Viable Scandium Extraction from Bauxite Residue at Pilot Scale †
Abstract
:1. Introduction
2. Experimental
3. Results
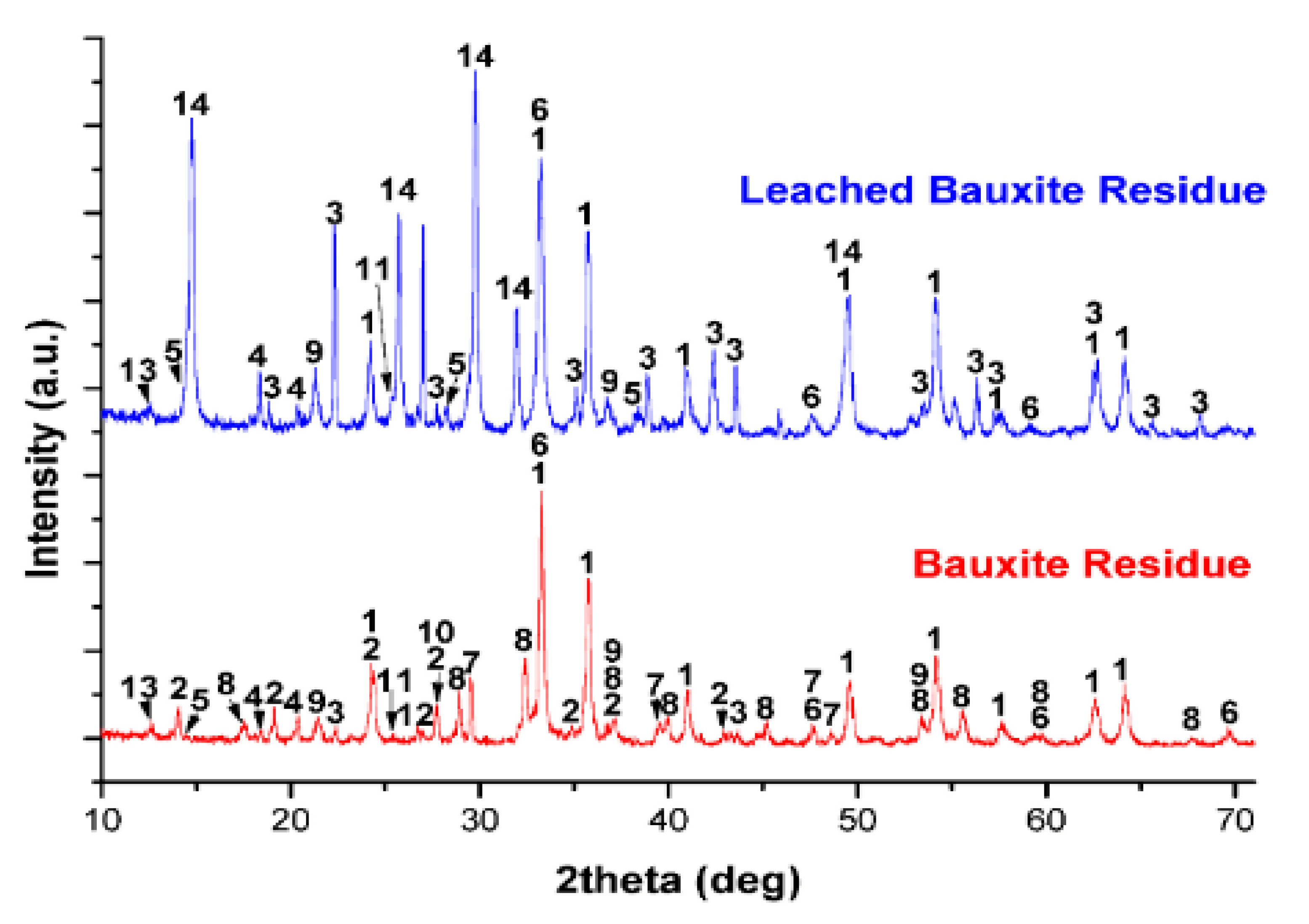
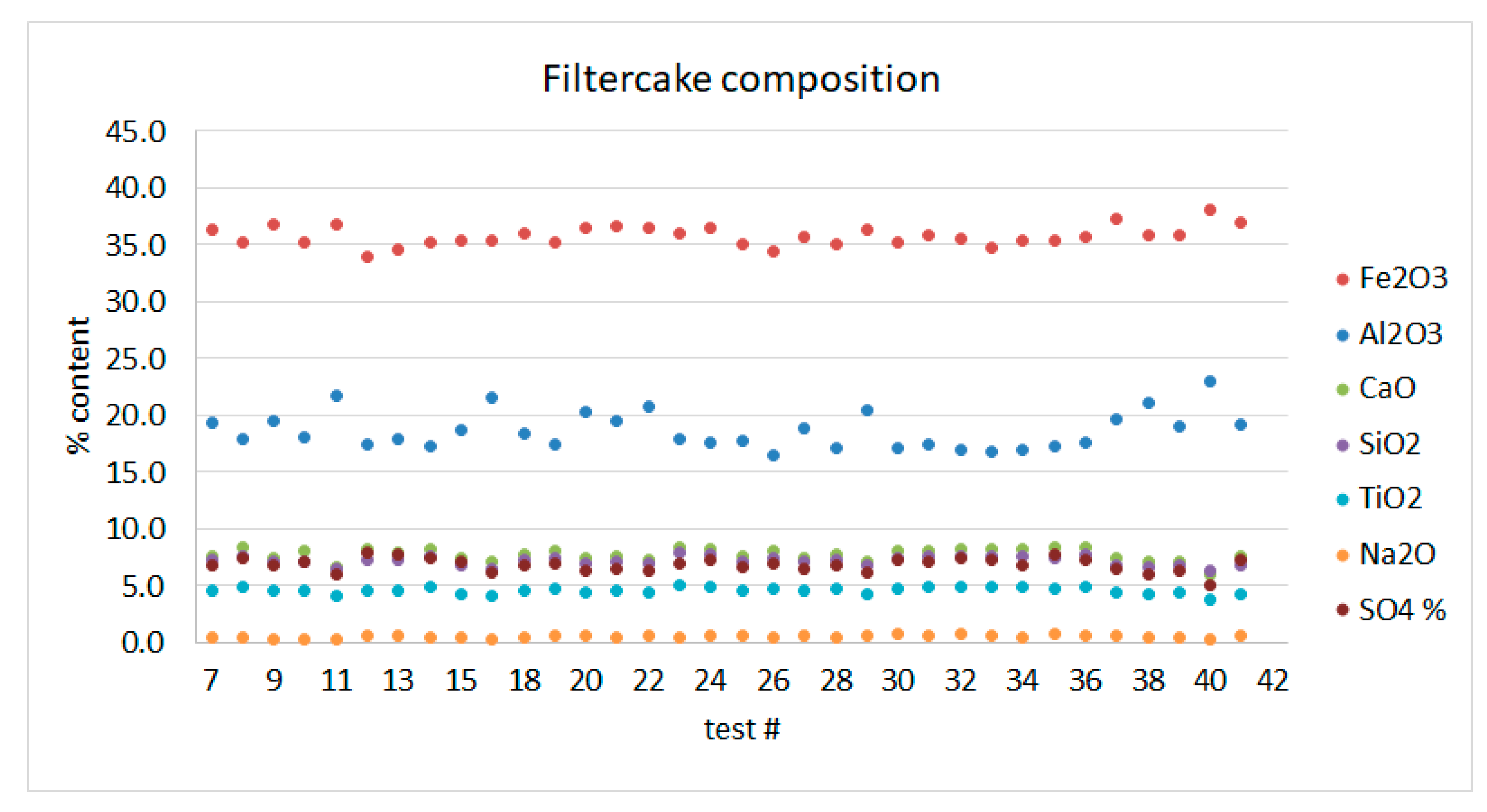
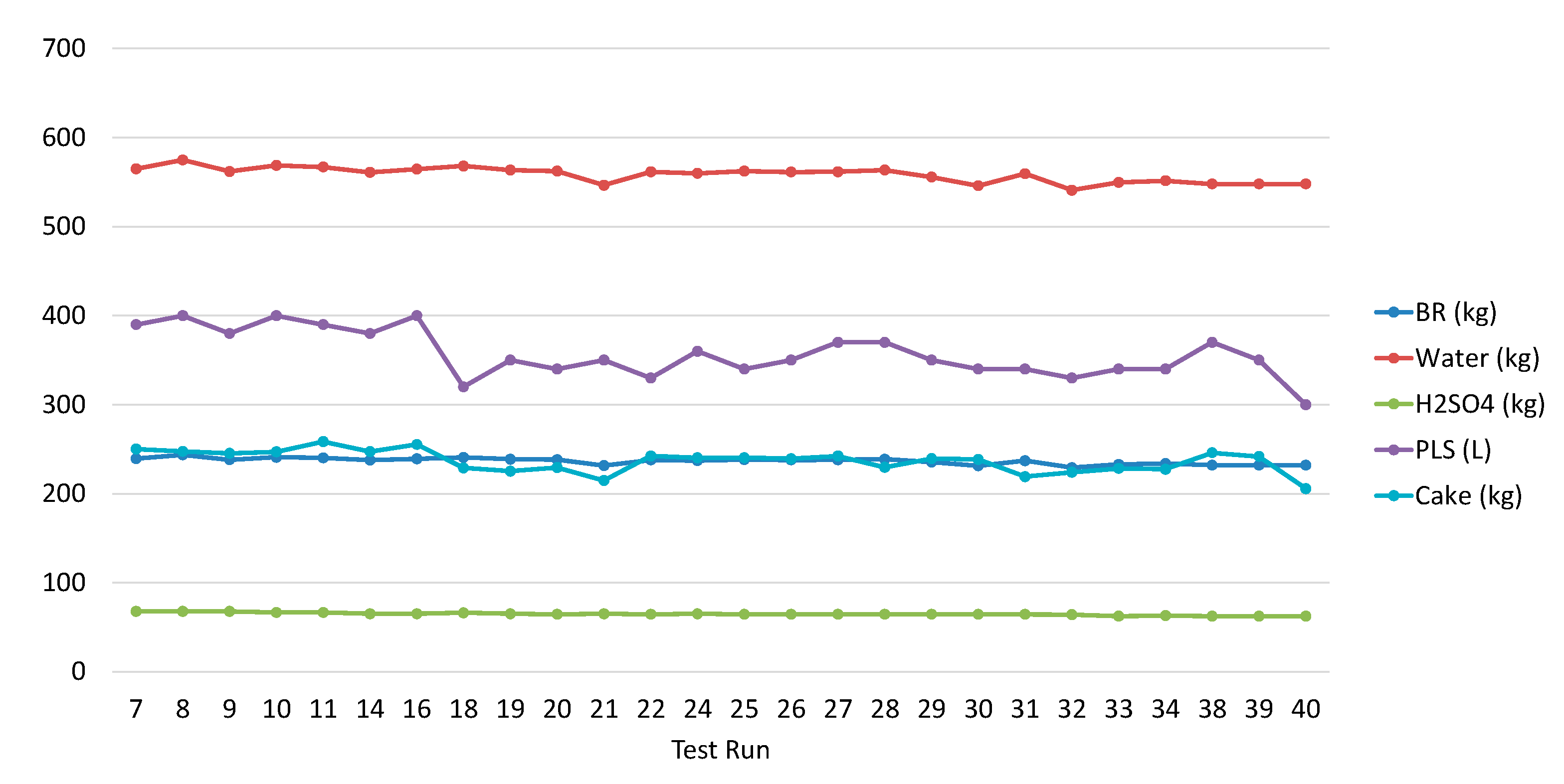
3.1. Bauxite Residue Leaching
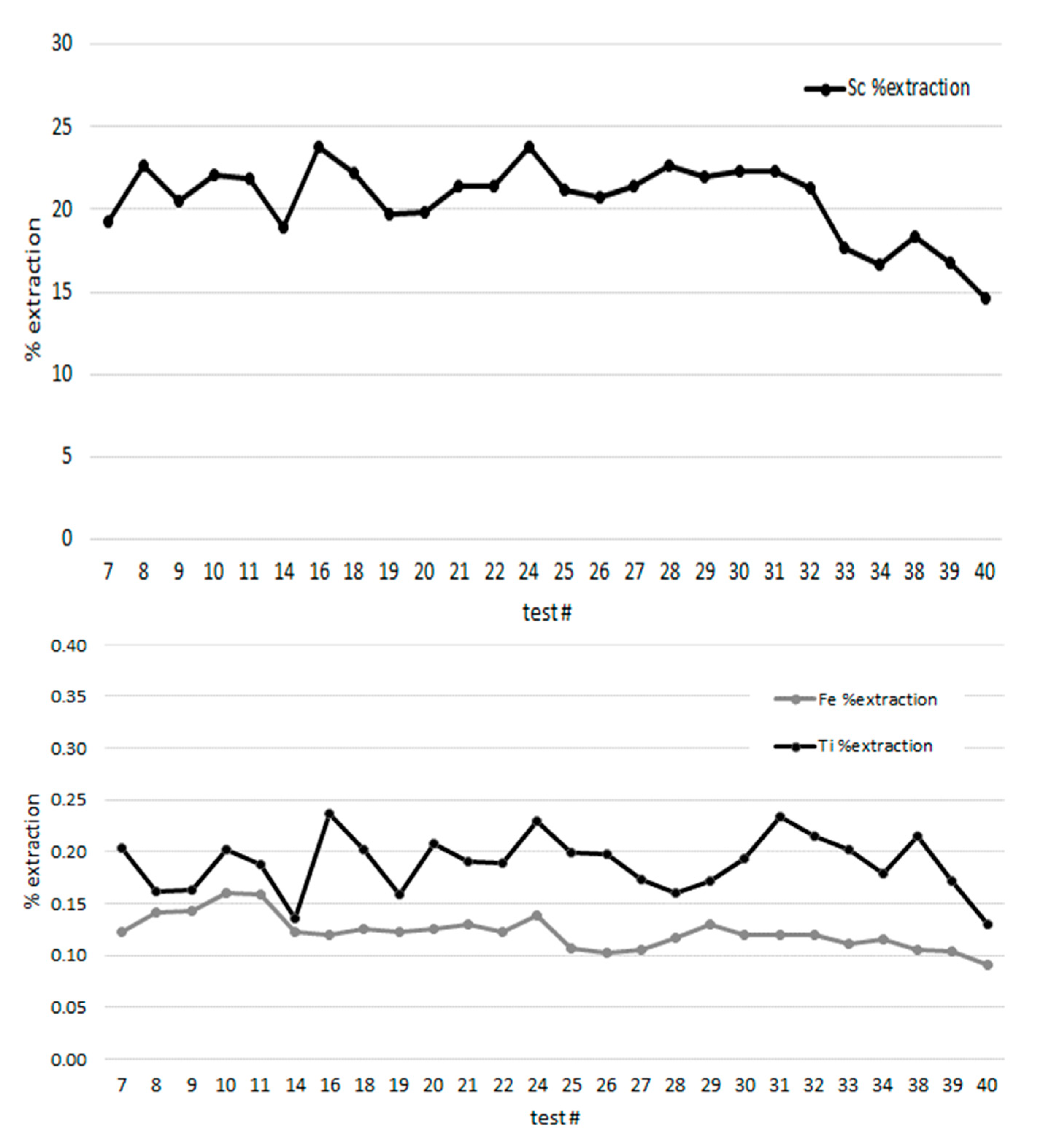
3.2. Processing PLS with SIR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Deady, É.A.; Mouchos, E.; Goodenough, K.; Williamson, B.J.; Wall, F. A review of the potential for rare-earth element resources from European red muds: Examples from Seydişehir, Turkey and Parnassus-Giona, Greece. Mineral. Mag. 2018, 80, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Vind, J.; Malfliet, A.; Blanpain, B.; Tsakiridis, P.E.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Rare Earth Element Phases in Bauxite Residue. Minerals 2018, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zong, Y.; Li, H.; Zhao, Z. Characterization of scandium and gallium in red mud with time of flight-secondary ion mass spectrometry (ToF-SIMS) and electron probe micro-analysis (EPMA). Miner. Eng. 2018, 119, 263–273. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.-X.; Cheng, H.-J.; Liu, X.-M. Electron probe microanalysis for revealing occurrence mode of scandium in Bayer red mud. Rare Met. 2017, 36, 295–303. [Google Scholar] [CrossRef]
