Abstract
Six ordinary Portland cement (OPC) clinkers and one white cement clinker were analyzed with the Rietveld method, using ZnO internal standard (IC), to determine the presence of amorphous matter (AM). All clinkers contain abundant AM and have lower silicate phase contents when compared with the same clinkers analyzed without IC, whereas the abundances of the aluminate and ferrate phases were not affected by AM. The white cement clinker had the highest AM content. Determination of AM is important for complete characterization of the OPC clinker and might contribute to a better understanding of the mechanical properties of the clinker.
1. Introduction
Amorphous matter is a common constituent in synthetic industrial products such as Portland cement clinker, pulverized fly ash, bottom furnace ash and slags [1]. Amorphous matter forms reaction products during hydration of phases with pozzolanic activity [2,3]. The latter may not necessarily be amorphous but may include poorly crystalline phases with very small coherent scattering domains to be detected by X-ray diffraction (XRD).
The detection and quantification of amorphous or poorly crystalline phases is a major task for complete characterization of industrial products such as ordinary Portland cement (OPC) and pozzolanic materials. These phases accelerate hydration and enhance reactivity of pozzolanic materials [4,5]. A useful technique recently used for quantitative analysis of amorphous or poorly crystalline materials is X-ray diffraction. Recent methods for quantification of the amorphous matter include full pattern fitting approaches by the Rietveld technique [6,7]. These approaches involve the use of a well-crystallized internal standard of known structure, such as corundum (α-Al2O3) or zincite (ΖnO), and have the advantage that they are not affected by peak overlapping [8].
The Rietveld approach has been adopted as the most reliable method for quantitative mineralogical analysis of cement [9,10]. In this work we applied Rietveld refinement to determine the mineralogical composition and quantify the amorphous matter in six OPC clinkers and one white cement clinker supplied by Titan Cement Co. The Rietveld data were compared with the mineralogical composition of the cement clinkers provided by Titan Cement Co. and with the normative Bogue composition determined from the chemical composition [11].
2. Materials and Methods
Six OPC clinkers and one white cement clinker corresponding to industrial products of different production days, rotary clinkers and different cement plants (Kamari, Patra and Thessaloniki) were investigated. The cement clinkers were ground with an agate pestle and mortar to pass through a 63 μm sieve and stored in a desiccator. Subsequently they were mixed with ZnO internal standard (IS) in a 1:2 IS:sample ratio (33:67%) and were further ground for 5 min in the agate mortar using acetone. After grinding, the samples were dried at 60 °C and mounted on Al- holders via side loading to avoid preferred orientation. A separate Rietveld analysis was performed without IS for terms of comparison.
The mineralogical composition of the clinkers was determined by X-ray diffraction (XRD). The samples were examined on a Bruker D8 Advance Diffractometer equipped with a Lynx Eye strip silicon detector, using Ni-filtered CuKα radiation (35 kV, 35 mA). Data were collected in the range 3–70° 2θ with a step size of 0.02° and counting time 1 s per strip step (total time 63.6 s per step). The XRD traces were analyzed and interpreted with the Diffract Plus software package from Bruker and the Powder Diffraction File (PDF). The quantitative analysis was performed by the Rietveld method using the BGMN computer code (Autoquan 2.80© software). The code performs quantitative analysis taking into account the particle size, the crystal microstrain and preferred orientation.
The chemical composition of the clinkers was determined on fusion beads by Energy Dispersive X-ray Fluorescence (ED-XRF) analysis with a Bruker S2 Ranger ED-XRF spectrometer. The fusion beads were prepared by mixing 1.5 g of the clinkers with 7.5 g of Li-metaborate/Li-tetraborate flux and heating the mixture at 1050 °C for 30 min.
