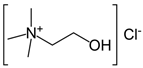
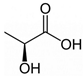
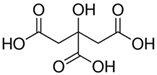
Abstract
The feasibility of using low-environmental-impact leaching media to recover valuable metals from lithium ion batteries (LIBs) has been evaluated. Several deep eutectic solvents (DES) were tested as leaching agents in the presence of different type of additives (i.e., H2O2). The optimization of Co recovery was carried out by investigating various operating conditions, such as reaction time, temperature, solid (black mass) to liquid (DES) ratio, additive type, and concentration. Leaching with final selected DES choline chloride (33%), lactic acid (53%), and citric acid (13%) at 55 °C achieved an extraction yield of more than 95% for the cobalt. The leaching mechanism likely begins with the dissolution of the active material in the black mass (BM) followed by chelation of Co(II) with the DES. The results obtained confirm that those leaching media are an eco-friendly alternative to the strong inorganic acids used nowadays.
Keywords:
batteries; lithium ion batteries; leaching; cobalt; critical metals; deep eutectic solvents; des; black mass 1. Introduction
Batteries are essential for the energy storage in the electronics used in our everyday lives, from small portable electronic devices (PEDs), from cell phones and laptops to medical devices or electric vehicles (EV). Batteries include a great variety of components, such as valuable metals (Li, Co, Ni, Mn, Cu, Al, …), graphite, and organic compounds. A battery cell functions by the reversible transportation of ions and electrons between the anode and cathode separated by a porous membrane generally made of plastics and filled with organic electrolyte containing additive salts. The anode and cathode are constructed by powder of active electrode materials (e.g., cobalt, nickel, manganese, and iron) attached on current cooper and aluminum collector foils.
Future waste production forecasts estimate 7.8 million tons of end of life (EOL) EV battery modules per year by 2040 [1], which is above the current global recycling capacity. Therefore, legislation in Europe has defined that all the spent batteries collected must undergo treatment and recycling. However, despite their continued extensive implementation, methods to recycle and reuse EOL battery materials are still under development.
Commonly used processes for recycling batteries can be divided into three different types: mechanical, pyrometallurgical, and hydrometallurgical processes. Currently, a combination of them is used for recovering at industrial scale the metals contained.
Recycling methods usually involve several steps. Firstly, after collection and selection of the batteries, the cells are discharged (immersion in salt solutions) and mechanically treated (i.e., shredded or crushed, sieved and air, water bath or magnetically separated). When breaking the structure of the batteries, a mixture of casing (steel), current collectors (Cu, Al), plastic separators, electrolyte, and active mass (AM) (i.e., LiCoO2) is obtained. After this pre-treatment, the material is converted in a black milled powder, called black mass (BM). The second step can be a pyrometallurgical treatment. Nowadays, industrial recycling processes involve three different steps: pyrolysis, reduction, and incineration. Finally, a hydrometallurgical treatment is applied. Usually, this last step involves leaching the obtained BM with inorganic acids, such as sulfuric acid, hydrochloric acid, and nitric acid. A final step is usually applied to recover the initial compounds of interest in the battery, by the addition of precipitating agents.
Above-mentioned recycling processes have several drawbacks. In particular, they produce a great negative impact in the environment because of the use of aggressive experimental conditions together with harmful and polluting reagents. Furthermore, these processes are not capable of recycling efficiently all metal substances, such as lithium, cobalt, nickel, manganese, and iron, contained in the spent batteries. Instead, low-environmental-impact leaching media to recover valuable metals contained in BM from several type of LIBs (consumer mixed LIBs, electric vehicle LIBs, etc.) are evaluated in the present research.
2. Materials and Methods
2.1. Chemical Reagents
Firstly, 65% ExpertQ® HNO3 and 37% ExpertQ® HCL supplied by Scharlab (Barcelona, Spain) were used for digestion and analysis of solid samples. For the preparation of the DES solutions, choline chloride ≥98%, citric acid ≥99.5%, and lactic acid 80%, supplied by Merck (Madrid, Spain), were used.
