1. Introduction
Since 2008, most diesel engine exhaust systems of vehicles comprise of a diesel particulate filter (DPF). Since 2014, the use of catalyzed diesel particulate filters (c-DPF) has become the most common method for capturing particulate matter. Such filters are made of a ceramic honeycomb and mainly consist of a cordierite substrate with alternately plugged channels. The filter removes particulate matter (soot and ash) from the exhaust gases of diesel engine vehicles. These particles are formed as a result of the incomplete combustion of diesel fuel that takes place in the engine. A catalyzed filter (c-DPF) not only traps particulate matter but also uses a patented catalytic technology (Pt as catalyst and Ce/Zr as washcoat) to further oxidize carbon monoxide (CO), hydrocarbons (HC), and diesel particulate matter [
1].
Over time, large particles are trapped and accumulate into the channels of the filter without being oxidized or burnt. As a result, the porous of the walls become plugged and the gas flow is not allowed to cross the filter. During the operation of the diesel engine, the filter is periodically regenerated, but due to poor vehicle maintenance this type of regeneration (passive regeneration) may not be profitable. Then, instead of replacement due to the high cost (a DPF/catalytic system costs up to 2000€ per part), the regeneration takes place in a regenerating compact machine. Nowadays, organic based solvents are used for dissolution of organic deposits. The solvents alongside remove the catalyst and the washcoat while revealing toxic wastes. As a result, there is a need for an innovative solvent which dissolves organic content but not the catalyst.
In this study, a novel environmentally friendly and low-cost alternative solvent was produced for regenerating diesel particulate filters. The innovative solvent which is developed and used dissolves and removes ash and soot (organic compounds) from blocked DPFs and c-DPFs, without dissolving platinum group metals (PGMs) (Pt) and rare earth elements (REEs) (Ce), which are essential for c-DPF’s catalytic operation.
The reagents of the new produced solution that were studied are citric acid (C.A.) as a mild acid, the deep eutectic solvent (DES) of choline chloride (Ch.Chl.) and lactic acid (L.A.), the surfactant sodium dodecyl-benzene sulfonate (SDBS) and sodium hypochlorite (NaOCl) as an antimicrobial factor. The optimized solution concentration was found to be Ch.Chl. 10% w/v, L.A. 5% v/v, C.A. 2% w/v, SDBS 0.1% w/v and NaOCl 1% v/v.
By this novel regenerating method, more than 90% of the organic loading is dissolved from DPF and c-DPF. At the same time, the critical elements are not affected, while their dissolution is less than 10% from the diesel filters during the regeneration. As a result, regeneration could be applied on diesel filters, since critical elements are not dissolved and removed. Since DPF and c-DPF filters remain intact, they preserve their functionality and high performance.
The method suggested herein was carried out in full scale, since real blocked DPF filter were regenerated in a compact machine using the novel produced solution.
2. Materials and Methods
2.1. Materials
For this study, catalyzed diesel particulate filters (c-DPFs) and non-catalyzed diesel particulate filters (DPFs) were collected, pre-processed and subjected to regeneration used. The reagents that were used are citric acid (99%), choline chloride (98%), lactic acid (DL-90%), sodium dodecyl-benzene sulfonate (technical grade), NaOCl (solution 14%) were provided by Sigma-Aldrich®, Darmstadt, Germany and were used without any further purification. Distilled water was used throughout the experiments, for regeneration steps.
2.2. Methods
2.2.1. Preprocessing
The spent or deactivated filters DPF and c-DPF, were collected by MONOLITHOS Ltd. After sorting, based on catalytic phase, filters were de-canned where metallic parts were removed and ceramic parts were grinded and milled under 250 µm. The powder was homogenized, and the particle size was determined with specific granulometry sieving. Finally, appropriate sampling was performed for X-ray Florescence Spectroscopy (XRF) analysis to place in order to determine PGMs content [
2]. Before the element quantification, the samples were dried in a © BINDER drying oven, Tuttlingen, Germany at 120 °C for 2 h to remove the humidity and avoid interference in the XRF results. The pre-processing method was described in detail by Yakoumis et al. for the three-way spent catalytic converters [
2].
