Abstract
A novel roofing tile was developed containing various types of nanoparticles of titanium dioxide (TiO2). Experiments were conducted using three types of TiO2 nanoparticles with and without polyethylene glycol (PEG). All types of newly developed nanomaterials were characterized using X-ray diffractometry. Particle size distribution analysis was performed and specific surface area was determined using the Brunauer–Emmet–Teller method. SEM imaging was used for the morphological characterization of nanoparticles. Commercial ceramic roofing tiles underwent a dip-coating procedure to obtain the desired photocatalytic surface. The TiO2 anatase samples exhibited greater surface areas of nanoparticles, thus providing potentially the highest photocatalytic efficiency.
1. Introduction
The need for products that are non-hazardous to the environment and able to improve living conditions has led to the development of the so-called cool roof. This comprises a clay roofing tile with a coating of nanomaterials on its surface. Due to its well-known photo reflecting and high photocatalytic properties under ultraviolet radiation, titanium dioxide (TiO2) is usually chosen as a raw material for coating cooling roof tiles. TiO2 is known for its photocatalysis, its zero toxicity, and its ability to degrade organic pollutants [1]. It is also widely available, low cost and can be stimulated directly by ultraviolet radiation.
It has been proved that titanium dioxide can photocatalytically degrade and mineralize a large variety of environmental pollutants, including organic and inorganic materials, CO2, H2O, and harmless inorganic anions [2]. The oxidation of nitrogen oxides to nitrate anions occurs very slowly under normal atmospheric conditions because of the low concentrations of nitrogen oxides. Photocatalytic TiO2 nanoparticles can speed up the photochemical oxidation process in air. The hydroxyl radicals •OH, produced by photoreaction, are powerful oxidizing agents and can oxidize nitrogen dioxide to nitrate anions directly on TiO2 surfaces [3]:
NO2 + •OH → H++NO3−
The photo-produced superoxide anions •O2− can oxidize nitrogen monoxide to nitrate ions during TiO2 photocatalysis:
NO+ •O2−→ NO3−
The produced ions (NO3−) are either washed away by rain or soak into concrete to form stable compounds [2].
Titanium dioxide catalysts have been used for various building materials, such as paving stones or paintings. Beeldens et al. [4] reported that concrete blocks with titanium have been applied as self-cleaning materials for air purification, more precisely for the conversion of NOx to NO3-. A decrease in pollutants has been observed when more blocks are used. Pollutant concentrations drop approximately 40% after light radiation and after five hours of radiation, NOx emissions cease for 30 min. Airborne pollutants such as volatile organic compounds and carbon dioxide can also be reduced.
A cool roof is defined as a roof covering system of a building that is capable of reflecting as much sunlight as possible and absorbing as little heat as possible compared to standard designed roofing products. As a result, the roof remains cool and the amount of heat transferred to the building decreases, thus maintaining a stable and cool temperature inside. Until now, it has been proved that titanium dioxide is capable of reflecting (diffuse reflectance spectroscopy) light as mentioned in the research of Colombo et al. [5]. This property makes titanium dioxide an appropriate material for the scope of the present project.
This research is being conducted as part of the research project entitled “Green Tile development-KERAMI” in collaboration with “KEBE S.A.–Northern Greece Ceramics”, and aims to develop a new bioclimatic product which will have photoreflective and photocatalytic properties for use in cool roofs. The preliminary steps of the present project which are presented in this paper are based on the development of different types of nanoparticles. After that, characterization techniques were followed for a better understanding of the properties of nanomaterials. The last part of this work is the insertion of the nanoparticles onto the upper surface of ceramic tiles and the investigation of their morphology.
2. Materials and Methods
2.1. Materials
Three types of TiO2 (titanium (IV) oxide anatase nanopowder; titanium (IV) oxide mixture of rutile and anatase nanoparticles in colloidal dispersion and titanium (IV) oxide rutile nanopowder <100 nm) and PEG (MW: 600) were purchased from Sigma-Aldrich. Two types of clay roofing tiles with and without silicon were manufactured and provided by the “KEBE S.A–Northern Greece Ceramics” Company.
2.2. Preparation and Characterization of the TiO2 Nanoparticles
TiO2 aqueous suspensions were prepared by mixing deionized water with 2.5 mass % of the three types of TiO2 and 0.208 mass % of polyethylene glycol (PEG 600), under vigorous magnetic stirring. A similar procedure was followed for the development of TiO2 nanoparticles without PEG. Subsequently, two types of ceramic roofing tiles type B and type C; with and without a silicon layer, respectively) underwent a dip-coating procedure to obtain the desired photocatalytic surface. Finally, the tiles were dried in an industrial furnace at 100 °C and 290 °C.