3. Results
3.1. Mineralogical Composition of the Clinkers
The mineralogical composition of the clinkers is listed in Table 1. Three C2S polymorphs (α, β and γ), one C3A polymorph (cubic) and one C3S polymorph (monoclinic) were detected and used in Rietveld analysis. The C2S contents in Table 1 correspond to the sum of the three polymorphs. The main phases in all the OPC are tricalcium silicate (C3S) and tetracalcium aluminate ferrite (C4AF). The clinkers contain 13.6–27.6 wt% amorphous matter, although the XRD traces do not show any hump in the 18–35° 2θ range. The mineral abundances and the abundance of the amorphous matter of the OPCs vary between narrow limits, indicating consistency in clinker production. Sample 14/10/19 RC1 from the Kamari plant has a slightly lower C3S content and a higher amorphous matter content than the remaining OPC counterparts. The higher amorphous matter content in this sample is due to the calcination conditions in the rotary clinker during production. More specifically, the material remained for a longer time in the clinker, and was thus affected by over-firing (Titan cement Co., pers. Comm) causing a greater production of amorphous matter.

Table 1.
Mineralogical composition of the cement clinkers (wt.%) determined by Rietveld refinement. The bold characters correspond to analyses with ZnO IS, the italics to analyses without IS and the plain characters to analyses provided by Titan cement Co. C3S = monoclinic tricalcium silicate, C2S = dicalcium silicate (sum of α, β and γ polymorphs), C3A = cubic tricalcium aluminate, C4AF = tetracalcium aluminate ferrite (brownmillerite), CH = calcium hydrate, M = periclase, C = lime, Am. Matter = amorphous matter, RC = rotary clinker. n.d. = not detected.
The white clinker has a distinct mineralogical composition, characterized by the absence of C4AF and the higher C2S content compared to its OPC counterparts. The absence of C4AF in the white clinker is due to the very low Fe2O3 content in this clinker (c.f. Table 2). Finally, the mineralogical analyses provided by Titan cement Co. were comparable to the Rietveld results without IS for all phases (Table 1). In contrast, the Rietveld analyses with IS yielded lower abundances for the silicate phases (C3S and C2S), and showed the presence of amorphous matter.

Table 2.
Chemical composition of the cement clinkers. n.d = not detected.
3.2. Chemical Composition of the Clinkers
The chemical composition of the cement clinkers is listed in Table 2. The composition of OPC clinkers varies between narrow limits. The white clinker has higher SiO2 and CaO contents and lower Fe2O3 and MgO contents than its OPC counterparts. These differences are in accordance with the mineralogical composition of the clinkers. Thus the white clinker is free of C4AF and periclase (M) and the higher C2S content in this sample is related to the higher SiO2 content compared to its OPC counterparts.
The Bogue normative composition of the clinkers, calculated from the chemical composition, is shown in Table 3. The calculated normative compositions of the clinkers are characterized by higher C3S, C2S and C3A contents and lower C4AF contents compared to the compositions obtained from Rietveld analysis. In addition, the white clinker has higher C3S and C3A contents and lower C2S contents than its OPC counterparts. Weak overall trends hold between C3S and C2S and between C3A and C4AF (data not shown). These trends were not observed in the compositions obtained from Rietveld analysis.

Table 3.
Bogue normative compositions of the cement clinkers.
4. Discussion
The Rietveld analysis of the seven cement clinkers showed the presence of amorphous or poorly crystallized matter in all samples, albeit there is lack of evidence of amorphous phase(s) in the XRD traces. This result is in accordance with recent work on well-crystallized natural and synthetic minerals [12] and seems to be a general observation for natural and synthetic materials. Hence, the lack of hump in the XRD traces is not indicative of a totally crystalline sample, inasmuch as up to 27% of amorphous matter might be present. The abundance of amorphous matter determined in this study is comparable to the values reported in the literature [1,13].
The combination of Rietveld analysis with IS with that without IS might contribute to inferring the composition of the amorphous phase. The main difference between the analyses with IS and the remaining analyses is the lower C3S and C2S contents of the former, whereas the aluminate and ferrite phases remained unaffected by the addition of IS, regardless of the abundance of the phases. This is also the case for the mineralogical data provided by Titan Cement Co., and is valid both for the OPC and the white clinker (Table 1). Hence, it is suggested that the amorphous matter might have a calcium silicate composition and might be associated with the heating and cooling history of the clinker, considering that the OPCs have very similar chemistry (Table 2). Nevertheless, more work would be necessary to verify this suggestion.