- Chassé, M.; Griffin, W.L.; O’Reilly, S.Y.; Calas, G. Scandium speciation in a world-class lateritic deposit. Geochem. Perspect. Lett. 2016, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Vind, J.; Malfliet, A.; Bonomi, C.; Paiste, P.; Sajó, I.E.; Blanpain, B.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Modes of occurrences of scandium in Greek bauxite and bauxite residue. Miner. Eng. 2018, 123, 35–48. [Google Scholar] [CrossRef]
- Suss, A.; Panov, A.; Kozyrev, A.; Kuznetsova, N.; Gorbachev, S. Specific Features of Scandium Behavior during Sodium Bicarbonate Digestion of Red Mud; Martin, O., Ed.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Petrakova, O.V.; Panov, A.V.; Gorbachev, S.N.; Klimentenok, G.N.; Perestoronin, A.V.; Vishnyakov, S.E.; Anashkin, V.S. Improved efficiency of red mud processing through scandium oxide recovery. In Light Metals 2015; Hyland, M., Ed.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018; pp. 93–96. [Google Scholar]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of Rare Earths and Other Valuable Metals From Bauxite Residue (Red Mud): A Review. J. Sustain. Met. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, S.; Gronen, L.; Stopic, S.; Friedrich, B. Novel approach for enhanced scandium and titanium leaching efficiency from bauxite residue with suppressed silica gel formation. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef] [PubMed]
- Hatzilyberis, K.; Lymperopoulou, T.; Tsakanika, L.-A.; Ochsenkühn, K.-M.; Georgiou, P.; Defteraios, N.; Tsopelas, F.; Ochsenkühn-Petropoulou, M. Process Design Aspects for Scandium-Selective Leaching of Bauxite Residue with Sulfuric Acid. Minerals 2018, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of Bauxite Residue (Red Mud) in View of Iron and Selective Rare Earths Recovery. J. Sustain. Met. 2015, 2, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Ochsenkühn-Petropoulou, M.T.; Hatzilyberis, K.S.; Mendrinos, A.L.N.; Salmas, C. Pilot-Plant Investigation of the Leaching Process for the Recovery of Scandium from Red Mud. Ind. Eng. Chem. Res. 2002, 41, 5794–5801. [Google Scholar] [CrossRef]
- Xu, W.-Q.; Mattera, V., Jr.; Abella, M.Y.R.; Abrenica, G.M.; Patkar, S. Selective Recovery of Rare earth Metals from an Acidic Slurry or Acidic Solution. U.S. Patent 20190078175A1, 16 November 2018. [Google Scholar]
