Sulphuric Acid Leaching of Spent Nickel Metal Hydride Car Batteries †
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
- The present work showed that metal recoveries of almost 100% can be achieved, during leaching, for Co, Ce, Y, Nd and La. The extraction of Ni did not follow this pattern and reached about 85% with leaching agents of 1 or 2M H2SO4. Maximum Ni recovery was obtained with a 2M sulphuric acid solution at a temperature of 95 °C, reaching 93%.
- The optimum conditions for the extraction of other than Ni elements were 2M H2SO4 concentration and temperature of 75 °C.
- A concentration of 0.5 M sulfuric acid for the tested liquid to solid ratio (20 L/kg) is not sufficient to achieve high metal recoveries.
- Increasing the sulphuric acid concentration favours the metals extraction.
- Increase in temperature does not seem to have a significant effect in the metal extraction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernardes, A.; Espinosa, D.; Tenório, J. Recycling of batteries: A review of current processes and technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Young, K.; Yasuoka, S. Capacity Degradation Mechanisms in Nickel / Metal Hydride Batteries. Batteries 2016, 2, 3. [Google Scholar] [CrossRef]
- Tarabay, J.; Karami, N. Nickel Metal Hydride battery: Structure, chemical reaction, and circuit model. In Proceedings of the Third International Conference on Technological Advances in Electrical, Electronics and Computer Engineering (TAEECE), Beirut, Lebanon, 29 April–1 May 2015; pp. 22–26. [Google Scholar] [CrossRef]
- Commision, E. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the 2017 List of Critical Raw Materials for the EU, Brussels, 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52017DC0490 (accessed on 1 April 2022).
- Porvali, A.; Ojanen, S.; Wilson, B.; Serna-Guerrero, R.; Lundström, M. Nickel Metal Hydride Battery Waste: Mechano-hydrometallurgical Experimental Study on Recycling Aspects. J. Sustain. Met. 2020, 6, 78–90. [Google Scholar] [CrossRef]
- Muller, T.; Friedrich, B. Development of a recycling process for nickel-metal hydride batteries. J. Power Sources 2006, 158, 1498–1509. [Google Scholar] [CrossRef]
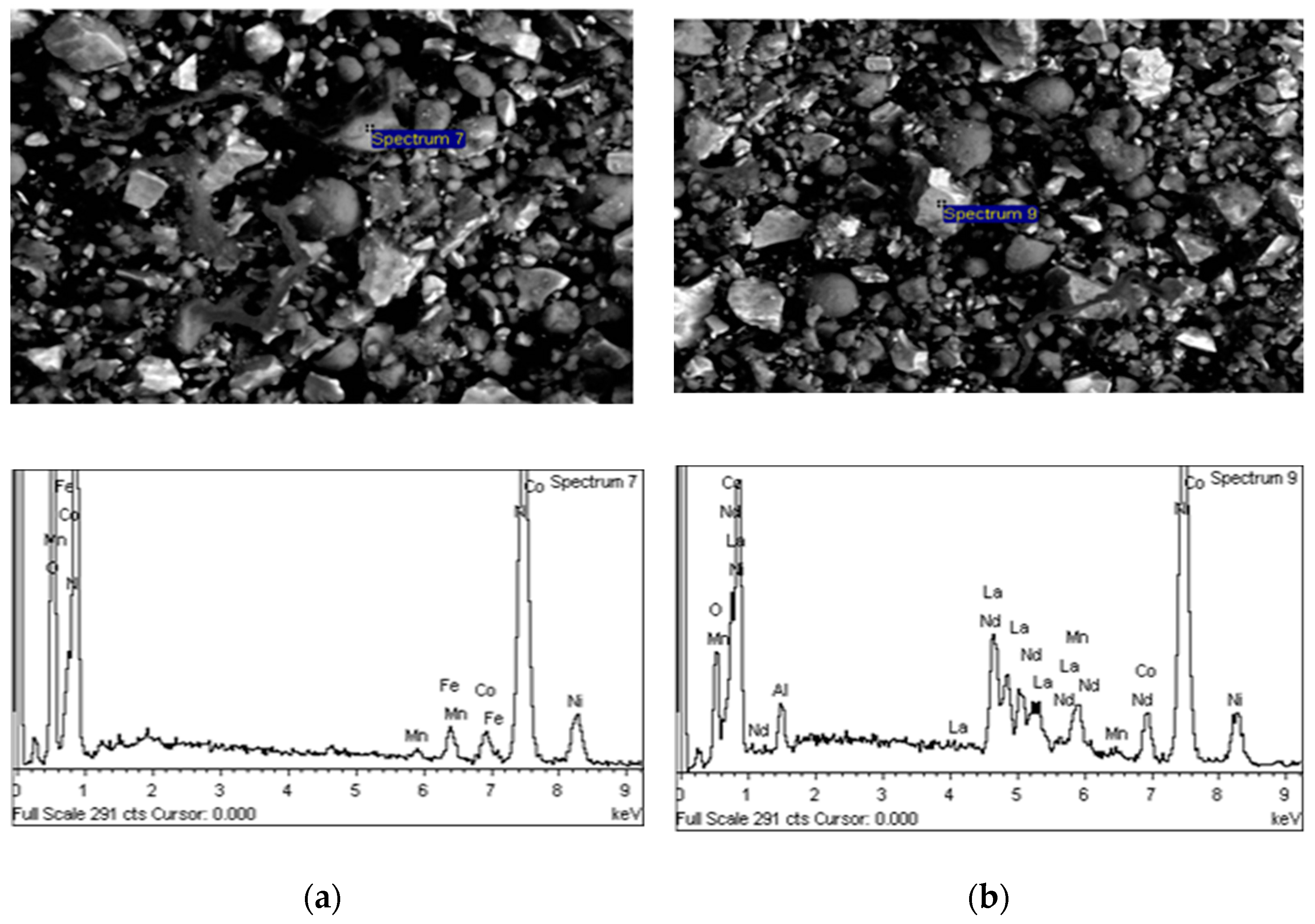
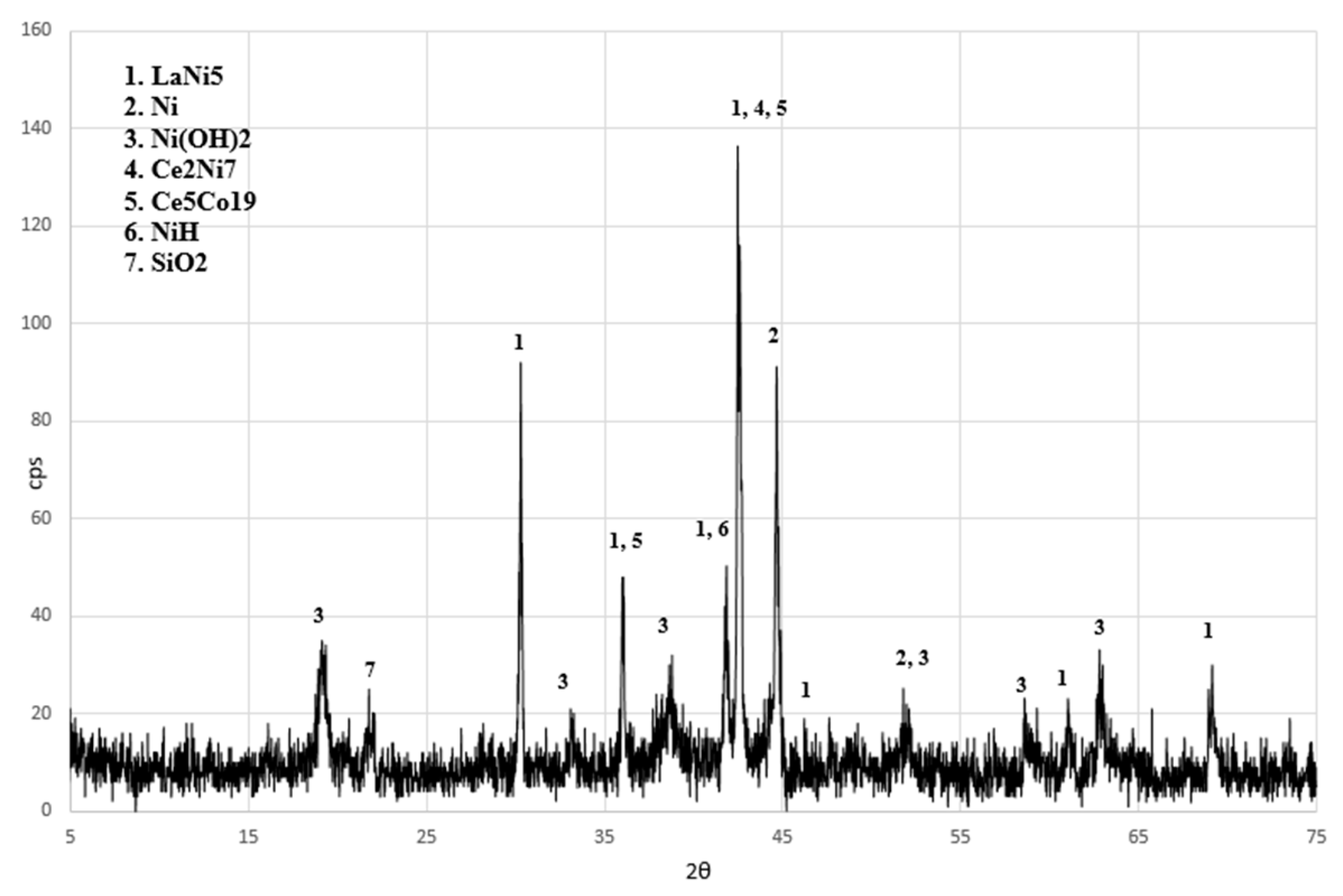
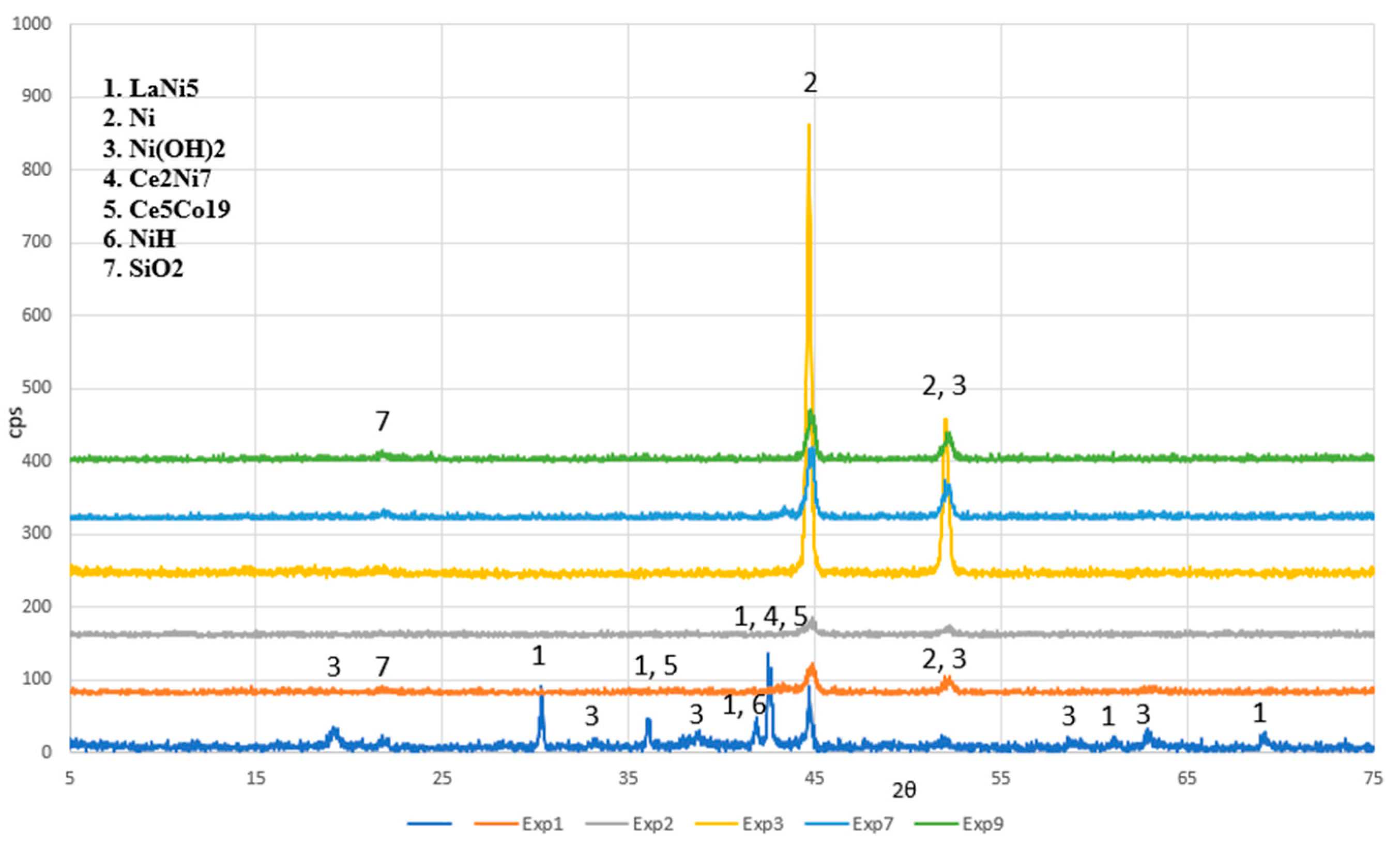
| Element | Content (% w/w) | ||||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Average | XRF | |
