Abstract
Semi-synthetic hydrogels made of carboxymethyl hyaluronic acid (CMHA) and polyacrylic acid (PAA) were synthesized using electron beam irradiation. CMHA, with a degree of substitution of 0.87 and a molecular weight of 149 kDa, was mixed with linear PAA and slightly crosslinked PAA (Carbopol). The equal weight ratio of the CMHA-Carbopol blends (10% CMHA, 10% Carbopol) was successfully crosslinked, even at a low irradiation dose of 20 kGy, producing a hydrogel with 60% gel fraction and 430 g/g degrees of swelling. The gel properties of this formulation showed good stability when exposed in PBS (pH 7.4) at 37 °C. Furthermore, the FT-IR spectra of the 10% CMHA, 10% Carbopol blends showed an increase in peak intensity at 1405 cm−1 due to the neutralization reaction between the COOH and COO- groups of PAA and CMHA polymers. The interaction effects between the concentration of CMHA and PAA and varying irradiation doses in the gel properties in CMHA-PAA hydrogels will be explored in a future study. Radiation crosslinking of biocompatible CMHA to other synthetic polymers, such as PAA, provides a cleaner method of producing biomaterials with tunable properties that are ideal for pharmaceutical, medical, and cosmetic applications.
1. Introduction
Semi-synthetic hydrogels are widely used biomaterials, which have been extensively studied for use in pharmaceutical, medical, and cosmetic applications. The benefits of mixing synthetic and natural polymers allow semi-synthetic hydrogels to be tailored for specific applications []. Rosiak et al. (1999) reported that one of the most efficient ways of producing semi-synthetic gels for biomedical applications is through radiation crosslinking []. Previously, we successfully produced radiation-crosslinked biocompatible hydrogels made from pure carboxymethyl hyaluronic acid (CMHA) [,]. Despite successful crosslinking of pure CMHA, it is worthwhile to explore semi-synthetic hydrogels using CMHA combined with other synthetic polymers.
Polyacrylic acid (PAA) and its derivatives belong to the most commonly used synthetic water-soluble polymers applied to the production of hydrogels. Because PAA hydrogels contain ionizable carboxylic moieties, their gel properties, such as the degree of swelling, are greatly affected by the degree of neutralization as well as the pH and the ionic strength of the dispersing medium [,]. As a biomaterial, it demonstrated good biocompatibility and bioadhesive properties [,]. However, using it alone has disadvantages when administered biomedically, and these issues are resolved through combination with other polymers.
In this study, CMHA was incorporated with linear and slightly crosslinked PAA to produce semi-synthetic hydrogels through the use of electron beam irradiation. The gel properties, such as the gel fraction and the degree of swelling, were evaluated. The FT-IR spectra and thermal properties, as well as the in-vitro biodegradability of the CMHA-PAA hydrogels, were evaluated.
2. Materials and Methods
2.1. Materials
Cosmetic-grade and 1800 kDa HA (Stanford Chemicals Company, Lake Forest, CA, USA) sodium hydroxide (99%, RCI Labscan, Bangkok, Thailand), chloroacetic acid (99.5%, LOBA Chemie, Mumbai, India), and isopropyl alcohol (ACS reagent, J.T. Baker, Radnor, PA, USA) were all used as received for the carboxymethylation process. Analytical-grade sodium chloride (AR, Univar, Downers Grove, IL, USA) was used to prepare the mobile phase for the GPC analysis. Linear PAA and cosmetic-grade Carbopol-940 were obtained from Merck Sigma-Aldrich and Alysons’ Chemical Enterprises, Inc. (Quezon City, Philippines), respectively. As with the in-vitro biodegradation experiment, a Phosphate Buffer Saline (PBS) tablet (Merck Sigma-Aldrich) was used.
2.2. Carboxymethylation of HA
Carboxymethyl hyaluronic acid was prepared using the previously reported protocol with a minor modification []. HA (20 g) was mixed with 50 mL of 45% w/v NaOH to form a paste and then mixed for 10 min. Isopropanol (2.0 L) was added and stirred for 1 h. About 60 g of solid chloroacetic acid was added and continuously stirred for 2.5 h. CMHA was collected by filtering the mixture through a non-woven fabric and then washed 3 times with isopropyl alcohol before being dissolved in 1.0 L deionized water. The CMHA solution was acidified until pH 7 with 12 N HCl and dialyzed using MWCO 12–14 kDa tubing for 3 days with frequent water changes. The solution was lyophilized, and the purified CMHA solid was stored at 4 °C for analysis and the preparation of hydrogels.