2.2. Spent Lithium Ion Battery Residues
Different pre-treated (mechanically or thermomechanically) BM residues from spent lithium ion batteries were subjected to leaching studies.
2.3. Leaching Procedure
The leaching media were prepared by mixing the solid and/or liquid organic compounds in a proper dilution ratio with deionized water. The lixiviant thus obtained was placed in a 500-mL jacketed reactor, provided with thermal control, a coil condenser, and an anchor impeller rotating at 300 rpm, until the desired temperature was reached. The BM to be treated was dosed slowly to the reactor and the resulting mixture maintained for a period of time from 30 min to 24 h depending on the experiment.
2.4. Analytical Techniques
2.4.1. Metal Composition
All the samples were characterized by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES), model Vista-MPX, CCD Simultaneous (Varian Ltd., Crawley, UK). Liquid samples were directly analyzed after dilution in 1M HCl solutions, while solid samples required an initial digestion with aqua regia.
2.4.2. Mineralogy
The phase and crystallinity were investigated using XRD (Bruker D8 Advanced X-Ray diffractometer). The 2θ interval was [15–90°] with a 0.03°/step and a 2 s/step measurement time.
Microstructure and composition of discrete particles were studied by scanning electron microscopy (SEM) images acquired using a JEOL JSM-5910LV, equipped with an EDX-INCA x-act (Oxford instruments). Samples were immersed in epoxy resin and polished before analysis in order to obtain homogeneous dispersion of particles.
3. Results and Discussion
3.1. Sample Characterization
Broad type of BM samples (A to Q) were characterized (elemental composition in Figure 1). The composition values show significantly wide metal content ranges for each metal (for example Mn 1–17%, Ni 1–21%, and Co 8–38%). This is a reflection of the different chemistries of the collected batteries involved in the production of each BM.
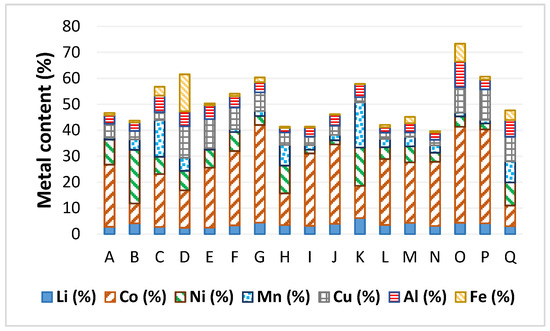
Figure 1.
Metal composition of the different treated BM.
XRD analysis was performed on the as-received BM (an example in Figure 2).
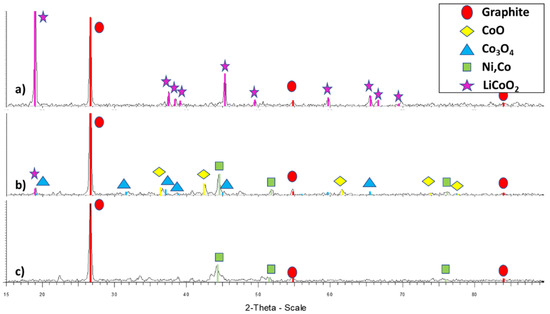
Figure 2.
Examples of XRD spectra for differently (mechanically or thermo-mechanically) pre-treated samples: (a) Sample I, (b) Sample M, and (c) Sample P.
As a result of the different XRD analyses performed, it was concluded that there were different types of Co containing compounds in the investigated pre-treated BM samples:
- -
- LiCoO2 containing BMs (samples A, E, H, I, J, and Q);
- -
- Co oxides (CoO, Co3O4 [CoO, Co2O3]) containing BMs (samples B, C, D, F, K, L, and N);
- -
- Metallic Co, Ni containing rich BMs (samples G, O, and P).