2.2.2. Regenerating Procedure
DPF and c-DPF powder samples were dried in the drying oven at 120 °C for 2 h for the humidity to be removed and for the Pt and Ce content to be accurately determined with XRF analysis. Suitable amount of the powder was weighted and then added to the beaker, then reagents of C.A., Ch. Chl., L.A., SDBS, NaOCl and H2O were added in a variety of concentrations to obtain certain experimental conditions (Ch.Chl. 10% w/v, L.A. 5% v/v, C.A. 2% w/v, SDBS 0.1% w/v and NaOCl 1% v/v). Regenerating temperature was 25 °C for an hour under stirring. The solid to liquid ratio remained stable at 10% w/v. The liquid and solid residue were separated via filtration. The wet solid residue was dried overnight so as for the water content to be removed and avoid humidity interference in XRF analysis.
2.2.3. Calcination
Before the regeneration process, appropriate sampling was performed from each batch of DPF and c-DPF. Representative samples were calcined at 800 °C for default time with a heating rate of 10 °C/min. During the calcination process, organic compounds were removed. Before and after the calcination, weighing was performed so as for the organic mass loss to be calculated (which equals the organic content of each solid). After the regeneration process, filters were subjected to the calcination process in order for organic compounds that have not been dissolved to be removed. Since the organic content is determined for each DPF and c-DPF, respectively, before and after regeneration, the organic mass was possible to be determined.
2.3. Methods
2.3.1. Optical Microscope Analysis
The morphology, structure and dimensions of c-DPF’s and DPF’s channels were determined by optical microscopy analysis. An optical microscope of Am Scope, ME520 Series Optical Microscope, Irvine, CA, USA was used for the performed analysis. Images were obtained with total microscope magnification 125×.
2.3.2. X-ray Fluorescence Spectroscopy
As for the qualitative and quantitative PGMs loading analysis of samples, X-ray Fluorescence spectrometer (portable VANTA Olympus 2017, Waltham, MA, USA) and software (Olympus OOSA, version 3.8.540.0) have been used. X-ray fluorescence (XRF) analysis provides accurate, fast, non-destructive, repeatable measurements, as well as no chemical preparation is required.
3. Results and Discussion
3.1. Optical Microscope Analysis
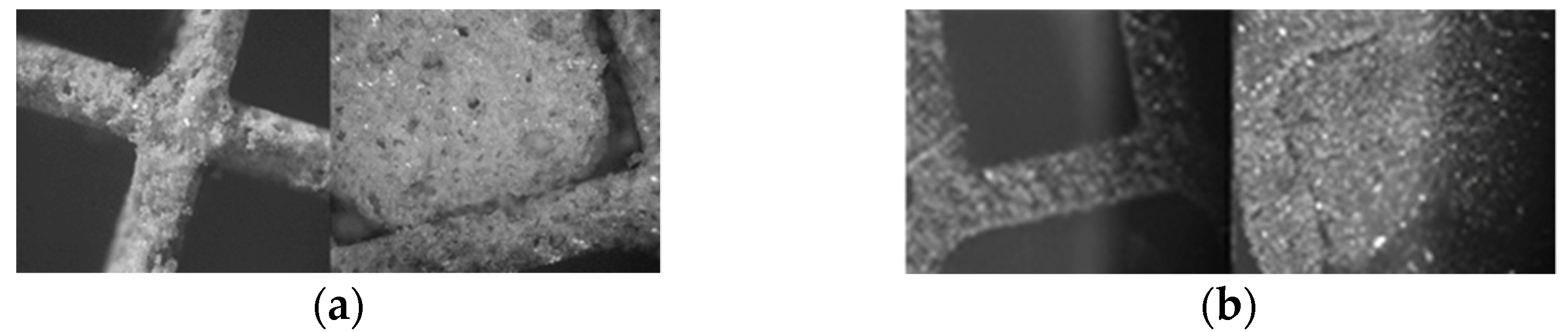
According to microscope images collected at c-DPF’s internal and external part (
Figure 1a), the cells’ walls and blocked channels are described in detail. The width of the wall was measured approximately 0.4547 mm.
Microscope images of the DPF’s internal and external part (
Figure 1a) describe in detail the structure of the filter. The external parts have alternately plugged channels and the internals have open channels. The wall width was measured as 0.3898 mm.
3.2. XRF Analysis
The PGMs and REEs content of DPF and c-DPF was determined by XRF analysis. Elemental and qualitative analysis is presented in
Table 1. The three main metals of substrate for both DPF and c-DPF are Fe, Zn and Cr. Platinum content was also detected for catalyzed filters, as expected, and Ce/Zr as a substrate (where it is the washcoat).