2.3. Determination of Molecular Weight and the Degree of Substitution
Potentiometric back titration was used to determine the degree of substitution (DS) of purified CMHA []. Briefly, cationic exchange resin (Amberlite H-form) was mixed in 5 mg/mL HA and 10 mg/mL CMHA aqueous solutions. The mixtures were swirled in a water bath for 2 h at 37 °C and then filtered through a stainless steel 200-mesh wire screen. The filtrates were passed through 0.45 μm and 0.22 μm syringe filters and then freeze-dried. About 0.1 g of dry acidified HA or CMHA was dissolved in 10 mL of standardized 0.1 M NaOH and diluted with 15 mL deionized water. This solution was then titrated with standardized 0.05 N HCl using phenolphthalein as an indicator. Blank titrations contained no sample. The DS was calculated based on the following equations:
where nCOOH = the mole of the carboxyl groups, Vb = the volume of HCl used to titrate the blanks, V = the volume of HCl used to titrate the sample, CHCl = the concentration of HCl, MWDSU = the molecular weight of the unsubstituted disaccharide unit, m = the mass of dry CMHA or HA, and MW1 = the molecular weight of the carboxymethyl group.
Gel permeation chromatography (Shimadzu Prominence), equipped with a refractive index detector, was used to determine the average molecular weight (MW) of the purified CMHA. The chromatography columns used were the Tosoh TSK gel guard column PWXL and four TSK gel, serially connected, analytical columns (G6000 PWXL, G4000 PWXL, G3000 PWXL, and G2500 PWXL). Elution was performed using 0.2 M NaCl at a flow rate of 0.5 mL/min while the temperature of both the detector and columns was set to 40 °C. A calibration curve was constructed using polyethylene oxide (PEO) and polyethylene glycol (PEG) as standards. Molecular weights reported in this study are based on standards and are not absolute.
2.4. Preparation and Irradiation of Polymer Blends
Polymer blends of CMHA and PAA were prepared by combining the freeze-dried CMHA with linear PAA (CMHA-PAA) or with slightly crosslinked PAA (CMHA-Carbopol). The CMHA-PAA and CMHA-Carbopol blends were thoroughly mixed with water before being kneaded in plastic PE sheets and were allowed to stand overnight to allow for complete dissolution. Afterward, the samples were kneaded again to ensure the homogenization of the polymer blends prior to vacuum-sealing. Electron beam irradiation was carried out using a 2.5 MeV accelerator with a 12 mA current and a conveyor speed of 4.3–8.5 m/min. The resulting radiation-crosslinked hydrogels were then freeze-dried. The formulation of the samples is listed in Table 1.

Table 1.
Formulation and irradiation doses of CMHA-PAA and CMHA-Carbopol blends.
2.5. Determination of Gel Properties
The gel fraction and the degree of swelling of the hydrogel samples were determined based on our previous study []. Approximately 0.2 g of the freeze-dried samples was immersed in 1.0 L of deionized water at room temperature for 72 h. Every 24 h, swollen samples were weighed and re-immersed in freshly changed deionized water. Swollen samples were then dried at 50 °C to a constant weight to obtain the gel fraction. The gel fraction and the degree of swelling were calculated as follows:
where Wd = the weight of the dried insoluble part after immersion for 3 days, Wi = the initial weight of the dried sample after irradiation, and Ws = the weight of the swollen gel. All measurements were completed in triplicates.
2.6. FT-IR and Thermogravimetric Analysis
The FT-IR spectra (600–4000 cm−1) of freeze-dried hydrogel samples without sol fraction were obtained using a PerkinElmer Spectrum One FTIR spectrophotometer in attenuated total reflectance (ATR) mode. TGA thermograms were collected using a Netzsch STA449 F3 Jupiter at temperatures ranging from 25 to 1000 °C and at a heating rate of 10 °C/min in a nitrogen atmosphere.
2.7. In-Vitro Biodegradability
About 20.0 mg of freeze-dried hydrogels were placed in 20-mL vials and submerged in 4.0 mL of PBS (pH 7.4) or a 1:200 ratio of dried hydrogel to PBS. Samples were allowed to swell, were incubated at 37 °C, and were weighed periodically (at 1, 3, and 5 days). The pH of the buffer was adjusted to 7.4 (1.0 mM HCl and NaOH solution) and replenished as needed. The degree of swelling in the PBS solution was calculated from the weight of the swollen gel and the initial weight of the freeze-dried sample, as shown in the equation:
where Ws,PBS = the weight of the swollen hydrogel in the PBS solution, while Wi = the weight of the freeze-dried hydrogel with insoluble fractions.