For the characterization of the samples, TiO2 aqueous suspensions with and without PEG were dried in an industrial furnace at 100 °C and 290 °C. A total number of 12 samples was prepared. Characterization techniques were carried out and determination of sample phase compositions was examined by XRD analysis. XRD measurements of dried and thermally treated samples were performed using a Brucker D8 Advance diffractometer operating in the reflection mode with Cu Ka radiation in the 2θ interval of 10–80°. Diffraction patterns of the powders were compared with the reference in the ICDD database.
The specific surface area of the samples was measured following the Brunauer–Emmett–Teller theory by low-temperature nitrogen adsorption at −196 °C using an experimental conformation instrument. All the samples were degassed before isotherm measurements.
Particle size distribution of the suspensions (2.5% TiO2 water suspension and the newly formed photocatalytic active suspension) was measured by a laser diffraction technique using a Nano ZS (Malvern Instruments, zeta-nano series). Water was used to disperse both suspensions in the sample cell. Approximately 0.1 mL of these suspensions was dispersed in 30mL of the dispersant (water). The refractive index (for TiO2 n = 2.5) of each solvent was used as a preference index for statistical calculation using the particle sizing program Dispersion Technology Software (DTS). To examine the variation in particle size distribution due to ultrasonic action, prior to the measurements, the samples were exposed to ultrasonic water action for 10 min.
Samples of coated roofing tiles were used for scanning electron microscopy (SEM) imaging by which morphological characterization of the nanoparticles on the surfaces of the tiles was performed. X-ray mapping was also carried out using a JSM-IT500 microscope (JEOL InTouchScope series model) to determine elemental distribution on the tiles’ surfaces. A synopsis of the methodology is presented in Figure 1.
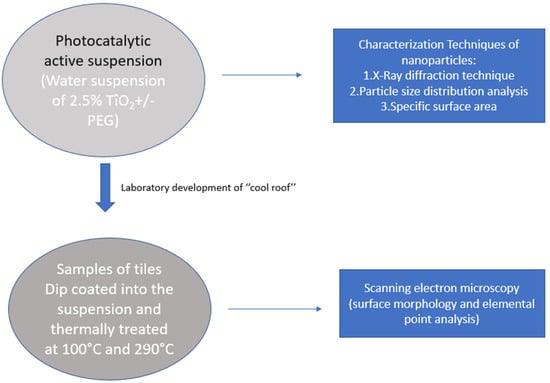
Figure 1.
Representation of the experimental methodology followed.
3. Results
Figure 2a,b present SEM images of the surface of the coated ceramic roof tiles. Two different types of furnaced tiles (type B and C) were tested in these experiments. A coating of silicon could be seen clearly on the type B tile, however the type C tile showed a better configuration of nanomaterials on its surface. SEM images of tiles of the latter type are shown in Figure 2a,b. In Figure 2b, different areas on the surface of samples, in which elemental analysis was conducted, are shown. Spectrum 1 and 2 are oxidized areas which emerged after the thermal degradation of polyethylene glycol. Spectrum 3 is a titanium dioxide nanoparticle and spectrum 4 represents the interface between a nanoparticle and a degraded molecule of PEG. Table 1 presents the results of the specific surface areas of all examined samples. As shown, the sample of TiO2 (anatase nanopowder) with polyethylene glycol, dried at 100 °C, had the highest specific surface area (91.5 m2/g) while the same type of sample dried at 290 °C exhibited a lower specific area (74 m2/g). This occurred due to the fact that PEG was thermally degraded at 290 °C. Additionally, samples with the same type of TiO2 but without the addition of PEG exhibited a lower specific area (77.1 and 77.7 m2/g). TiO2 samples consisting of a mixture of anatase and rutile, with and without the addition of PEG, also presented differentiations between their specific surface areas. The sample with PEG dried at 100 °C exhibited the highest specific surface area (58.9 m2/g) among the samples with this type of TiO2 while the ones developed without PEG exhibited the lowest (49.9 and 49 m2/g). At last, as can been seen in Table 1, the third type of TiO2 nanoparticles (rutile nanopowder) exhibited the lowest specific surface areas. In this type of samples, the addition of PEG increased the surface area from 31 m2/g to 36.3 m2/g.
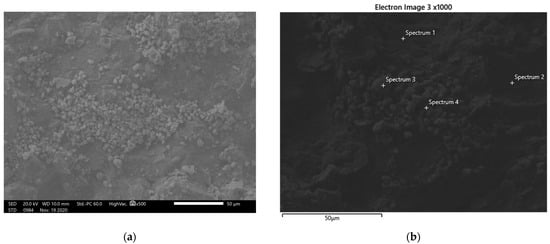
Figure 2.