3.3. Effect of Reagents
The current study aimed to form a biocompatible solution to solubilize soot and ash (organic compounds). In order the solvent to be feasible, it was important that the content of the catalyst (Pt) and REEs (Ce, Zr) remain intact. The studied solutions were expected to affect each filter not in the similar way, due to the fluctuations in the concentration and the variation of the metals in the surfaces. However, the optimized selected solution should be efficient in the regenerating process for both of DPF and c-DPF. Therefore, the efficiency of the regenerating process was defined by the high organic mass loss and simultaneous low Pt and Ce loss. Citric acid is a mild acid with the ability to form chemical compounds with metals, often used in soaps and detergents applications. The studied concentrations were 0.5, 2, 4, and 6% w/v in an aqueous solution.
The DES of choline chloride and lactic acid exhibits excellent biodegradability, low volatility, non-toxicity, ease of preparation and low cost. The effect of the ratio of choline chloride to lactic acid and the concentration of these reagents in the produced solution were studied (10/5%–10/20% (w/v)/(v/v)).
Sodium dodecyl-benzene sulfonate is an anionic surfactant used in various detergents to remove organic compounds. The studied concentrations were 0.1, 0.5 and 1% w/v in an aqueous solution.
Sodium hypochlorite (NaOCl) is mainly used as a disinfectant or a bleaching agent and is added as an antimicrobial factor. The concentration that was examined was 1% v/v.
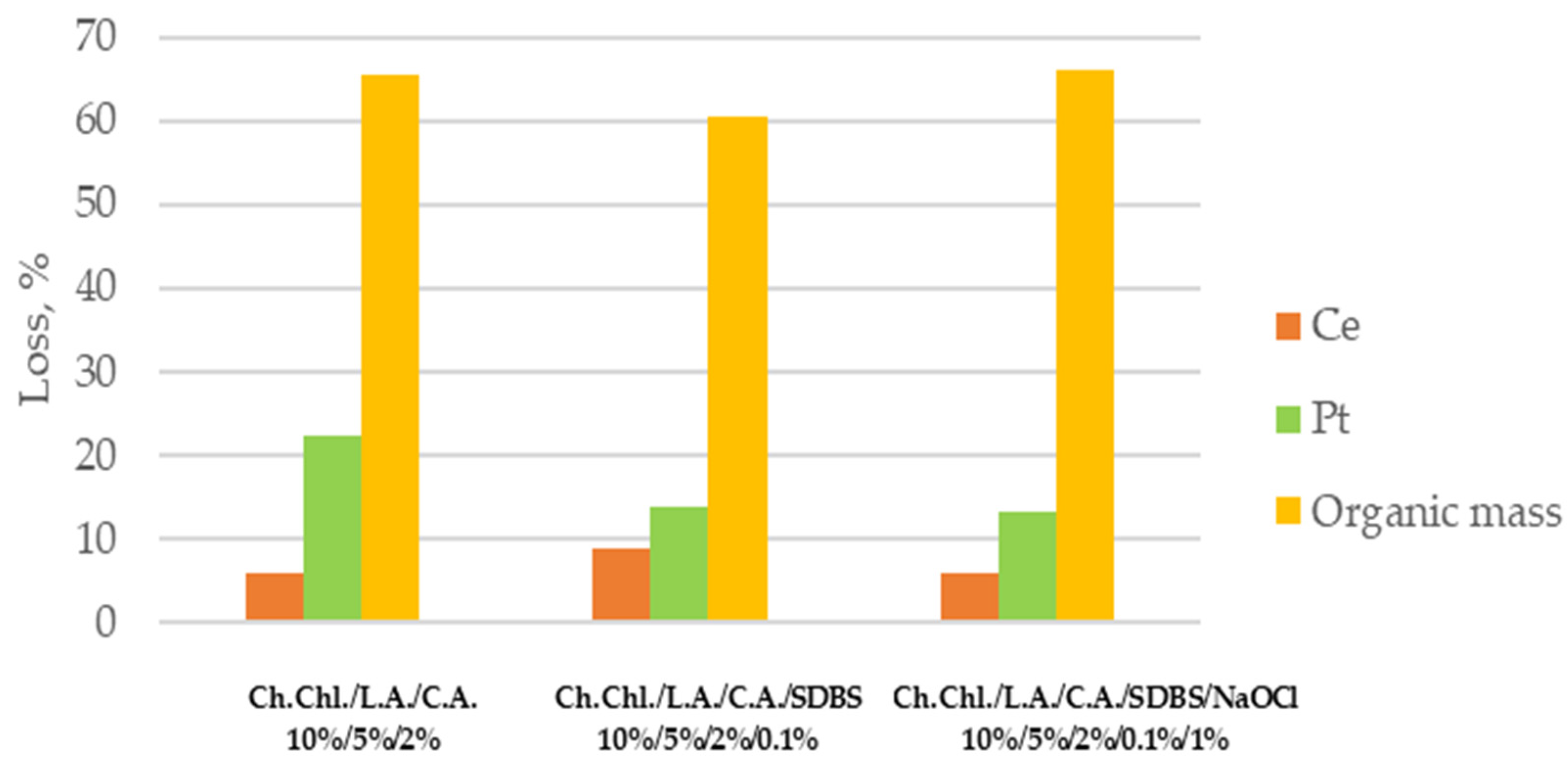
The efficiency of the process is compared with the addition of the surfactant and then the NaOCl in the DES/citric acid solution. Different reagent combinations were studied in order to select the more efficient solution for regenerating c-DPF. According to
Figure 2, the solution that consists of 1%
w/
v NaOCl is more efficient due to the higher organic mass loss and lower Pt and Ce losses.
