | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorge, N.; Teixeira, A.R.; Marchão, L.; Tavares, P.B.; Lucas, M.S.; Peres, J.A. Application of Ferrocene in the Treatment of Winery Wastewater in a Heterogeneous Photo-Fenton Process. Mater. Proc. 2022, 9, 12. https://doi.org/10.3390/materproc2022009012
Jorge N, Teixeira AR, Marchão L, Tavares PB, Lucas MS, Peres JA. Application of Ferrocene in the Treatment of Winery Wastewater in a Heterogeneous Photo-Fenton Process. Materials Proceedings. 2022; 9(1):12. https://doi.org/10.3390/materproc2022009012
Chicago/Turabian StyleJorge, Nuno, Ana R. Teixeira, Leonilde Marchão, Pedro B. Tavares, Marco S. Lucas, and José A. Peres. 2022. "Application of Ferrocene in the Treatment of Winery Wastewater in a Heterogeneous Photo-Fenton Process" Materials Proceedings 9, no. 1: 12. https://doi.org/10.3390/materproc2022009012
APA StyleJorge, N., Teixeira, A. R., Marchão, L., Tavares, P. B., Lucas, M. S., & Peres, J. A. (2022). Application of Ferrocene in the Treatment of Winery Wastewater in a Heterogeneous Photo-Fenton Process. Materials Proceedings, 9(1), 12. https://doi.org/10.3390/materproc2022009012