The quantitative mineralogical analyses provided by Titan Cement Co. yielded comparable abundances of the clinker phases with the Rietveld method without IS, which in turn display a positive trend with the abundances determined by the Bogue equations. Figure 1 shows the trend observed for the C3S and C2S. In the case of C2S the white cement deviates from the overall trend. In all OPC clinkers, except for the Patras plant one, the C3S contents determined by Rietveld method are higher and those of C2S lower than the values determined by the Bogue equations in accordance with previous work [14]. Three interesting features of the OPC samples are observed in Figure 1: (a) the trends for C3S and C2S are subparallel; (b) the OPC clinker from the Patras plant is not projected in the same area with the remaining OPC clinkers, showing a higher C3S and a lower C2S content than the remaining clinkers; and (c) the C3S and C2S contents calculated by Titan Cement Co. are projected with the Patras plant clinker. The deviation from the overall trend of the Patras plant clinker is due to the Bogue contents for the C3S and C2S. In contrast, the C3S and C2S contents of the Patra clinker determined by the Rietveld method both with and without IS are within the range of the remaining OPC clinkers (Table 1, Figure 1). In addition, this sample has the highest CaO content among all the OPC clinkers (Table 1) and has by far the highest CH content, indicating that it contained free lime, which did not combine with free SiO2 during the sintering process (Table 1). Since free C and CH are not taken into account in the Bogue calculations, the excess CaO is used up to the calculation of the C3S, thereby raising the abundance of this phase. The excess CaO binds a higher amount of SiO2, allowing a lesser amount of SiO2 to form C2S.
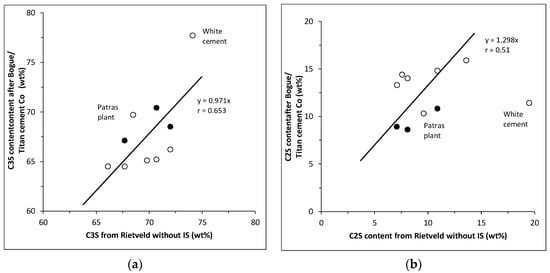
Figure 1.
(a) C3S content (wt%) and (b) C2S content (wt%) of the clinkers determined by the Rietveld approach without IS and the Bogue equations (open circles). The closed circles indicate the C3S and C2S contents of the clinkers determined by the Rietveld approach without IS and the Titan Cement Co.
The method used by Titan Cement Co. for quantitative analysis of the clinker seems to underestimate slightly the C3S content of the clinker and to overestimate slightly the C2S content (Table 1, Figure 1). In any case, those analytical methods which do not use IS for quantitative analysis tend to overestimate the silicate phases, because of the existence of amorphous and/or poorly crystalline phases that are not detectable by XRD. Nevertheless, inasmuch as the hydration products of the clinkers have adequate mechanical strength, the presence of amorphous/poorly crystalline matter might be associated with the hydration rate of the clinkers.
5. Conclusions
Application of the Rietveld method, after addition of ZnO as IS, for quantitative analysis of six OPC clinkers and one white clinker from different cement plants of the Titan Cement Co., produced on different production dates, showed that all clinkers contain amorphous matter ranging from 13.6 to 27.6 wt%. The white cement had the highest amorphous matter content. Determination of amorphous matter caused a decrease of the abundance of C3S and C2S compared to the clinker compositions without IS, whereas the aluminate and ferrate phases were not affected. The use of IS in the Rietveld method in association with standardless analyses and application of the Bogue equation for determination of the normative mineralogical composition may provide useful information about the properties of OPC clinkers. Nevertheless, the different crystallite size of OPC phases, which is related to the firing and cooling conditions during production of the OPC and the properties of raw materials (quartz particle size in silica sand) may affect the accuracy of the method. More work is necessary to verify these points.