- Xu, W.-Q.; Mattera, V., Jr.; Abella, M.Y.R.; Abrenica, G.M.; Patkar, S. Composite Extract-ant-Enhanced Polymer Resin, Method of Making the Same, and Its Usage for Extraction of Valuable Metal(s). U.S. Patent 2017/074921A1, 25 October 2016. [Google Scholar]
- Hatzilyberis, K.; Tsakanika, L.-A.; Lymperopoulou, T.; Georgiou, P.; Kiskira, K.; Tsopelas, F.; Ochsenkühn, K.M.; Ochsenkühn-Petropoulou, M. Design of an advanced hydrometallurgy process for the intensified and optimized industrial recovery of scandium from bauxite residue. Chem. Eng. Process. Process. Intensif. 2020, 155, 108015. [Google Scholar] [CrossRef]
- Ochsenkuehn-Petropoulou, M.; Tsakanika, L.-A.; Lymperopoulou, T.; Ochsenkuehn, K.-M.; Hatzilyberis, K.; Georgiou, P.; Stergiopoulos, C.; Serifi, O.; Tsopelas, F. Efficiency of Sulfuric Acid on Selective Scandium Leachability from Bauxite Residue. Metals 2018, 8, 915. [Google Scholar] [CrossRef] [Green Version]
- Lymperopoulou, T.; Georgiou, P.; Tsakanika, L.-A.; Hatzilyberis, K.; Ochsenkuehn-Petropoulou, M. Optimizing Conditions for Scandium Extraction from Bauxite Residue Using Taguchi Methodology. Minerals 2019, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Balomenos, E.; Nazari, G.; Davris, P.; Abrenica, G.; Pilihou, A.; Mikeli, E.; Panias, D.; Patkar, S.; Xu, W.