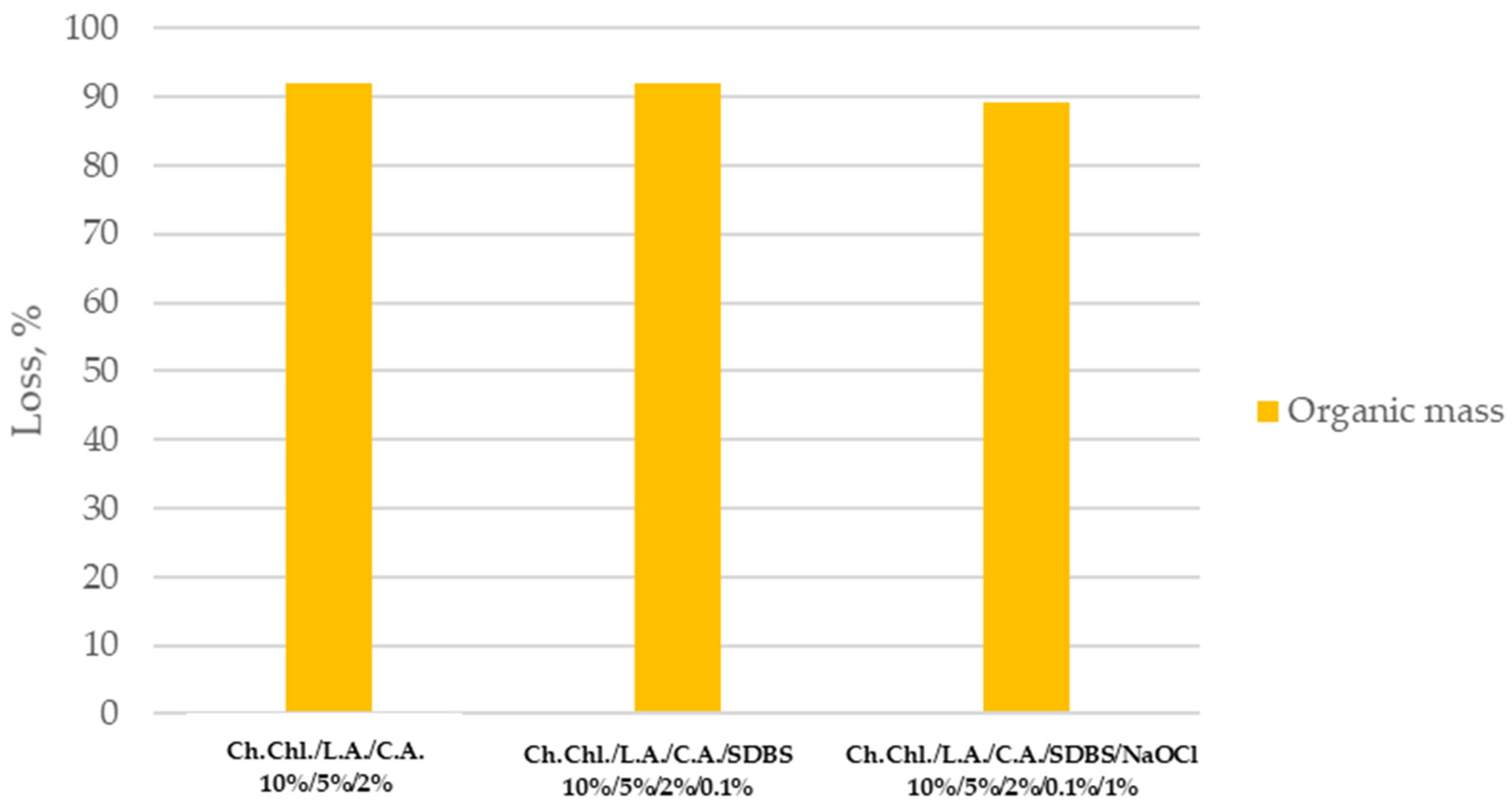
According to
Figure 3, the organic mass loss, related to three different solutions for the regeneration of the DPF is presented. In case of c-DPF’s, the Ch.Chl./L.A./C.A./SDBS/NaOCl solution exhibits high organic mass and disinfectant action.