The LiCoO2 structure (present in the cathodic active material in working batteries) is transformed, after being heated over 200 °C [2,3], into an oxide mixture containing Co(II) and Co(III). If the thermal treatment is carried out above 500 °C in reducing conditions, part of the cobalt and other metals present in the BM can be reduced to metallic state [4,5]. This was proven after performing SEM-EDS analysis of thermally treated BM samples, where part of the active cathodic oxides started to transform in metallic particles, as seen in Figure 3 for sample L. EDS mapping was performed, showing their metallic alloy nature (while grey ones had different Co:O ratios), and that bright areas were free of oxygen.
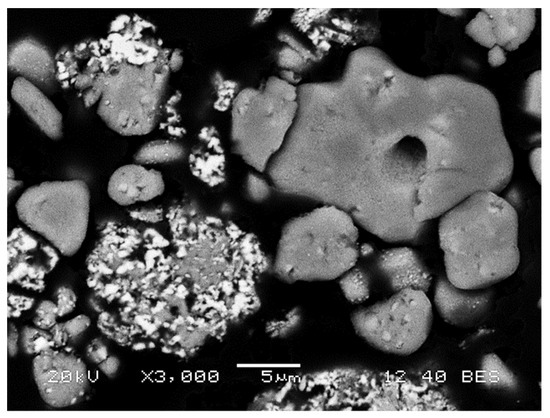
Figure 3.
SEM image of the sample L showing metallic (bright) and oxidized (grey) particles.
3.2. Leaching Experiments
After performing the characterization of all received materials, the leaching systems for preliminary screening tests were selected among validated SOA organic acids [6,7,8,9,10,11] and knowledge in DES leaching systems gained in earlier research studies and European projects (REE4EU [12] and COLABATS [13]).
Initially, a ternary deep eutectic solvent mixture, based on choline chloride (a cheap chemical widely used as an additive for chicken feed that acts as hydrogen bond acceptor) and two organic acids (lactic acid and citric acid, which act as hydrogen bond donors and as good chelating compounds) was used (Table 1).

Table 1.
Ternary DES formulation (compound amount in initial DES formulation).
The parameters studied were leaching time, leaching temperature, BM:DES ratio, water dilution effect, and additives. The ternary DES system contains a certain amount of water due to the fact that the organic compounds are highly hygroscopic and the commercial lactic acid used is 80% in water solution. In addition, the effect of added water in the leaching system was also studied. The stirring speed was tested to achieve appropriate DES–residue contact; 300–320 rpm is the adequate range for the reactor used.
Initial leaching tests were performed at high temperature (85 °C) using low solid to liquid ratio ([BM]:[DES] = 1:50) and low water addition (DES:water = 50:8). Due to viscosity limitations, a certain amount of water dosage (20%) was needed to ease ion mobility and, consequently, to improve the leaching yield.
After performing screening tests over the sample A from 55 °C to 85 °C range, the lowest temperature was proven good enough due to the high Co recovery yield achieved (>90%) (see Table 2). In addition, high temperatures promoted undesirable leaching of metals, such as Cu, which decrease the selectivity of the process.

Table 2.
Results for the temperature screening tests over sample A using 1:50 BM:DES ratio.
BM:DES ratio was evaluated, making it possible to obtain similar Co extraction yields lowering from the initial 1:50 (BM:DES) to a final 1:5, but only if the water dilution was notably increased. In those cases, the dilution of the system leads to a notable decrease in the leaching time. For example, in the case of BM A, where 7.5 h were needed to get 94% Co extraction, the time was reduced to 3 h to achieve the same extraction yield. When the DES content was reduced below this value, the leaching performance of the system decreased, leading to lower Co extractions (80% in 3 h).
The high metal concentration in the 1:5 (BM:DES) leaching systems led to lower extraction yields in the same leaching times, probably due to the notable increase in the viscosity of the DES system and the metal saturation of the solution. The water dilution of 5:12 (DES:water) was able to reduce the viscosity and metal concentration, while the extraction yields were maintained constant. Using these leaching conditions, it was possible to achieve more than 90% of Co extraction in a 3 h reaction.