| Al | 1.22 | 1.26 | 1.22 | 1.23 | 0.54 |
| Co | 4.86 | 4.97 | 4.59 | 4.81 | 5.76 |
| Fe | 2.26 | 2.76 | 2.29 | 2.44 | 1.21 |
| Mn | 2.40 | 2.15 | 2.33 | 2.29 | 2.26 |
| Ni | 49.05 | 48.8 | 46.45 | 47.43 | 50.7 |
| La | 9.53 | 9.48 | 8.18 | 9.06 | 7.79 |
| Ce | 5.08 | 4.82 | 4.87 | 4.92 | 4.56 |
| Nd | 1.53 | 1.47 | 1.53 | 1.51 | 1.52 |
| Y | 0.74 | 0.76 | 0.73 | 0.74 | 1.02 |
| Experiment | H2SO4 (M) | T (°C) | Extraction (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Co | Fe | Ni | Mn | La | Ce | Nd | Y | |||
| 4 | 0.5 | 50 | 52.09 | 92.36 | 41.37 | 73.94 | 100.00 | 87.80 | 93.44 | 94.02 | 92.21 |
| 5 | 0.5 | 75 | 19.34 | 92.32 | 53.60 | 74.50 | 97.81 | 85.11 | 90.96 | 94.16 | 94.47 |
| 6 | 0.5 | 95 | 24.08 | 84.87 | 52.08 | 65.93 | 83.16 | 73.68 | 77.83 | 80.80 | 87.85 |
| 1 | 1 | 50 | 94.39 | 97.36 | 94.53 | 84.57 | 100.00 | 90.46 | 95.88 | 100.00 | 96.73 |
| 2 | 1 | 75 | 93.21 | 99.00 | 95.35 | 86.74 | 100.00 | 93.09 | 99.11 | 100.00 | 98.32 |
| 3 | 1 | 95 | 90.76 | 95.69 | 95.28 | 84.23 | 100.00 | 87.37 | 92.63 | 100.00 | 98.50 |
| 7 | 2 | 50 | 87.27 | 98.16 | 96.52 | 83.53 | 100.00 | 92.64 | 98.29 | 100.00 | 97.83 |