-Q. Scandium Extraction from Bauxite Residue Using Sulfuric Acid and a Composite Extractant-Enhanced Ion-Exchange Polymer Resin. In Rare Metal Technology 2021; Springer International Publishing: Cham, Switzerland, 2021; pp. 217–228. [Google Scholar] [CrossRef]


| wt% | ppm (mg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | Al2O3 | SiO2 | CaO | Na2O | TiO2 | LOI | Ce | La | Y | Sc |
| 38.73 | 24.13 | 7.65 | 8.03 | 3.58 | 5.00 | 10.10 | 657 | 110 | 132 | 71 |
| 1 t BR Input | 1 t Cake Output | 1.66 t PLS | % Extraction in PLS | |||||
|---|---|---|---|---|---|---|---|---|
| Al (kg) | 127.71 | kg | 116.58 | kg | 11.12 | kg | 8.71 | Al |
| Fe (kg) | 270.84 | kg | 270.51 | kg | 0.33 | kg | 0.12 | Fe |
| Ca (kg) | 57.36 | kg | 54.90 | kg | 2.46 | kg | 4.28 | Ca |
| Si (kg) | 35.72 | kg | 35.36 | kg | 0.36 | kg | 1.01 | Si |
| Ti (kg) | 29.99 | kg | 29.94 | kg | 0.06 | kg | 0.19 | Ti |
| Na (kg) | 26.56 | kg | 3.93 | kg | 22.63 | kg | 85.20 | Na |
| Sc (g) | 83.28 | g | 66.13 | g | 17.15 | g | 20.59 | Sc |
| (mg/L) | Fe | Ti | Al | Si | Ca | Sc | Ce | Y | La |
|---|---|---|---|---|---|---|---|---|---|
| Tank 2a PLS | 275 | 33 | 6530 | 127 | 590 | 12.8 | 8.9 | 7.5 | 3.4 |