The in-vitro degradation of the hydrogel blends was evaluated by determining the weight loss of the freeze-dried hydrogel exposed in the PBS solution (pH 7.4) at 37 °C. Submerged hydrogels, in a 1:200 ratio of dried hydrogel to PBS, were collected periodically at intervals of 1, 3, and 5 days. Swollen samples were washed with PBS and then freeze-dried. The degradation was calculated as follows:
where Wd,PBS = the weight of the freeze-dried CMHA submerged in PBS at day 1, 3, or 5.
3. Results and Discussion
3.1. Degree of Substitution and Molecular Weight of Carboxymethyl Hyaluronic Acid
The carboxymethylation of hyaluronic acid introduces a new functional group, which offers many possible prospects for the synthesis of new products from this simple polysaccharide with interesting chemical and biological properties. In radiation processing, the chemistry of carboxymethyl groups leads to the formation of radical intermediates, which are helpful in forming chemical bonds with other polymer chains, resulting in high-purity hydrogels. We previously attempted to make pure carboxymethyl hyaluronic hydrogels using high-energy radiation and discovered that higher DS, MW, concentration, and irradiation doses are required to make biocompatible CMHA hydrogels [,]. In this particular study, the DS and MW of the CMHA produced were 0.87 and 149 kDa, respectively. This will be used to investigate milder irradiation conditions for the formation of hydrogels by combining CMHA with linear and slightly crosslinked PAA. The sections that follow discuss the characterization of the polymer blends, such as their gel properties, molecular structure, thermal stability, and in-vitro degradation using PBS (pH 7.4).
3.2. Gel Properties of CMHA-PAA and CMHA-Carbopol Blends
Hydrogels for biomedical uses undergo initial characterization through the determination of their gel content and swelling properties. These two properties can be used as a means to assess the stability of hydrogels in aqueous solutions []. The gel properties of CMHA-PAA are shown in Figure 1. The gel fractions substantially increased as the absorbed dose became higher, from 20 to 40 kGy (Figure 1a). Hydrogel blends of 35% CMHA, 5% PAA had higher gel fractions compared to the 37.5% CMHA, 2.5% PAA blend for both doses. These values show that higher irradiation doses and higher concentrations of PAA result in more crosslinking, hence the higher gel fractions.
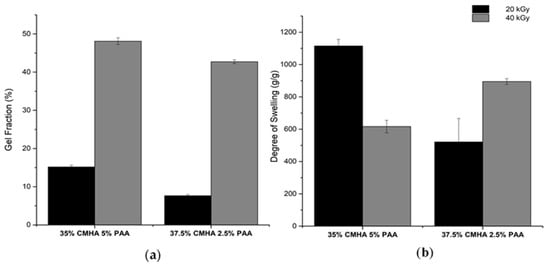
Figure 1.
Gel fraction (a) and degree of swelling (b) of CMHA-PAA hydrogels.
Different trends for the degree of swelling were observed as the dose increased (Figure 1b). The degree of swelling of the 35% CMHA, 5% PAA blend decreased from 1115 to 616 g/g, while the formulation with 37.5% CMHA, 2.5% PAA increased from 522 to 895 g/g. From the results of the gel fraction experiment, higher degree of swelling are expected for the hydrogels with more PAA and those that are irradiated at higher doses, due to more crosslinking. However, the swelling ratio obtained for the 35% CMHA, 5% PAA did not follow the same trend at 40 kGy, possibly because the higher degree of crosslinks caused the hydrogel to be more rigid, hence restricting its ability to swell and hold water in its network.
For the CMHA-Carbopol hydrogel blends, it was also observed that gel fractions increased proportionally with the dose for all samples (Figure 2a). However, the gel fractions decreased when CMHA was mixed with Carbopol, with the 10% pure Carbopol samples obtaining gel fractions in the range of 95–99%, while the 10% Carbopol, 10% CMHA obtained 60–81%. The soluble fraction from the 10% Carbopol, 10% CMHA hydrogel may have predominantly originated from the degraded CMHA chains. This could be due to the fact that some of the CMHA polymeric chainswere degraded since polysaccharides, including hyaluronic acid and their derivatives, are susceptible to chain scission when exposed to high-energy radiation. This radiation-induced chain scission is more apparent at low CMHA concentrations (i.e., 10% CMHA).

Figure 2.
Gel fraction (a) and degree of swelling (b) of CMHA: Carbopol blends.