| 8 | 2 | 75 | 91.52 | 98.96 | 99.03 | 87.30 | 100.00 | 95.25 | 100.00 | 100.00 | 98.87 |
| 9 | 2 | 95 | 93.39 | 99.03 | 84.49 | 99.34 | 100.00 | 91.34 | 97.51 | 100.00 | 98.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polychronopoulou, E.; Oustadakis, P.; Tsakiridis, P.; Betsis, K.; Xenidis, A. Sulphuric Acid Leaching of Spent Nickel Metal Hydride Car Batteries. Mater. Proc. 2021, 5, 126. https://doi.org/10.3390/materproc2021005126
Polychronopoulou E, Oustadakis P, Tsakiridis P, Betsis K, Xenidis A. Sulphuric Acid Leaching of Spent Nickel Metal Hydride Car Batteries. Materials Proceedings. 2021; 5(1):126. https://doi.org/10.3390/materproc2021005126
Chicago/Turabian StylePolychronopoulou, Elli, Paschalis Oustadakis, Petros Tsakiridis, Konstantinos Betsis, and Anthimos Xenidis. 2021. "Sulphuric Acid Leaching of Spent Nickel Metal Hydride Car Batteries" Materials Proceedings 5, no. 1: 126. https://doi.org/10.3390/materproc2021005126
APA StylePolychronopoulou, E., Oustadakis, P., Tsakiridis, P., Betsis, K., & Xenidis, A. (2021). Sulphuric Acid Leaching of Spent Nickel Metal Hydride Car Batteries. Materials Proceedings, 5(1), 126. https://doi.org/10.3390/materproc2021005126