Scanning electron microscopy (SEM) images showing: (a) nanoparticles of titanium dioxide on coated type C roof tiles; (b) different spectrum areas in which elemental analysis was made.

Table 1.
Samples and results of their specific surface area.
The contents of anatase and rutile phases in the powders were determined using X-ray diffraction and Figure 3 shows the results of the crystalline phase of sample numbers 1_100 °C, 1_290 °C, 2_100 °C and 2_290 °C. The XRD patterns for all powders exhibited strong diffraction peaks at 25.2°, 37.9°, 47.9° and 54.0°, corresponding to anatase (101), (004) (200). In addition to the other crystal planes, there was an indication of TiO2 in the anatase crystal phase. All the peaks were in good agreement with the standard spectrum ICDD. There was no visible change concerning the thermal treatment or the addition of PEG 600, therefore it can be concluded that all the samples are in the anatase crystal phase which is the most photocatalytic form of TiO2 [6].
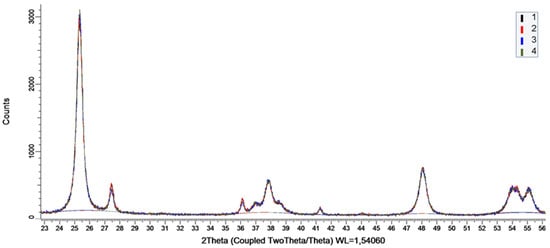
Figure 3.
XRD patterns for sample numbers 1_100, 1_290, 2_100 and 2_290.
4. Discussion
Titanium dioxide has many polymorphs, the two most significant of which are the stable rutile and the metastable anatase. These polymorphs exhibit different properties and consequently different photocatalytic performances. Anatase has high photoreactivity and can reflect long wave ultraviolet (UVA) and visible light. Rutile has a more stable structure than anatase and rutile nanoparticles absorb visible violet light [7]. All the polymorphs used in the present study showed coherent specific surface area results with an average particle size of 70 to 110 nm.
5. Conclusions
The Brunauer–Emmet–Teller method, used for the determination of the specific surface area of the samples, reported coherent results while the sample 1_100 exhibited the highest specific surface area. As the photocatalytic reaction occurs on the surface of the tiles, greater surface areas of nanoparticles provide faster rates. This means that sample 1_100 will potentially show the highest photocatalytic efficiency, although this needs to be verified by future photocatalytic experiments. A suitable experimental set-up has already been developed by this research team and comprises a reactor, an ultra-violet light source, two target gas pollutants (the NO pollutant and a supply of synthetic air) and several flow-rate valves.
Cool and eco-friendly roof tiles are ideal to enhance environmental protection, reduce air pollution and greenhouse gas emissions, and also promote reduced energy consumption. Their future usage could lead to less use of air conditioning units, thus improving thermal comfort for building occupants and helping reduce the urban heat-island phenomena.
Funding
This research study was funded by the Operational Program Competitiveness, Entrepreneurship and Innovation 2014–2020 (EPAnEK) of the Hellenic Ministry of Economy and Development (project code T1EDK-03326), and aims to develop a new bioclimatic product which will have photoreflective and photocatalytic properties for use in cool roofs.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bahnemann, D. Photocatalytic Detoxification of Polluted Waters Environmental Photochemistry; Springer: Berlin/Heidelberg, Germany, 1999; pp. 285–351. [Google Scholar]
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Dalton, J.S.; Janes, P.A.; Jones, N.; Nicholson, J.A.; Hallam, K.R.; Allen, G.C. Photocatalytic oxidation of NOx gases using TiO2: A surface spectroscopic approach. Environ. Pollut. 2002, 120, 415–422. [Google Scholar] [CrossRef]
- Beeldens, A. An environmental friendly solution for air purification and self-cleaning effect: The application of TiO2 as photocatalyst in concrete. In Proceedings of the Transport Research Arena Europe–TRA, Göteborg, Sweden, 12–15 June 2006; pp. 12–16. [Google Scholar]
- Colombo, D.P., Jr.; Bowman, R.M. Femtosecond diffuse reflectance spectroscopy of TiO2 powders. J. Phys. Chem. 1995, 99, 11752–11756. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase characterization of TiO2 powder by XRD and TEM. Agric. Natl. Resour. 2008, 42, 357–361. [Google Scholar]
- Simons, P.; Dachille, F. The structure of TiO2II, a high-pressure phase of TiO2. Acta Crystallogr. 1967, 23, 334–336. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).