Author Contributions
G.E.C., conceptualization, supervision, data interpretation, writing—review and editing, M.D., methodology, investigation, data interpretation, writing—review and editing; G.T., methodology, data interpretation, writing—review and editing, C.T., resources, supervision, data interpretation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. The study did not involve humans or animals.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank Titan Cement Co. for supplying the cement clinkers. A. Stratakis performed the XRD analysis. The constructive comments of an anonymous reviewer improved the text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Whitfield, P.S.; Mitchell, L.D. Quantitative Rietveld analysis of the amorphous content in cements and clinkers. J. Mater. Sci. 2003, 38, 4415–4421. [Google Scholar] [CrossRef] [Green Version]
- Suherman, P.M.; Van Riessen, A.; O’Connor, B.; Li, D.; Bolton, D.; Fairhurst, H. Determination of amorphous levels in Portland cement clinker. Powder Diffr. 2002, 17, 178–185. [Google Scholar] [CrossRef]
- Snellings, R.; Machiels, L.; Mertens, G.; Elsen, J. Rietveld refinement strategy for quantitative phase analysis of partially amorphous zeolitised tuffaceous rocks. Geol. Belg. 2010, 13, 183–196. [Google Scholar]
- Blanco Valera, M.; Martínez Ramírez, S.; Ereña, I.; Gener, M.; Carmona, P. Characterization and pozzolanicity of zeolitic rocks from two Cuban deposits. Appl. Clay Sci. 2006, 33, 149–159. [Google Scholar]
- Mertens, G.; Snellings, R.; Van Balen, K.; Bicer-Simsir, B.; Verlooy, P.; Elsen, J. Pozzolanic reactions of common natural zeolites with lime and parameters affecting their reactivity. Cem. Conc. Res. 2009, 39, 233–240. [Google Scholar] [CrossRef]
- De La Torre, A.G.; Bruque, S.; Aranda, M.A.G. Rietveld quantitative amorphous content analysis. J. Appl. Cryst. 2001, 34, 196–202. [Google Scholar] [CrossRef]
- Bish, D.L.; Ploetze, M. X-ray powder diffraction with emphasis on qualitative and quantitative analysis in industrial mineralogy. In Advances in the Characterization of Industrial Minerals; Christidis, G.E., Ed.; Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 2011; Volume 9, pp. 35–76. [Google Scholar]
- Post, J.E.; Bish, D.L. Rietveld refinement of crystal structures using powder X-ray diffraction data. Rev. Mineral. 1989, 20, 277–308. [Google Scholar]
- Le Saout, G.; Kocaba, V.; Scrivener, K. Application of the Rietveld method to the analysis of anhydrous cement. Cem. Conc. Res. 2011, 41, 133–148. [Google Scholar] [CrossRef]
- Aranda, M.A.G.; De la Torre, A.G.; Leon-Reina, L. Rietveld quantitative phase analysis if OPC clinkers, cements and hydration products. Rev. Mineral. Geochem. 2012, 74, 169–209. [Google Scholar] [CrossRef] [Green Version]
- Bogue, R.H. Calculation of the compounds in Portland cement. Ind. Eng. Chem. Anal. Ed. 1929, 1, 192–197. [Google Scholar] [CrossRef]
- Christidis, G.E.; Paipoutlidi, K.; Marantos, I.; Perdikatsis, V. Determination of amorphous matter in industrial minerals with X-ray diffraction, using Rietveld refinement. Bull. Geol. Soc. Greece 2020, 56, 1–15. [Google Scholar] [CrossRef]
- Lerch, W.; Brownmiller, L.T. Method for approximating the glass content of portland cement clinker. J. Res. Nat. Bur. Stand 1937, 18, 609–622. [Google Scholar] [CrossRef]
- Plötze, M. Quantitative phase analysis of Portland cement clinker with the Rietveld method. In Proceedings of the 6th International Congress on Applied Mineralogy (ICAM), Göttingen, Germany, 17–19 July 2000; Rammlmair, D., Mederer, J., Oberthür, T., Heimann, R.B., Pentinghaus, H., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 2000; pp. 879–882. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).