After studying the use of additives, it was determined that, only in some cases, the dosage of external additives to the leaching system was needed (i.e., oxidizing agents, such as H2O2, as well as reducing agents, such as Al or Cu) (see Table 3).

Table 3.
Comparison of Co leaching yields for a selection of samples when using proper additives.
4. Conclusions
The viability of leaching most of the Co present in a BM residue from LIBs (>99%) in less than 3 h and at low working temperature (55 °C) was demonstrated by using diluted DES mixtures composed by mild organic compounds. The obtained results indicate that the use of additives is not necessary if an appropriate combination of BM pre-treatment and leaching media is selected. Consequently, knowledge of the nature of the black mass to select the adequate operating conditions of the treatment is required.
Author Contributions
Introduction, J.H. and A.S.; Materials and Methods, J.H. and L.Y.; Results and Discussion, J.H. and L.Y.; Conclusions, L.Y. and A.S.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s EU Framework Programme for Research and Innovation Horizon 2020, grant number 776473 (CROCODILE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in ZENODO repository at reference number 10.5281/zenodo.5838166 (https://doi.org/10.5281/zenodo.5838166).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Holland, A.; Jiao, N. End-of-Life Electric Vehicle Batteries: Recycling or Second-Life? Idtechex. 2021. Available online: https://www.idtechex.com/de/research-article/end-of-life-electric-vehicle-batteries-recycling-or-second-life/20900 (accessed on 6 February 2021).
- Lin, X.; Salari, M.; Arava, L.M.R.; Ajayan, P.M.; Grinstaff, M.W. High temperature electrical energy storage: Advances, challenges, and frontiers. Chem. Soc. Rev. 2016, 45, 5848–5887. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Veintemillas-Verdaguer, S.; Bomati-Miguel, O.; Morales, M.P.; Xu, H.B. Thermal history dependence of the crystal structure of Co fine particles. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71, 024106. [Google Scholar] [CrossRef]
- Cendrowski, K.; Zenderowska, A.; Bieganska, A.; Mijowska, E. Graphene nanoflakes functionalized with cobalt/cobalt oxides formation during cobalt organic framework carbonization. Dalt. Trans. 2017, 46, 7722–7732. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Nasim, F.; Choucair, M.; Ullah, S.; Badshah, A.; Nadeem, M.A. Cobalt oxide nanoparticle embedded N-CNTs: Lithium ion battery applications. RSC Adv. 2016, 6, 1129–1135. [Google Scholar] [CrossRef]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Zhang, Y.; Dong, P.; Wang, D.; Zhou, Z.; Duan, J.; Li, X.; Lin, Y.; Meng, Q.; Pai, K.V.; et al. An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. J. Environ. Chem. Eng. 2019, 7, 102854. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Lu, Z.; Xu, Z. A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries. Green Chem. 2020, 22, 4473–4482. [Google Scholar] [CrossRef]
- Tran, M.K.; Rodrigues, M.T.F.; Kato, K.; Babu, G.; Ajayan, P.M. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat. Energy 2019, 4, 339–345. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Zhang, Y.; Dong, P.; Wang, D.; Pai, K.V.; Manjanna, J.; Santhosh, G.; Duan, J.; Zhou, Z.; Xiao, J. Effective and environmentally friendly recycling process designed for LiCoO2cathode powders of spent Li-ion batteries using mixture of mild organic acids. Waste Manag. 2018, 78, 51–57. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Cobalt and Lanthanide Recovery from Batteries (COLABATS). Available online: https://cordis.europa.eu/project/id/603482/es (accessed on 7 June 2021).
- European Commission. Integrated High Temperature Electrolysis (HTE) and Ion Liquid Extraction (ILE) for a Strong and Independent European Rare Earth Elements Supply Chain (REE4EU). Available online: https://cordis.europa.eu/project/id/680507/es (accessed on 7 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).