As previously discussed, the degree of swelling decreases for samples with a high PAA concentration at higher doses due to the formation of more crosslinks within the hydrogel (Figure 2b). Higher degrees of swelling of 10% Carbopol, 10% CMHA hydrogel blends were observed compared to the pure 10% Carbopol samples, which may have been caused by the presence of more flexible polymer chains having a network of lesser crosslinks.
The hydrogel blends based on CMHA and polyacrylic acid polymers produce interesting gel properties that can be controlled by modifying either the polymer concentrations, the irradiation dose, or both. It is also possible to produce gels using milder irradiation conditions by considering concentrations with an equal or lower weight ratio of CMHA to PAA polymers (1:1 or lower than 7:1).
3.3. FT-IR and TGA Analysis
One way to determine the successful crosslinking or blending of polymer chains within the hydrogel is by analyzing their FT-IR spectra and comparing them from their pristine raw materials []. The FT-IR characteristic peaks of the raw materials HA, CMHA, PAA, and Carbopol, as well as the CMHA-PAA hydrogel blends, are shown in Figure 3. The 35% CMHA, 5% PAA hydrogel irradiated at 40 kGy has shown more resemblance to the spectrum of its dominant polymer CMHA, but with a new peak around 1705 cm−1 due to the C=O carboxylic group of PAA. The FT-IR spectra of 10% CMHA, 10% Carbopol irradiated at 20 kGy showed a significant deviation from its pristine material. Specifically, the C=O carboxylate group from PAA showed a strong peak at 1410 cm−1, obscuring the C=O carboxylic groups of hyaluronic acid as well as the decrease in peak intensity at 1040 cm−1 corresponding to the C-OH alcohol moiety from CMHA. It was also observed that the change in the shape of the characteristic bands of -OH at 3000–2850 cm−1 changes depending on the weight ratio of the polymers.

Figure 3.
FT-IR spectra HA, CMHA, crosslinked CMHA, crosslinked CMHA-PAA, and crosslinked CMHA-Carbopol hydrogels.
The thermograms of the CMHA-PAA and CMHA-Carbopol hydrogels without soluble fractions are shown in Figure 4. The TG and DTG profiles depicted the multistep decomposition of the samples. The possible dehydration of 35% CMHA, 5% PAA 40 kGy was 71.7 °C, which is generally lower than the 164.5 °C of 10% CMHA, 10% Carbopol 20 kGy hydrogels (Figure 4). Previous studies have also observed a great difference in the dehydration temperature between their PAA-based samples []. At 185–315 °C, the decarboxylation and depolymerization of both the PAA and CMHA main chains of the hydrogel blends occur [,]. The observed mass losses greater than 315 °C were potentially from crosslinked CMHA chains, CMHA-PAA chains, andPAA chains with different crosslink densities formed after irradiation [].

Figure 4.
TGA and DTG profiles of (a) 35% CMHA, 5% PAA irradiated at 40 kGy and (b) 10% CMHA, 10% Carbopol irradiated at 20 kGy hydrogels.
3.4. In-Vitro Biodegradation of Hydrogels in Phosphate Buffer Solution (PBS)
By using the simulated ionic and pH conditions of biological fluids, such as PBS (pH = 7.4), at 37 °C, the stability of the samples could be initially assessed []. Figure 5 shows that a formulation with the equal weight ratio of 10% CMHA, 10% Carbopol irradiated in 20 kGy has a stable crosslinking network exhibiting a stable swelling property without a significant increase in the degradation percentage of PBS. Biomedical hydrogels with stable gel properties in biological fluids can potentially be used for applications that need longer exposure when implanted in the body.
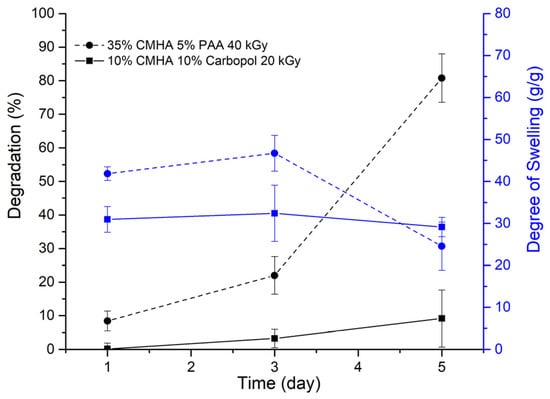
Figure 5.
In-vitro degradation and swelling properties of CMHA with poly(acrylic) acid hydrogels in PBS (pH 7.4) at 37 °C.