The most efficient solution for regenerating both c-DPF and DPF is selected (the 10%/5%/2%/0.1%/1% ratio of Ch. Chl./L.A./C.A./SDBS /NaOCl). The one selected solution for c-DPF and DPF is based on removing the highest soot and ash content (up to 90%) with least PGMs (Pt) and REEs (Ce) removal while revealing disinfectant action.
3.4. Industrial Scale Experiment
The scope of this study is to evaluate the developed method of MONOLITHOS Ltd. for regenerating diesel filters using the novel solution on an industrial scale. The catalytic systems are placed in regeneration machine (
Figure 4) without being dismantled.
The regeneration process is performed according to the instructions of the machines supplier. The total residence time in the cleaning machine is 30 min (where time of drying of the filter is included). The effectiveness of the process is tested through the pressure exerted on the filter. As the particulate matter is removed, the pressure in the filter is decreased. Thus, the pressure of the catalytic system was measured before and after the completion process (
Table 2).
The decreased pressure in the catalytic systems, using both regenerating solutions, is attributed to the unblocked filter channels. The efficiency of the novel solution was equal to the commercial’s solvent.
4. Conclusions
In this study, an innovative, eco-friendly and low-cost method for the regeneration of used diesel particulate filters is presented. Spent and deactivated c-DPFs and DPFs were collected by MONOLITHOS Ltd. and the morphology, structure and loading of PGMs and REEs with optical microscopy and XRF analysis were studied. The structural analysis and physicochemical characterization of DPF and c-DPF was carried out before the regeneration process. Then, the optimized conditions for the regeneration of the filters were described, alongside with the presentation of the relative charts. Concerning the regeneration of c-DPF, high organic mass loss and low removal of Pt and Ce are desirable. Respectively, the optimized process’s conditions should be attributed to high organic mass loss during regenerating DPF. Among the studied additives, the selected solution (choline chloride 10% w/w, lactic acid 5% v/v, citric acid 2% w/w, SDBS 0.1% w/w, NaOCl 1% w/w), proved to be efficient in the regeneration of c-DPF and DPF while simultaneously not affecting the morphology, structure, and PGM/REE content of the filter. Therefore, this solution seems promising for the effective regeneration of DPF and was proven to be effective on an industrial scale compared to a commercially available regenerating solution. Finally, the performance of the innovative method was as efficient as the commercial process while being ecofriendly, low-cost, and less harmful to the filter. In addition, the novel regeneration process for diesel filters has the potential to be applied in gasoline particulate filters’ regeneration.
Author Contributions
Conceptualization, I.Y.; methodology, A.M.M. and I.Y.; validation, A.M.M., K.M.S. and I.Y.; formal analysis, all authors; investigation, H.P., S.P. and A.M.M.; resources, K.M.S. and I.Y.; data curation, H.P., S.P. and A.M.M.; writing—original draft preparation, H.P., S.P. and A.M.M.; writing—review and editing, all authors; visualization, K.M.S. and I.Y.; supervision, I.Y.; project administration, K.M.S.; funding acquisition, K.M.S. and I.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by RPF of Cyprus (Research Promotion Foundation) under the program framework of Restart Research 2016–2020, REGFILT project, CONCEPT/0618/0065.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Papagianni, S.; Moschovi, A.-M.; Polyzou, E.; Yakoumis, I. Platinum Recovered from Automotive Heavy-Duty Diesel Engine Exhaust Systems in Hydrometallurgical Operation. Metals 2022, 12, 31. [Google Scholar] [CrossRef]
- Yakoumis, I.; Moschovi, A.; Panou, M.; Panias, D. Single-Step Hydrometallurgical Method for the Platinum Group Metals Leaching from Commercial Spent Automotive Catalysts. J. Sustain. Metall. 2020, 6, 259–268. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).