| Tank 2b PLS | 278 | 29 | 6400 | 159 | 603 | 10.8 | 9.9 | 8.0 | 4.3 |
| Sc | Ti | Fe | Na | Al | Si | LOI | |
|---|---|---|---|---|---|---|---|
| %wt | 22.0 | 15.2 | 0.54 | 1.0 | 1.3 | 5.8 | 32.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davris, P.; Balomenos, E.; Nazari, G.; Abrenica, G.; Patkar, S.; Xu, W.-Q.; Karnachoritis, Y. Viable Scandium Extraction from Bauxite Residue at Pilot Scale. Mater. Proc. 2021, 5, 129. https://doi.org/10.3390/materproc2021005129
Davris P, Balomenos E, Nazari G, Abrenica G, Patkar S, Xu W-Q, Karnachoritis Y. Viable Scandium Extraction from Bauxite Residue at Pilot Scale. Materials Proceedings. 2021; 5(1):129. https://doi.org/10.3390/materproc2021005129
Chicago/Turabian StyleDavris, Panagiotis, Efthymios Balomenos, Ghazaleh Nazari, Gomer Abrenica, Shailesh Patkar, Wen-Qing Xu, and Yiannis Karnachoritis. 2021. "Viable Scandium Extraction from Bauxite Residue at Pilot Scale" Materials Proceedings 5, no. 1: 129. https://doi.org/10.3390/materproc2021005129
APA StyleDavris, P., Balomenos, E., Nazari, G., Abrenica, G., Patkar, S., Xu, W.-Q., & Karnachoritis, Y. (2021). Viable Scandium Extraction from Bauxite Residue at Pilot Scale. Materials Proceedings, 5(1), 129. https://doi.org/10.3390/materproc2021005129