4. Conclusions
Semi-synthetic hydrogels based on CMHA with polyacrylic acid (CMHA-PAA) and CMHA-Carbopol) were produced using electron beam irradiation without the use of toxic initiators or crosslinkers. The gel properties of the produced gels can easily be modified by changing the polymer weight ratio and the absorbed radiation dose according to the results. Crosslinking between CMHA and PAA polymer chains was assessed by changes in the IR spectra and thermal stability of the samples. Moreover, the in-vitro biodegradability study showed the stability of the produced hydrogels. Since hyaluronic acid is ubiquitous to the human body, CMHA-based hydrogels have a strong potential for use in biomedical applications.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by A.K.R.G. and A.P.S. Study investigation and supervision were led by L.S.R. The first draft of the manuscript was written by A.K.R.G. and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Mignon, A.; De Belie, N.; Dubruel, P.; Van Vlierberghe, S. Superabsorbent polymers: A review on the characteristics and applications of synthetic, polysaccharide-based, semi-synthetic and ‘smart’ derivatives. Eur. Polym. J. 2019, 117, 165–178. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Yoshii, F. Hydrogels and their medical applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1999, 151, 56–64. [Google Scholar] [CrossRef]
- Relleve, L.S.; Gallardo, A.K.R.; Abad, L.V. Radiation crosslinking of carboxymethyl hyaluronic acid. Radiat. Phys. Chem. 2018, 151, 211–216. [Google Scholar] [CrossRef]
- Relleve, L.S.; Gallardo, A.K.R.; Tecson, M.G.; Luna, J.A.A. Biocompatible hydrogels of carboxymethyl hyaluronic acid prepared by radiation-induced crosslinking. Radiat. Phys. Chem. 2021, 179, 109194. [Google Scholar] [CrossRef]
- Gallardo, A.K.R.; Relleve, L.S.; Barba, B.J.D.; Cabalar, P.J.E.; Luna, J.A.A.; Tranquilan-Aranilla, C.; Madrid, J.F.; Abad, L.V. Application of factorial experimental design to optimize radiation-synthesized and biodegradable super water absorbent based on cassava starch and acrylic acid. J. Appl. Polym. Sci. 2021, 139, 51451. [Google Scholar] [CrossRef]
- Shin, M.S.; Kim, S.J.; Park, S.J.; Lee, Y.H.; Kim, S.I. Synthesis and characteristics of the interpenetrating polymer network hydrogel composed of chitosan and polyallylamine. J. Appl. Polym. Sci. 2002, 86, 498–503. [Google Scholar] [CrossRef]
- Nho, Y.C.; Park, J.S.; Lim, Y.M. Preparation of poly(acrylic acid) hydrogel by radiation crosslinking and its application for mucoadhesives. Polymers 2014, 6, 890–898. [Google Scholar] [CrossRef]
- Biswas, G.R.; Majee, S.B.; Roy, A. Combination of synthetic and natural polymers in hydrogel: An impact on drug permeation. J. Appl. Pharm. Sci. 2016, 6, 158–164. [Google Scholar] [CrossRef][Green Version]
- Edsman, K.; Nord, L.I.; Öhrlund, Å.; Lärkner, H.; Kenne, A.H. Gel properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2012, 38, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, B.; Kaczmarska, K.; Bobrowski, A.; Kurleto-Kozioł, Z.; Szymański, L. Crosslink the Novel Group of Polymeric Binders BioCo by the UV-radiation. Arch. Foundry Eng. 2016, 16, 85–88. [Google Scholar] [CrossRef]
- Dil, N.N.; Sadeghi, M. Free radical synthesis of nanosilver/gelatin-poly (acrylic acid) nanocomposite hydrogels employed for antibacterial activity and removal of Cu(II) metal ions. J. Hazard. Mater. 2018, 351, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.D.; Hsieh, Y.L.; Krochta, J.M.; Kurth, M.J. Study on molecular interaction behavior, and thermal and mechanical properties of polyacrylic acid and lactose blends. J. Appl. Polym. Sci. 2001, 82, 1921–1927. [Google Scholar] [CrossRef]
- Relleve, L.S.; Aranilla, C.T.; Barba, B.J.D.; Gallardo, A.K.R.; Cruz, V.R.C.; Ledesma, C.R.M.; Nagasawa, N.; Abad, L.V. Radiation-synthesized polysaccharides/polyacrylate super water absorbents and their biodegradabilities. Radiat. Phys. Chem. 2020, 170, 108618. [Google Scholar] [CrossRef]
- Deshmukh, M.; Singh, Y.; Gunaseelan, S.; Gao, D.; Stein, S.; Sinko, P.J. Biodegradable poly (ethylene glycol) hydrogels based on a self-elimination degradation mechanism. Biomaterials 2010, 31, 6675–6684. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).